Abstract
Background: There are currently limited systematic reviews of mobile health interventions for middle-aged and elderly patients with prediabetes from trial studies. This review aimed to gather and analyze information from experimental studies investigating the efficacy of mobile health usability for outcomes among middle-aged and elderly patients with prediabetes. Methods: We conducted a literature search in five databases: Clinicaltrials.gov, the International Clinical Trials Registry Platform (ICTRP), PubMed, ProQuest, and EBSCO, with a date range of January 2007 to July 2022 written in English, following a registered protocol on PROSPERO (CRD42022354351). The quality and possibility of bias were assessed using the Jadad score. The data extraction and analysis were conducted in a methodical manner. Results: A total of 25 studies were included in the qualitative synthesis, with 19 studies using randomized trial designs and 6 studies with non-randomized designs. The study outcomes were the incidence of diabetes mellitus, anthropometric measures, laboratory examinations, measures of physical activity, and dietary behavior. During long-term follow-up, there was no significant difference between mobile health interventions and controls in reducing the incidence of type 2 diabetes. The findings of the studies for weight change, ≥3% and ≥5% weight loss, body mass index, and waist circumference changes were inconsistent. The efficacy of mobile health as an intervention for physical activity and dietary changes was lacking in conclusion. Most studies found that mobile health lacks sufficient evidence to change hbA1c. According to most of these studies, there was no significant difference in blood lipid level reduction. Conclusions: The use of mobile health was not sufficiently proven to be effective for middle-aged and elderly patients with prediabetes.
Keywords:
prediabetic state; telemedicine; m-Health; middle-aged; elderly; intervention; systematic review 1. Introduction
Impaired Fasting Glucose (IFG), Impaired Glucose Tolerance (IGT), or both, and/or elevated levels of Hemoglobin A1c (HbA1c) are all signs of prediabetes [1]. In 2015, there were over 318 million cases of IGT worldwide, and by 2040, there are expected to be about 481 million cases [2]. Prediabetes has become more common as people become older [3]. The transition from normoglycemia to prediabetes or diabetes, or from prediabetes to diabetes, was prevalent in middle-aged and elderly people, suggesting the need for continuing treatment [4]. Individuals with prediabetes are four times more likely than individuals with normal glucose tolerance to develop Type-2 Diabetes Mellitus (T2DM) [5]. Prediabetes can lead to major complications, whether or not patients have T2DM. Microalbuminuria is about twice as common in patients with prediabetes as it is in healthy people, and it can lead to significant problems such as chronic kidney disease (CKD) and macrovascular disease. Prediabetes, on the other hand, can cause many types of diabetic neuropathy, including peripheral neuropathy, polyneuropathy, small-fiber neuropathy, and autonomic neuropathy [6].
Changes in lifestyle, such as daily physical activity and a healthy diet, can reduce the risk of prediabetes, improve the health of diabetic patients, and prevent complications [7]. Consultations with health care professionals at medical facilities are commonly used to modify a person’s lifestyle. Personal coaching for physical activity and personal consultations for healthy eating recommendations are also not generally offered. As a result, mobile health or m-Health interventions could be some of the solutions for improving self-management awareness and compliance with prediabetes interventions including lifestyle changes and healthy foods [8].
Mobile phone ownership is on the rise, with an estimated 90% of the world’s population having one by 2020. According to the most recent statistics, 80% of older adults own a mobile phone (aged 65 years and older) [9]. As the number of mobile phone owners increases, so does the number of mobile phone software applications (apps), including mobile health apps [10]. Health-related mobile phone apps are more preferable since they are less expensive, handier, and more engaging [11]. Their use has been applied in both developed and developing countries, and results demonstrated that they can provide great promise when it comes to providing individualized medical recommendations [8].
Previous systematic reviews on the efficacy of mobile health interventions to improve health outcomes concluded that interventions have the potential to improve health outcomes as well as diet, physical activity, and clinical metabolic parameters [12,13,14]. There were various types of mobile health interventions to achieve positive health outcome change [15,16], such as app-based mobile interventions [16,17,18,19], text message-based interventions [16,20,21,22], website-based interventions [23,24], wearable devices [16,25,26], and so on. Several studies [15,18,23] suggested that future research be conducted to determine the best number and combination of different technology features to maximize intervention efficacy, utilization patterns, and participant engagement. Despite this, evidence on the effectiveness of mobile health interventions for some chronic conditions and non-communicable diseases has been inconsistent and remains weak [27,28].
There are currently limited systematic reviews of mobile health interventions from trial studies for middle-aged and elderly patients with prediabetes [29,30,31]. Elavsky et al. [31] published a systematic review of interventions utilizing mobile technology to change physical activity, sedentary behavior, and sleep among adults aged 50 and older. The review did not include a specific focus on prediabetes management through mobile health interventions and health outcomes. Inconsistent findings, effects on health outcomes, and intervention details remain to be investigated [29,31]. Therefore, the goal of this study was to compile and analyze evidence from experimental studies investigating the efficacy of mobile health usability for outcomes among middle-aged and elderly patients with prediabetes.
2. Methods
2.1. Study Design and Research Sample
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (Supplementary File S1) [32]. The protocol has been registered in the Prospective Registry of Systematic Reviews (PROSPERO) database. The registration number is CRD4202235435. We used PubMed, ProQuest, EBSCO, Clinicaltrials.gov, and the International Clinical Trials Registry Platform (ICTRP) to conduct a systematic search. The scope of the search was limited to English-language publications published between January 2007 and July 2022. Duplicate records were eliminated, and all records were imported into Mendeley Desktop from the database search.
The following keywords were used to search the database: ((prediabetes AND (minimize risk factor OR physical activity OR body mass index OR weight loss OR healthy diet OR smoking OR alcohol consumption) AND (mobile phone application OR mobile phone app OR telemedicine OR telehealth OR mobile health OR health, mobile OR health OR mobile) AND (middle-aged OR elderly)).
2.2. Inclusion and Exclusion Criteria
The following were the inclusion criteria for the studies that were included: (1) Participants had prediabetes according to American Diabetes Association (ADA) criteria, which included impaired fasting glucose (IFG) 5.6–6.9 mmol/L, impaired glucose tolerance (IGT) 7.8–11.0 mmol/L, or HbA1c (hemoglobin A1c) 5.7–6.4% without a history of T2DM and diabetes medications (insulin or oral anti hyperglycemic agents); (2) Participants were 40 years of age or older; (3) Mobile health intervention is a major component of the study; (4) the mobile health provided lifestyle modification (e.g., physical activity and healthy diets); (5) the study measured participants’ glycated hemoglobin (HbA1c), weight, fasting blood sugar, body mass index(BMI), or other health-related outcomes; (6) the study design is a randomized controlled trial (RCT) or other types experimental study; and (7) the full-text of the article was available. We excluded studies that: (1) Participants had gestational diabetes mellitus (GDM); (2) the study was not a primary study; (3) protocol publication; (4) and did not meet one or more of the inclusion criteria.
2.3. Study Selection
Three reviewers (Y.A.J., R.N. and D.D.E.) worked independently to identify articles by looking at the titles, then studying the abstracts, and finally reading the full-text form. The full articles of any possibly relevant studies were then obtained for a thorough review by all reviewers. In the event of a disagreement, a consensus decision was achieved.
2.4. Data Extraction
Three reviewers (Y.A.J., R.N. and D.D.E.) retrieved data from the included studies and entered it into a spreadsheet independently. Author, year, country, sample size, study design, details of intervention and control, outcomes of interest, and the other key results were among the data retrieved from the studies. If the needed data were not reported in an article, we contacted the article’s corresponding author to obtain any missing information. If within four weeks of being contacted, the corresponding author does not respond, then the study was not included.
2.5. Quality Assessment
Using an adapted version of the Jadad score, two reviewers (R.N. and D.D.E.) independently assessed the quality and risk of bias of the included studies [33]. In the event of a disagreement, another author (Y.A.J.) joined the debate to assist in the resolution of the issues.
2.6. Data Synthesis
The Cochrane methodologies for data analysis and syntheses [34] were used to accomplish the data synthesis (narrative synthesis). Data from both qualitative and quantitative sources were described, analyzed, and classified individually. The characteristics of the research, as well as the significant disparities between them, were meticulously recorded. Topics that were relevant were highlighted, and the data were translated into a descriptive style. As stated in the original studies, the outcome results are described in detail. Due to a paucity of studies with similar settings, interventions and results, a meta-analysis could not be performed.
3. Results
3.1. Search Results
For our investigation, we selected five electronic search engines (PubMed, ProQuest, EBSCO, Clinicaltrials.gov, and the International Clinical Trials Registry Platform (ICTRP)) accessed on 8 July 2022. There were 11,938 electronic database documents discovered in all. A total of 11,871 articles were removed from the study due to being irrelevant (title, conference, published, editorial, news, comments, and reviews). In addition, 11 articles were eliminated from the study because they had a title that was duplicated across four search engines. Following that, 16 papers were eliminated since the abstracts did not meet the inclusion requirements. A total of 41 full articles were reviewed for eligibility and 25 were included in the qualitative synthesis (Figure 1).
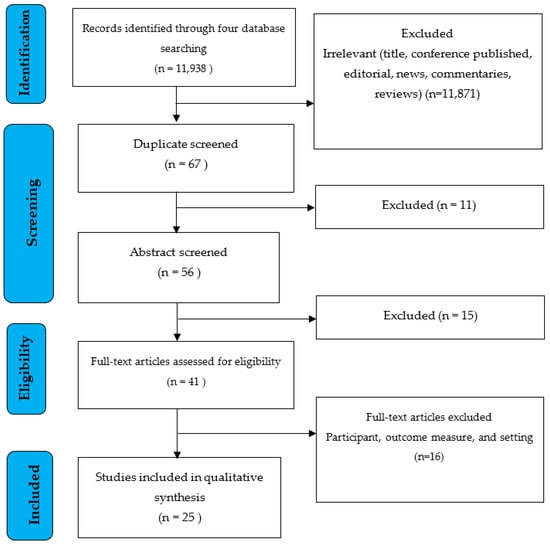
Figure 1.
PRISMA flow diagram [35].
3.2. Characteristics of the Studies
This systematic review was comprised of 19 studies with randomized trial designs [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] and 6 studies [55,56,57,58,59,60] with other types of trial designs (pragmatic trial and quasi-experimental). The features of the studies are detailed in Table 1. The characteristics of the participants and the mobile health intervention are summarized in Table 2. A total of 7600 participants from 19 trials were randomized, with 6318 completing the study, indicating that ±17% of the participants did not finish the randomized trials. A total 1866 (94.7%) completed the studies from non-randomized trial design. There are eight studies [38,47,49,50,56,58,59,60] that samples of less than 100 participants. There were three studies with a sample size of more than 1300, two RCT studies [52,61] and 1 pragmatic study [57]. The majority of studies reported mobile health as an early intervention for individuals with prediabetes. Chen’s [55] study reported not only personal counseling as an early intervention but also risk scoring as screening.
A total of thirteen studies [36,39,40,41,43,44,46,48,50,51,53,57,58] only used hbA1c as a diagnostic tool for prediabetes. Research conducted by Ramachandran [36] only used an oral glucose tolerance test (OGTT) as a diagnostic standard for prediabetes. Three other studies, namely Chen [55], Block [37], and Muralidharan [54], only used plasma fasting glucose (FG) tests. There were seven other studies that use a combination examination to establish the diagnosis of prediabetes. Bender, Sharit, Kunthi, Stewart, Sevilla, and Moravcova [42,45,49,56,59,60] used three tests, namely HbA1c, OGGT, and Fasting Glucose (FG) levels. Meanwhile, Fukuoka [47] used a combination of HbA1c and FG assays. There was one study that diagnosed prediabetes by not using laboratory tests but using risk category scoring from the ADA [38].
The youngest average age of 37.8 ± 9.2 years was found in Muralidharan’s [54] study, while the oldest average age was found in McLeod’s [53] study, which is 61.8 ± 9.5 years old. Hamdan’s [41] study only examined female subjects and Ramachandran’s [36] only examined male subjects. Bender’s study only intervened in Filipino Americans because they had a higher risk of developing type 2 diabetes [49]. The shortest studies lasted 3 months, and were found in the Sevilla, Kondo, and Griauzde [46,50,60] studies. Khunti’s study had the longest intervention which was 48 months. There were three types of interventions used, namely text message-based, website-based, and mobile phone apps.
Staite [39] used all three intervention combinations. Block [37] used websites and mobile phone apps. Stewart [59] used text message-based and mobile phone apps. There were 15 studies using only mobile phone apps, 3 other studies using websites, and 4 studies using text messages only. There were 6 studies that use wearable devices as part of the intervention [39,43,49,50,59]. The study outcomes were classified into five categories, which included the incidence of diabetes mellitus (DM), anthropometric measures, laboratory examinations, measures of physical activity, and eating behavior. Ramachandran [36], Khunti [42], and Nanditha [52] examined all of the five outcome categories. Kondo and Chen examined four of the five categories except incidence of DM. Meanwhile Fischer [57] (anthropometric measures) and Francis [40] (physical activity measures) only assessed one of the five clinical outcome parameters.
3.3. Quality Assessment
The Jadad score [33,62] was used to measure the quality of the trials, which reflects the quality of research based on their description of randomization, blinding, and dropouts (withdrawals). The methodology features of the Jadad score are shown in Table 3. Each study in this systematic review received a score (Table 4). In the Jadad score, the scale runs from 0 to 5, with a score of ≤2 indicating a low-quality report and a score of ≥3 indicating a high-quality report [33]. Six [55,56,57,58,59,60] studies have low quality, according to the analysis. This is due to the trial’s insufficiency of randomization in selecting participants. Concerning “blinding,” we understand that many studies do not receive points because the nature of the intervention does not allow for “blinding”.

Table 1.
Characteristics of the studies.
Table 1.
Characteristics of the studies.
| Author, Year | Subject Criteria | Number of Subjects | Duration of Intervention and Study Location | Type of Mobile Health | Functions Used | Methods | Outcome Measures | Measurement Methods Detail |
|---|---|---|---|---|---|---|---|---|
| Ramachandran et al., 2013 [36] | Impaired glucose tolerance (OGTT: 140–199 mg/dL) Age: 35–55 years BMI: ≥23 kg/m² or more. Sex: Male | Randomized Participants: 537 Analyzed Participants: 537 Completed: 527 | 24 months Southeast India | Text message based: Short message Service (SMS), Text Message based on the transtheoretical model stage | Encouraged lifestyle change could reduce incident type 2 diabetes | RCT (prospective,2-arm parallel-group) | Primary Outcome
| Primary Outcome Incident T2DM: The estimated cumulative incidence of T2DM was calculated using unadjusted Cox regression analysis. Secondary Outcome Body Mass Index (BMI), Waist circumference, and Blood Pressure (systolic and diastolic): Mean of two readings obtained at each visit using standardized methods. Physical activity evaluation using a questionnaire Total dietary intake: dietary intake as determined by a 24-h recall Lipid profile (total cholesterol, triglycerides (TG), and HDL): fasting venous plasma sampling HOMA-IR: ([fasting insulin (mU/L) × fasting glucose (mmol/L)] formula/22·5) |
| Chen et al., 2014 [55] | Impaired Fasting Glucose (FG:100–125 mg/dL) Age: 40–70 years BMI: Not Reported Sex: Male and Female | Eligibility Participant: 253 Analyzed Participant: 231 Completed: 231 | 6 months Lu’an Anhui, China | Website Based: SWAP-DM2 | Risk scoring and personalized counseling lifestyle management | Quasi-Experimental Mixed Methods |
| not reported in detail |
| Block et al., 2015 [37] | Impaired Fasting Glucose (FG: 100–125 mg/dL) and or Prediabetes (A1c: 5.7–6.4%) Age: 30–69 years BMI: ≥ 27 kg/m (BMI ≥25 kg/m for Asian participants) Sex: Male and Female | Randomized Participants: 340 Analyzed Participants: 339 Completed: 292 | 6 months Berkeley USA | “Hybrid” Mobile phone apps and Website Based: The Alive-PD | To improve glycemic control and reduce diabetes risk through lasting changes in physical activity and eating habits | RCT (wait-list controlled Trial) | Primary Outcome
| not reported in detail |
| Fukuoka et al., 2015 [38] | Impaired Fasting Glucose (FG: 100–125 mg/dL) and or Prediabetes (A1c: 5.7–6.4%) Age: ≥35 years BMI: BMI of at least 25 kg/m (BMI > 23 kg/m for Asian-Pacific Islanders participants) Sex: Male and Female | Randomized Participants: 61 Analyzed Participant: 61 Completed: 56 | 5 months San Francisco and Berkeley, CA, USA | Mobile phone apps based: The mDPP trial app | to supplement the in-person sessions and was intended to enhance their effect. Electronic diaries for self-monitoring of weight, activity, and caloric intake, daily reminders. | RCT (2-arm, parallel groups) | Primary Outcome
| All outcomes are objectively measured and reported by participants. but not in detail |
| Fischer et al., 2016 [39] | Prediabetes (HbA1c from 5.7–6.4%) and Obesity (BMI 25–50 kg/m2) Age: ≥18 years BMI: 25–50 kg/m2 Sex: Male and Female | Randomized Participants: 163 Analyzed Participants: 157 Completed Participants: 157 | 12 months Denver, Colorado, USA | Text message based: Text message using the National DPP (Diabetes Prevention Program) curriculum content (SMS4PreDM) | To support weight loss | RCT (parallel groups) | Primary Outcome
| not reported in detail |
| Bender et al., 2018 [40] | Prediabetes (HbA1c from >5.6%) or Impaired Fasting Glucose (FG; 100–125 mg/dL) Or Impaired glucose tolerance (OGTT: 140–199 mg/dL) Age: ≥18 years BMI: >23 kg/m2 Sex: Male and Female Notes: Self-identified as Filipino | Randomized Participants: 67 Analyzed Participants: 61 Completed Participants: 61 | 6 months San Francisco, USA | Mobile phone apps based: (Fit and Trim) App Plus Wearable Devices (Fitbit Devices) | Weight loss lifestyle intervention including virtual social support | RCT (2-arm, wait-list controlled Trial) | Primary outcome:
| not reported in detail |
| Sharit et al., 2018 [56] | Prediabetes (HbA1c from 5.7–6.4%) or Impaired Fasting Glucose (FG: 100–125 mg/dL) Or Impaired glucose tolerance (OGTT: 140–199 mg/dL) Age: ≥20 years BMI: 25–42 kg/m2 Sex: Male and Female | Eligibility Participant: 38 Analyzed Participants: 38 Completed Participants: 38 | 13 weeks A large city in the USA | Website based: Practice on use of Track Health (TH) Journals and Vitals + Readings features as a basis for promoting positive Physical Activity (PA) and dietary lifestyles | Promoting positive Physical Activity (PA) and dietary lifestyles | Quasi-Experimental (pilot 3 month clinical trial pre-post design) | Primary outcome:
| not reported in detail |
| Griauzde et al., 2019 [41] | Prediabetes (HbA1c from 5.7–6.4%) Age: >18 years old BMI: Not Reported Sex: Male and Female | Randomized Participants: 69 Analyzed Participants: 55 Completed Participants: 55 | 12 weeks Ann Arbor, MI, USA | Mobile phone apps based: App-Only And App-Plus Wearable device (Fitbit Devices) | To help individuals gain awareness of and control over the factors that influence their health behaviors (Sleep; Presence; Activity; Creativity; Eating) (S.P.A.C.E). | RCT (parallel, 3-arm) Mixed Methods |
| not reported in detail |
| Fischer et al., 2019 [57] | Prediabetes (HbA1c from 5.7 06.4%) Age: > 18 years old BMI: Not Reported | Eligibility Participant: 1.518 Analyzed Participants: 1.518 Completed Participants: 1.492 | 12 months Denver, Colorado, USA | Text message based: Text message using the National DPP (Diabetes Prevention Program) curriculum content (SMS4PreDM) | To support weight loss | Pragmatic trial |
| For SMS4PreDM participants, a repeating measure analysis used all weights available in the EHR from routine healthcare visits within a year of the individual’s start date, or matched identification dates for controls. |
| Ramos et al., 2020 [42] | Prediabetes (HbA1c from 5.7–6.4% within 3 months before study enrollment) Age: >18 years old BMI: NA Sex: Male and Female Notes: referral from the patient’s physician | Eligibility Participant: 1.513 Randomized Participants: 202 Analyzed Participants: 202 Completed Participants: 155 | 12 months New York, NY, USA | Mobile phone apps based: Noom’s App With the National DPP (Diabetes Prevention Program) curriculum content | To support weight loss | RCT (two-arm, parallel RCT) | Primary outcome:
Program engagement
| The DCA Vantage (Siemens) point-of-care (POC) HbA1c machine was used in primary care and endocrinology clinics. |
| Nandhita et al., 2020 [43] | Prediabetes (HbA1c from 6.0–6.4%) Age: 35–55 years (India) 40–74 years (UK) BMI: ≥23 kg/m2 (India) Sex: Male and Female Notes: Pre-screening: having three or more risk factors, including age 35–55 years, BMI ≥ 23 kg/m2, waist circumference ≥90 cm in men and ≥80 cm in women, first-degree family history of type 2 diabetes, history of hypertension or prediabetes, or habitual sedentary behavior | Randomized Participants: 2062 Analyzed Participants: 2062 Completed Participants: 1.763 | 24 months UK and India | Text message based: Short message Service (SMS), Text Message based on the transtheoretical model stage | To provide additional education and motivation. The messages provided tips, suggestions, and positive reinforcement for healthy behaviors including goal setting, physical activity, dietary planning, and personal strategies for a lifestyle change. | RCT (two-arm, parallel RCT) | Primary outcome:
| HbA1c international criteria for fasting plasma glucose or HbA1c at any study review visit or in any healthcare setting (UK); incident type 2 diabetes determined solely by HbA1c (India) Body Mass Index (BMI), Waist Circumference, and Blood Pressure (Systolic and Diastolic): the average of two readings taken by standard procedures at each visit. The serum lipid profile (total cholesterol, low-density lipoprotein, HDL-cholesterol, and triacylglycerols) and HbA1c were measured using standard enzymatic procedures with quality control. |
| Mcleod et al., 2020 [44] | Prediabetes (HbA1c from 5.9–6.6% current or tested in preceding 3 months) Age: 18–75 years BMI: ≥20–40 kg/m2 Sex: Male and Female Notes: participant included diabetes | Randomized Participants: 225 Analyzed Participants: 201 Completed Participants: 201 | 12 month The greater Wellington and Waikato regions of the North Island of New Zealand, | Mobile phone apps based: BetaMe/Melon App | a structured, in-person lifestyle modification program focused on weight reduction | RCT (parallel-group 2-arm single-blinded superiority trial) | Primary outcome:
| The Protocol describes standardized measurement procedures. |
| Muralidharan et al., 2020 [45] | Prediabetes Impaired Fasting Glucose (FG 100–125 mg/dL) Age: 20–65 years BMI: ≥25 kg/m2 Sex: Male andFemale | Randomized Participants: 741 Analyzed Participants: 561 Completed Participants: 561 | 4 month Chennai, Bangalore and New Delhi, India. | Mobile phone apps based: mDiab | To support Diabetes prevention program (DPP) and culturally modified it to suit the Indian population | RCT (parallel-group two-arm) |
| Omron HBF-306 Body Fat Monitor was used to calculate the percentage of body fat. Every day, the waist circumference was measured with a standard, non-stretchable, 1-inch tape measure that was calibrated. While calibrating, it was ensured that the difference between the two readings was no greater than 0.2 cm. If the measuring tape was damaged, whether stretched or twisted, or if graduations had been erased, it was replaced with a new one. The study participants were instructed to stand with their feet together and their arms by their sides, palms facing inward. The technician then determined the midpoint by locating the inferior margin of the last rib and the crest of the ileum. After ensuring the proper placement of the tape around the waist, a measurement to the nearest 0.1 cm was taken. Two measurements were taken, and the average of the two was taken. |
| Xu et al., 2020 [46] | Prediabetes (High risk for diabetes, measured by the American Diabetes Association (ADA) screening tool (score of ≥5 or) Age: ≥18 years BMI: NA Sex: Male and Female Notes: access to WeChat | Randomized Participants: 81 Analyzed Participants: 76 Completed Participants: 76 | 6 month Beijing, China | Mobile phone apps based: DHealthBar | improving eating habits and physical activity, | RCT (A pragmatic, parallel-group, 2-arm) | Primary Outcome:
| Participants filled out self-reported questionnaires. |
| Staite et al., 2020 [47] | Prediabetes (HbA1c from 5.7–6.4%) Age: 18–65 years BMI: ≥25 kg/m² (≥23 kg/m² if of Asian ethnicity) Sex: Male and Female Notes: being ambulatory | Randomized Participants: 200 Analyzed Participants: 156 Completed Participants: 156 | 12 month London, United Kingdom. | “Tribrid” Mobile phone Apps based & Web-based & Text Messages based: the wristband and the associated study-specific smartphone app (Buddi wristband) | to support participants in forming healthy intentions, encourage self-monitoring of lifestyle behaviors, and promoting social support | RCT (two-arm, parallel, single-blind) | Primary outcome:
| Using a stadiometer, weight was measured in light clothing, without shoes, to 0.01 kg, and height was measured to 0.1 cm (Class 3 Tanita SC240). BMI (kg/m2) was calculated based on weight and height measurements. Using a non-extensible steel tape against the bare abdomen, the waist circumference (cm) was measured horizontally halfway between the lowest rib and the upper prominence of the pelvis. The waist-to-hip ratio was also calculated by measuring hip circumference. Diastolic and systolic blood pressure (BP), as well as resting heart rate, were measured using digital Omron BP monitors (Omron M7) and standardized procedures for the average of two readings taken one minute apart while seated. |
| Francis et al., 2021 [48] | Prediabetes (HbA1c from 5.7–6.4%) And ≥25 kg/m2 Or Person with ≥30 kg/m2 Only Age: >18 years old BMI: ≥25 kg/m2 Sex: Male and Female | Randomized Participants: 430 Analyzed Participants: 388 Completed Participants: 388 | 6 months Iowa City, IA, USA | Mobile phone apps based: MapTrek-Plus Fitbit Devices | To promotes walking | RCT (two-arm, parallel) | Primary outcome:
| The Fitbit Zip activity monitor objectively measures the number of daily steps. |
| Hamdan et al., 2021 [49] | Prediabetes (HbA1c from 5.6–6.9%) Age: 18–60 years BMI: ≥25 kg/m2 Sex: Female Notes: active users of android or IOS-based | Cluster Randomized: 3 primary care Randomized Participants:110 Analyzed Participants: 110 Completed Participants: 483 | 6 months Riyadh, SA | Mobile phone apps based: Al-Nahdi Mobile App And Social Media | Lifestyle modifications emphasizing the importance of weight loss, healthy diet and physical activity. | RCT (multicenter, 3-arm cluster randomized, Multi-intervention) | Primary outcome:
| Anthropometric measurements were taken at baseline and after 6 months in all three arms of the study. Height (to the nearest cm) and weight (to the nearest 100 g) were measured in light clothing without shoes on calibrated scales. Waist circumference (cm) was measured at the umbilical level without clothing after exhaling in a relaxed standing position. The traditional mercurial sphygmomanometer was used to measure blood pressure (mmHg) after a sufficient rest at each visit, and the average was recorded. Body mass index (BMI) is calculated by dividing weight in kg by height in meters squared (kg/m2). All measurements were taken by trained and licensed nurses and dietitians. |
| Summers et al., 2021 [58] | Prediabetes (HbA1c from 5.6–6.9%) Age: ≥18 years BMI: Not reported Sex: Male and Female Notes: Diabetes and prediabetes | Eligibility Participant: 27 Analyzed Participants: 27 Completed Participants: 21 | 12 months Norwood Surgery in Southport, United Kingdom. | Mobile phone apps based: The Low Carb Program App | Educates and supports sustainable dietary changes focused on carbohydrate restriction | Single-Arm Prospective Study | Primary outcome:
| not reported in detail |
| Khunti et al., 2021 [50] | Prediabetes (HbA1c from 6–6.4% within the last 5 years.) or Impaired Fasting Glucose (FG 100–125 mg/dL) Or Impaired glucose tolerance (OGTT: 140–199 mg/dL) Age: 40–74 years, or 25–74 years if they were South Asian BMI: Not Reported Sex: Male and Female | Randomized Participants: 1366 Analyzed Participants: 1366 Completed Participants: 986 | 48 month Leicester, United Kingdom. | Mobile phone apps based: an integrated mobile health | to support the maintenance of behavior change within Walking Away | RCT (three-arm, parallel-group, pragmatic, superiority) | Primary outcome:
| Estimated conversion rate at the lower level reported for traditionally defined prediabetes Height, body weight, body fat percentage, and waist circumference were all measured to the nearest 0.5 cm, 0.1 kg, 0.5%, and 0.1 cm, respectively. The waist circumference was measured with a soft tape measure halfway between the lowest rib and the iliac crest. When the participant was seated, the arterial blood pressure was taken from the right arm. Venous sampling was used to assess standard biomedical outcomes such as HbA1c, a lipid profile (triglycerides, HDL, LDL, and total cholesterol), urea and electrolytes (sodium, potassium, urea, and creatinine), and liver function tests (albumin, total bilirubin, alkaline phosphatase, and alanine transaminase). |
| Katula et al., 2022 [51] | Prediabetes (HbA1c from 5.7–6.4%) Age: ≥19 years BMI: ≥25 kg/m2 (≥22 kg/m2 if participant self-identified as Asian) Sex: Male and Female | Randomized Participants: 599 Analyzed Participants: 599 Completed Participants: 483 | 12 months Omaha, Nebraska., USA | Mobile phone apps based: App-Plus Wearable Devices | To support weight loss | RCT (two-rm, parallel single-blind) | Primary outcome:
| All study measures were collected at the University of Nebraska Medical Center in a 4-week assessment window by staff who were blinded to study group assignment at each assessment point (baseline, 4 months, and 12 months). Sociodemographic data and health literacy were only collected at the start of the study. HbA1c levels were determined using nonfasting blood samples. Blood was drawn via venipuncture and processed at the University of Nebraska Medical Center’s central diagnostic testing laboratory for cardiovascular disease (lipid panels) using the boronate affinity analytical technique. Weight was measured in stocking feet on a calibrated medical-grade scale with the participant fasting. |
| Stewart et al., 2022 [59] | Prediabetes (HbA1c from 5.7–6.4%) Age: Not Reported BMI: Not Reported Sex: Male and Female Notes: One method of participant recruitment from primary care manager (PCM) referral | Eligibility Participant: 33 Analyzed Participants: 33 | 12 month Augusta, Georgia, USA | “Hybrid” Mobile phone apps and text message-based: Mobile phone App Plus Wearable Devices (FitBit) And The combination of the daily text messages of DPP content | to support participants in losing weight (5–7%) and being active (150 physical activity minutes/week) | Quasi-experimental (pre/post design) |
| Baseline biometric/survey measures and the electronic medical record Physical activity as self-reported |
| Lim et al., 2022 [52] | Prediabetes (HbA1c from 6–6.4%) or Impaired Fasting Glucose (fasting glucose 100–125 mg/dL) Or Impaired glucose tolerance (oral glucose tolerance test: 140–199 mg/dL) Age: 21–75 years BMI: ≥23 kg/m2 Sex: Male and Female | Participant Eligible: 217 Randomized Participants: 148 Analyzed Participants: 148 Completed Participants: 140 | 6 month Singapore | Mobile phone apps based: the nBuddy Diabetes app | To empower individuals through prompts and cues and achieve clinically meaningful weight loss of >5% | RCT (multicenter concurrent parallel RCTs) | Primary outcome:
Secondary outcome:
| Body weight was measured in the clinic by research staff using a standard digital weighing scale (Omron HN-289, Japan) after an overnight fast, with participants dressed lightly and without shoes. To calculate BMI, height was measured without shoes to the nearest centimeter. Venous blood samples were collected after 8–12 h of overnight fasting and processed at CAP-accredited laboratories (National University Hospital Department of Laboratory Medicine or National Healthcare Group Diagnostics). Plasma glucose was determined using a photometric assay using the hexokinase method, and HbA1c was measured using high-performance liquid chromatography. An enzymatic colorimetric assay was used to determine serum lipids and creatinine levels. Self-reported questionnaires were used to collect participants’ physical activity levels in minutes per week at baseline, 3 months, and 6 months. At the baseline, 3-, and 6-month visits, dietary intake was collected using a 2-day food diary and analyzed using the nBuddy Dashboard’s nutrient analysis platform, which includes over 14,000 food items and incorporates the Singapore energy and nutrient composition of food, Malaysian Food Composition, and USDA food databases, as well as nutritional information from food packaging and nutrient analysis of recipes. |
| Sevilla et al., 2022 [60] | Prediabetes (HbA1c from 6–6.4%) or Impaired Fasting Glucose (FG 100–125 mg/dL) Or Impaired glucose tolerance (OGTT: 140–199 mg/dL) Age: 18–65 years BMI: ≥20–40 kg/m2 Sex: Male and Female Notes: Some participants were randomly prescribed 750 mg extended release metformin every 12 h to evaluate the “medication” module of the web platform | Eligibility Participant: 122 Randomized Participants: 77 Analyzed Participants: 77 Completed Participants: 77 | 3 month Mexico City, Mexico | Web-based: Vida Sana | Lifestyle modification counseling with the goal of reaching a weight loss of >3%. | Prospective Interventional Study |
| Dual-energy X-ray absorptiometry is used to determine body composition. Waist and hip circumferences (to the nearest 0.5 cm) were measured at the midpoint between the lower ribs and the iliac crest and at the level of the trochanter major, respectively. Participants who met the inclusion criteria were invited back the following week for an intervention visit. Using colorimetric enzymatic methods, measurements from the oral glucose tolerance test included glucose levels, insulin levels, and lipid profiles (Unicel DxC 600). Beckman Coulter Synchron Clinical System. A chemiluminescence assay was used to measure insulin levels (Access 2, Beckman Coulter). HbA1c was measured using a Variant II Turbo system (BIORAD) and a 4-mL peripheral blood sample was drawn via venipuncture using the standardized technique. Diet and physical activity questionnaires were administered. |
| Moravcova et al., 2022 [53] | Prediabetes (HbA1c from 6–6.4%) or Impaired Fasting Glucose (FG 100–125 mg/dL) Or Impaired glucose tolerance (OGTT: 140–199 mg/dL) Or Insulin resistance (IR) (HOMA-IR > 2.7) Age: 18–60 years BMI: ≥30 kg/m2 Sex: Male and Female Notes: participant included diabetes | Randomized Participants: 100 Analyzed Participants: 78 (3 months) 51 (6 months) Completed Participants: 78 (3 months) 51 (6 months) | 6 month Mexico City, Mexico | Mobile phone apps based: Vitadio application | to provide individualized support in lifestyle modification and self-management | RCT (prospective, double-armed) | primary outcome:
only the 3-month laboratory measurement is evaluated in this paper. The 6-month attrition rate was higher dropout were associated with the COVID-19 pandemic. | The study procedure includes four visits: at baseline, three months later, six months later, and twelve months later, with anthropometric and laboratory examinations performed at each visit. Blood samples were collected after a 12- to 14-h fast. A glucose hexokinase method was used to measure glucose. An enzymatic colorimetric test was used to examine lipids. A two-step sandwich enzyme immunoassay using monoclonal antibodies was used to measure serum insulin. Matthews’ formula was used to calculate HOMA-IR. Body composition was determined using bioelectrical impedance analysis with the InBody 370 and 15 impedance measurements at 5 body segments, as well as a tetrapolar 8-point tactile electrode system. |
| Kondo et al., 2022 [54] | Prediabetes Impaired Fasting Glucose (FG: 100–125 mg/dL) Age: 40–75 years BMI: ≥25 kg/m2 Sex: Male and Female Notes: participant has metabolic syndrome | Randomized Participants: 122 Analyzed Participants: 75 Completed Participants: 74 | 3 months Tokyo, Japan. | Mobile phone apps based: DialBeticsLite | Daily recording of several physical parameters, in addition to tracking lifestyle behavior, that is, diet and exercise | RCT (open-label, 2-arm, parallel-design | Primary Outcome:
| VFA was calculated by distinguishing visceral fat from abdominal subcutaneous fat using current flow from two different routes (DU-ALSCAN, HDS-2000, Fukuda Colin). The HDS-2000 underestimates VFA when compared to computed tomography scans, but the correlation was very strong (r = 0.89). Because of its simplicity and noninvasiveness, the HDS-2000 can be a good option for evaluating VFA. To avoid variation in measurement procedures across raters, the VFA of all participants was measured by the same individual with sufficient experience. The person was able to see again after the group intervened. Secondary outcomes included changes in physical and metabolic parameters from baseline to the 3-month follow-up. Physical parameters included BW, WC, BMI, and BP. Blood tests were used to determine metabolic parameters such as cholesterol, triglyceride, fasting plasma glucose, and HbA1c levels. |

Table 2.
Summary of participant characteristics and mobile health intervention.
Table 2.
Summary of participant characteristics and mobile health intervention.
| Results | Chen et al., 2014 [55] | Fischer et al., 2019 [57] | Sevilla et al., 2022 [60] | Sharit et al., 2018 [56] | Stewart et al., 2022 [59] | Summers et al., 2021 [58] | Bender et al., 2019 [40] | Block et al., 2015 [37] | Fischer et al., 2016 [39] | Francis et al., 2021 [48] | Fukuoka et al., 2015 [38] | Griauzde et al., 2019 [41] | Hamdan et al., 2021 [49] | Katula et al., 2022 [51] | Khunti et al., 2021 [50] | Kondo et al., 2022 [54] | Lim et al., 2022 [52] | Mcleod et al., 2020 [44] | Moravcova et al., 2022 [53] | Muralidharan et al., 2020 [45] | Nandhita et al., 2020 [43] | Ramachandran et al., 2013 [36] | Ramos et al., 2020 [42] | Staite et al., 2020 [47] | Xu et al., 2020 [46] | ||
| Type of Trial | Non-Randomized Intervention Trial | Randomized Trial | |||||||||||||||||||||||||
| Number of Samples Included | <100 participant | 77 | 38 | 33 | 27 | 67 | 61 | 69 | 90 | ||||||||||||||||||
| 100–500 participant | 253 | 340 | 163 | 430 | 253 | 122 | 148 | 225 | 100 | 202 | 208 | ||||||||||||||||
| 501–1000 participant | 599 | 833 | 537 | ||||||||||||||||||||||||
| >1000 participant | 1518 | 1366 | 2062 | ||||||||||||||||||||||||
| SEX | Both | Both | Both | Both | Both | Both | Both | Both | Both | Both | Both | Both | Female | Both | Both | Both | Both | Both | Both | Both | Both | Male | Both | Both | Both | ||
| Mean AGE (years) ** | Range: 50–70 | IG: 45.5 (12.2); CG: 48.4 (14.6) | IG: 48 ± 12.0; CG: 48.4 ± 10.8 | 57.7 ± 7.7 | 44 ± 8.5 | 52.42 ± 13.43 | IG: 42.1 ± 12.2; CG: 41.3 ± 12.1 | 55 ± 8.9 range: 31–70 | IG: 47.7 ± 12.4; CG: 45.2 ± 10.6 | IG: 46.9 ± 13.2; CG: 45.8 ± 13.8 | 55.2 ± 9.0 | IG: 52.1 ± 12.0; IG- plus 51.6 ± 11.1; CG: 51.3 ± 11.0 | IG: 43.7 8.1 CG positive: 42.9–12.2: CG negative: 50.9–7.1 | IG: 55.3 ± 12.9; CG: 55.6 ± 12.6 | IG: 59.3 ± 9.1; CG positive: 59.4 ± 9.4; CG negative: 59.4 ± 8.8 | IG: 49.3 ± 6.1; CG: 48.5 (5.3) | IG: 51.9 ± 8.7; CG: 54.3 (9.9) | IG: 61.8 ± 9.5; CG: 62.4 ± 8.7 | IG: 43.3 ± 10.5; CG: 43.3 ± 8.4 | IG: 37.8 ± 9.2; CG: 37.8 ± 9.6 | CG: 52.0 ± 10.2; IG: 52.1 ± 10.3 | IG: 46.1 ± 4.6; CG: 45.9 ± 4.8 | IG: 55.7; CG: 57.5 | IG: 51.76 ± 7.68; CG: 52.78 ± 8.20 | IG: 46.0; CG: 47.5 | ||
| Mean AGE (years) ** | MIDDLE AGE (40–60) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||
| ELDERLY (>60) | √ | √ | √ | ||||||||||||||||||||||||
| Mean BMI (kg/m2) ** | IG: 24.80 ± 3.21; CG: 23.36 ± 2.95 | Not Reported | IG: 30.8 ± 16.5 CG: 30.6 ± 4.2 | 33.6 ± 3.9 | Pre:38.8 ± 1.8; Post: 37.6 ± 1.9 | Not Reported | IG30.5(±3.9) CG 30.5(±4.9) | 31.1 ± 4.4 | Not Reported | IG: 36.4 ± 6.2; CG: 37.0 ± 6.8 | 33.3 ± 6.0 | IG only: 33.0 ± 10.4; IG plus 30.7 ± 9.3; CG: 33.4 ± 7.8 | IG: 30 ± 5.1; CG Positive: 34.8 ± 9.0; CG Negative: 31.6 ± 5.8 | IG: 35.8 ± 6.1; CG: 35.8 ± 6.1 | IG: 28.4 CG Positive: 28.2; CG negative: 28.5 | IG: 27.4 3.0Con: 26.6 (2.2) | IG: 29.8 ± 4.2; CG: 29.8 ± 3.9 | IG: 33.5 ± 7.7; CG: 33.1 ± 7.1 | IG: 40.5 ± 7.1 CG: 39.7 ± 5.1 | IG: 29.4 ± 3.8; CG: 29.3 ± 4.2 | IG: 28.7 ± 4.7CG: 28.9 ± 4.8 | IG 25.8 ± 3.0; CG: 25.8 ± 3.3 | IG: 31.25 ± 6.43; CG: 30.94 ± 7.23 | Not Reported | IG: 25.3; CG: 24.7 | ||
| Diagnostic Criteria | HbA1c | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||
| HbA1c Only | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||
| OGGT | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||
| OGGT Only | √ | ||||||||||||||||||||||||||
| FG | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||
| FG Only | √ | √ | √ | ||||||||||||||||||||||||
| Combination | √ | √ | √ | √ | √ | √ | √ | * | |||||||||||||||||||
| Duration of study | <6 month | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||||||
| 6–12 month | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||
| 13–24 month | |||||||||||||||||||||||||||
| >24 month | √ | √ | √ | ||||||||||||||||||||||||
| Type of mobile health | Text Message-Based | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||
| Web Based | √ | √ | √ | √ | √ | ||||||||||||||||||||||
| Mobile Phone Apps | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||
| Combination (Hybrid or Tribrid) | √ | √ | √ | ||||||||||||||||||||||||
| Wearable Devices | √ | √ | √ | √ | √ | √ | |||||||||||||||||||||
| Outcome Measures | Incidence | √ | √ | √ | |||||||||||||||||||||||
| Anthropometric Measures | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| Laboratory Parameters | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||
| Physical Activity | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||
| Dietary Behavior | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||||
√: Reported.  : Non-Randomized Interventional Trial.
: Non-Randomized Interventional Trial.  : Randomized Trial. *: Diagnosed by American Diabetes Association (ADA) screening tool (score of ≥ 5 or). **: Age (years) by mean ± SD or range age of participants. IG: Intervention Group; CG: Control Group.
: Randomized Trial. *: Diagnosed by American Diabetes Association (ADA) screening tool (score of ≥ 5 or). **: Age (years) by mean ± SD or range age of participants. IG: Intervention Group; CG: Control Group.

Table 3.
Jadad Score Calculation [33,62].
Table 3.
Jadad Score Calculation [33,62].
| METHODOLOGICAL FEATURE | ITEM | ANSWER | SCORE |
|---|---|---|---|
| Randomization | Was the study described as randomized? | Yes | 1 |
| No | 0 | ||
| Was the method used to generate the sequence of randomization described and appropriate? | Yes | 1 | |
| No | 0 | ||
| Described and inappropriate | −1 | ||
| Blinding | Was the study described as double-blind? | Yes | 1 |
| No | 0 | ||
| Was the method of double blinding described and appropriate? | Yes | 1 | |
| No | 0 | ||
| Described and inappropriate | −1 | ||
| withdrawals and dropouts | Was there a description of withdrawals and dropouts? | Yes | 1 |
| No | 0 |

Table 4.
Jadad quality score of experimental studies in this systematic review.
Table 4.
Jadad quality score of experimental studies in this systematic review.
| Author, Year | Ramachandran et al., 2013 [36] | Chen et al., 2014 [55] | Block et al., 2015 [37] | Fukuoka et al., 2015 [38] | Fischer et al., 2016 [39] | Bender et al., 2019 [40] | Sharit et al., 2018 [56] | Griauzde et al., 2019 [41] | Fischer et al., 2019 [57] | Ramos et al., 2020 [42] | Nandhita et al., 2020 [43] | Mcleod et al., 2020 [44] | Muralidharan et al., 2020 [45] | Xu et al., 2020 [46] | Staite et al., 2020 [47] | Francis et al., 2021 [48] | Hamdan et al., 2021 [49] | Summers et al., 2021 [58] | Khunti et al., 2021 [50] | Katula et al., 2022 [51] | Stewart et al., 2022 [59] | Lim et al., 2022 [52] | Sevilla et al., 2022 [60] | Moravcova et al., 2022 [53] | Kondo et al., 2022 [54] |
| Randomization | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | |
| Blinding | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Withdrawals and Dropouts | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total Score | 3 | 1 | 4 | 4 | 3 | 4 | 1 | 3 | 1 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 1 | 4 | 4 | 1 | 4 | 1 | 4 | 4 |
Red: minimum randomization criteria score. Yellow indicates the lowest possible score for the blinding criteria. Green indicates the highest possible score for all criteria. Pink: 1 point off the total score.
3.4. Mobile Health Interventions
Six studies [36,39,48,52,57,59] used text messaging or short message service (SMS) as an intervention. Furthermore, Staite [39] and Stewart [59] include interventions other than text messaging, such as mobile phone apps and website-based interventions. Ramachandran [36] and Nanditha [52] reported on text messages based on the trans-theoretical model stage. Despite this, Staite [39] reported text messages that were based on the theory of planned behavior; Stewart [59] reported text messages that were based on Bandura’s self-efficacy: toward a unifying theory of behavioral change; and Fischer [48,57] did not report any details about the theory that underpins text messages to participants. Four studies [36,39,52,59] that used messages as interventions reported that content-text messages were created using curriculum material from the National Diabetes Prevention Program (DPP).
In a different manner, five studies [37,39,55,56,60] used website-based interventions. Besides website-based interventions, participants also received intervention via mobile phone apps [37]. Chen Study [55] with SOPs underlying SWAP-DM2 and Block Study [37] with DPP Curriculum demonstrate a distinct variation in the content of each website-based intervention in each study. The website-based intervention adopted the methods and theoretical frameworks, such as The SWAP-DM2 provided diabetes prevention services, ranging from uncomplicated educational websites and record-keeping to quite complex risk-scoring and individualized counseling [55]. Despite this, the Alive-PD was a fully automated and flexible online behavior change strategy [37]. The system includes tools for weight monitoring, eating, and physical activity, as well as weekly health information on diabetes and prevention strategies, quizzes, social support through virtual teams and a participant messaging program, feedback on diet and activity reports, and also goal achievement of success or failure, weekly reminders, and other features [37]. Practice the use of website-based was conducted as a basis for promoting positive Physical Activity (PA), dietary lifestyles [56] and mental resilience [39].
Eighteen [38,40,41,42,44,45,46,48,49,50,51,52,53,54,58] studies used mobile phone apps as an intervention. There were twelve mobile phone apps [37,40,42,45,46,47,51,52,53,54,59] developed from the Diabetes Prevention Programs (DPP) curriculum for adults at risk of T2DM. Despite this, another mobile phone app was developed by other methods such as for a low-carb program [58]. Most mobile phone apps aim to change dietary habits and increase physical activity. Reminder functions [37,38], daily recording [40,42,52], peer support [37,44], personal health coaching [44], and even gamified individual goals [48] were used to achieve the target parameters.
Six studies [40,41,47,48,51,59] were conducted on wearable devices, which may be useful for tracking and recording users’ activity in near-real time [47] and providing feedback [48]. Fitbit accounts for five out of six wearable devices [40,41,51,59,63].
3.5. Outcomes Reported
Anthropometric measures, such as weight loss, changes in BMI, and waist circumference were used as the primary outcomes in twelve studies [38,39,42,45,47,52,53,54,55,56,57,59]. Changes in HbA1c were the main result in five studies [37,44,49,51,58]. In addition, three studies [46,48,50] examined physical activity and its changes as the primary outcome of the research. In Ramachandran [36] and Nanditha’s [43] studies, the primary outcome was the incidence of type 2 diabetes. In the study conducted by Griauzde [41], Bender [40], and Sevilla [60], the primary outcomes were the feasibility and acceptability of the mobile health intervention. In Xu’s [46] studies, the primary outcome was a change in dietary behaviors and physical activity. The summary of intervention outcomes is shown in Table 5.
3.5.1. Incidence of T2DM
The findings from Ramachandran [36] showed that mobile phone messaging (SMS) could be an effective technique for lifestyle modification to reduce the incidence of type 2 diabetes. The cumulative incidence of T2DM at 24-month follow-up was lower in those who received mobile phone messages than in controls [36]. Despite this, Khunti [50] and Nanditha [43] found that SMS and mobile phone apps did not significantly reduce the cumulative incidence of T2DM at the 12, 24, and 48-month follow-ups. There were other factors that could influence these inconsistent results, such as the content of the intervention and the role of the tool or other intervention (wearable device) [50] that has the potential to have an effect. Differences in examination methods and the provision of a prediabetes diagnosis were also factors that needed to be considered in these findings.
3.5.2. Anthropometric Measures
An analysis of twenty studies using weight change as an outcome found that eight studies [39,43,44,47,49,50,53,60] showed no significant differences between intervention and control. Despite this, a significant difference was observed between the intervention and control groups in nine other studies [37,38,42,45,51,52,54,57,59]. Furthermore, the Sharit [56], Chen [55], and Summers [58] studies found significant changes in body weight following the intervention. Seven [37,38,42,45,51,57,59] of the 12 studies that found a significant difference in weight change from mobile health apps were based on content based on DPP.
There were two studies [39,57] that include a ≥3% weight loss as a research outcome. The results of the two are completely contradictory. According to the Fischer study in 2016, there was a significant difference in achieving ≥3% weight loss between the intervention and control groups [39], Meanwhile Fischer in 2019 [57] stated the opposite. Respondent characteristics, such as ethnicity, were suspected to be important considerations in these findings. There was a significant difference between the control and intervention groups and pre-post intervention in three [40,51,52] of the four studies that included achieving ≥5% weight loss as an outcome. Fischer [39], however, stated that there was no difference between the intervention and control groups. Two studies [40,51] used DPP-based content and mobile health apps as interventions.
Changes in BMI were noted in 17 studies [36,37,38,40,42,43,45,46,47,49,50,51,53,54,55,56,60]. BMI changes were shown in two studies [55,56] pre-and post-intervention, but the findings were not conclusive. The other fifteen studies [36,37,38,40,42,43,45,46,47,49,50,51,53,54,60] examined the comparison of changes in BMI in the intervention and control groups. Seven [37,38,42,45,46,51,54] of the fifteen studies reported a significant difference in BMI changes between the control and intervention groups. However, the findings of eight [36,40,43,47,49,50,53,60] other studies found, there was no significant difference in BMI changes between the intervention and control groups.
Waist circumference was observed as an outcome in fifteen studies. Six studies [36,43,47,49,50,53] showed no significant effect of intervention on waist circumference. Despite this, eight studies [37,38,40,45,46,51,54,60] reported a significantly reduced waist circumference between the intervention group and the control. In addition, Sharit’s study described a different mean reduction in waist circumference between baseline and post-intervention [56].

Table 5.
Summary of Intervention Outcome.
Table 5.
Summary of Intervention Outcome.
| Results of Intervention Outcome | Chen et al., 2014 [55] | Fischer et al., 2019 [57] | Sevilla et al., 2022 [60] | Sharit et al., 2018 [56] | Stewart et al., 2022 [59] | Summers et al., 2021 [58] | Bender et al., 2019 [40] | Block et al., 2015 [37] | Fischer et al., 2016 [39] | Francis et al., 2021 [48] | Fukuoka et al., 2015 [38] | Griauzde et al., 2019 [41] | Hamdan et al., 2021 [49] | Katula et al., 2022 [51] | Khunti et al., 2021 [50] | Kondo et al., 2022 [54] | Lim et al., 2022 [52] | Mcleod et al., 2020 [44] | Moravcova et al., 2022 [53] | Muralidharan et al., 2020 [45] | Nandhita et al., 2020 [43] | Ramachandran et al., 2013 [36] | Ramos et al., 2020 [42] | Staite et al., 2020 [47] | Xu et al., 2020 [46] | |
| Type of Trial | Non-Randomized Interventional Study | Randomized Trial | ||||||||||||||||||||||||
| Incidence of type 2 DM | 0 | 0 | 1 | |||||||||||||||||||||||
| anthropometry measures | Weight Change | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | |||||
| Achieving ≥ 3% Weight loss | 0 | 1 | ||||||||||||||||||||||||
| Achieving ≥ 5% | 1 | 0 | 1 | 1 | ||||||||||||||||||||||
| BMI Change | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | |||||||||
| Waist circumference | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | |||||||||||
| Physical Activity | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | |||||||||||||||
| Dietary Behavior | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | ||||||||||||||||||
| Laboratory measures | HbA1C Reduction | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |||||||||
| Blood Glucose Level Reduction | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | ||||||||||||||||||
| Fasting Plasma Glucose | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| OGGT | 1 | 0 | 0 | |||||||||||||||||||||||
| Blood Lipid Level Reduction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||
3.5.3. Physical Activity
Eleven [36,38,43,48,50,51,52,54,55,56,59] studies reported changes in physical activity as an outcome measure of the study. Five of the 11 studies stated that the mobile health intervention showed significant differences in increased physical activity between the control and intervention groups [48,51,54,59] and pre-post intervention [55]. Despite this, six studies reported that there were no significant differences in increases in physical activity between the control and intervention groups [36,38,43,50,52] and pre-post intervention [56].
3.5.4. Dietary Behaviors
Eight [36,43,46,49,50,54,55,60] studies reported dietary behavior as an outcome measure. Five [36,43,46,54,55] of the eight studies stated that the mobile health intervention showed significant differences in changes to healthy dietary behavior between the control group and the intervention and pre-post-intervention groups [55]. Despite this, three [49,50,60] studies reported that there was no significant difference in changes in dietary behavior between the control and intervention groups.
3.5.5. Laboratory Measures
In sixteen studies [37,38,39,40,42,43,44,47,49,50,51,52,53,54,58,60], the HbA1c test was used as an outcome. Four studies found that mobile health interventions significantly improved HbA1c reduction, both in pre-post [51,58] studies and control intervention [37,52] studies. On the other hand, twelve [38,39,40,42,43,44,47,49,50,53,54,60] other studies found that there was no significant difference in HbA1c reduction between the control and intervention groups.
Reduction in blood glucose levels was one of the measures used as an outcome in eight studies [36,37,38,40,43,53,54,60]. Three [36,37,60] studies explained that there were significant differences in reduction in blood fasting glucose levels between the intervention and control groups and 1 h glucose levels in pre-post intervention. Whereas no significant difference in reduction in blood fasting glucose levels was found between the intervention and control groups in the other five studies [38,40,43,53,54]. In addition, the reduction in blood glucose levels was also significantly different in the oral glucose tolerance test (OGTT) in the control and intervention groups in the study on Vida Sana apps [60].
Seven studies [36,37,38,43,45,49,52] reported blood lipid level reduction as an outcome of the study. All of these studies stated that the mobile health intervention did not show a significant difference in blood lipid level reduction between the control and intervention groups [36,37,38,43,45,49,52].
4. Discussions
Traditional face-to-face treatment for achieving and sustaining weight or BMI can be replaced with technologies that are practical, affordable, and scalable [64]. Mobile phone intervention has gained widespread acceptance among people of all ages and socioeconomic backgrounds, and it provides numerous opportunities in health care, including self-management and T2DM prevention [65]. Despite growing concerns about data privacy in the fields of access, patient confidentiality, and data storage [66]. The variety of definitions and use of diagnostic tools used in these study results indicate that prediabetes was a complex condition that triggers a burden on clinical services and public health policies [3]. The use of combined diagnostic tools is expected to be able to detect conditions of undiagnosed prediabetes and diabetes [67]. Depending on the diagnostic tools used as standards, the characteristics of the intervention each subject requires, and the treatment’s results can change.
During long-term follow-up, there was no significant difference between mobile health interventions and controls in reducing the incidence of T2DM [43,50]. Despite this, there was variation in outcomes that may be brought about by variances in the intervention’s underlying theory and the message’s content to participants, and length of observation. Participants in twenty trials used technology to reduce weight, according to this systematic review. Studies show that mobile phone messaging is an effective and acceptable method to deliver advice and support towards dietary behavior to prevent T2DM in individuals at high risk. The findings [57] showed that although SMS4PreDM was relatively low-cost to deliver and demonstrated high retention, weight loss outcomes may not be sufficient to serve as a population health strategy. It can happen because pre-diabetes is influenced by many factors [55].
In these studies, website-based interventions were used to promote positive health outcomes such as physical activity, dietary habits, and mental resilience. These interventions differed in their complexity, ranging from simple educational websites to more complex individualized counseling. The differences in the details of the form of intervention, may be one factor making the research results inconclusive.
As a simple and effective measure of central obesity, waist circumference is a major predictor of increased risk of hypertension, diabetes mellitus, dyslipidemia, metabolic syndrome, and coronary heart disease [68]. Waist circumference could potentially be used as a clinical equivalent for visceral adipose tissue, which impairs insulin sensitivity and predisposes to prediabetes when excessive [69]. A systematic review and meta-analysis study looked at the impact of technology on waist circumference reduction and found a mean change of 02.99 cm (95% CI: 03.68 to 02.30) [68]. Self-management by chronic healthcare customers can be enhanced via mobile apps, according to users of health apps. This might be used for patients with prediabetes, particularly in terms of lifestyle adjustments [70].
Using text messages instead of other reminders had some advantages. Text messages could be sent to patients at the same time, they were always available, cost-effective, and required less staff [71]. Despite all the benefits and features of text messaging on a mobile phone, there are some drawbacks, such as: the staff could not be certain that text messages were received by all participants, mobile phone numbers could have changed [71], and participants sometimes blocked their status or stopped reading the text messages [72]. A few intervention strategies were bidirectional, allowing T2DM patients to receive continuous and personalized support via SMS while also allowing T2DM patients to interact with healthcare professionals and diabetes care educators to strengthen their T2DM self-management abilities and knowledge [65].
In other circumstances, mobile health technology (including social media) may provide benefits such as low or no cost, high scalability, self-tracking, and tailored feedback, image and video use for improved health literacy, wide range, and data sharing for large-scale analytics [73]. Mobile phone apps are widely utilized all around the world. Health and medical apps are increasingly being employed in a range of scenarios, according to evidence. Many authors in the medical and public health literature have highlighted the benefits that laypeople and healthcare professionals may contribute to health and medical apps [74]. Chronic disease or patients’ conditions must be monitored for a long time. Using mobile phone apps for chronic self-care could be beneficial, allowing patients to monitor and regulate their illnesses [70]. Despite the fact that mobile health intervention prompts were well-received by patients, there are conflicting results regarding their influence on behavioral, laboratory, and T2DM incidence [75].
There are some limitations in this study that need to be addressed. First, in our study, we only used American Diabetes Association (ADA) criteria for prediabetes, although many other prediabetes studies used the World Health Organization (WHO) criteria. Second, although few studies on mobile health interventions with a sample adequate for prediabetes intervention used randomized controlled trials (RCTs) as their study design, we propose that further studies on mobile health apps for prediabetes intervention use RCT design. Third, we were unable to depict all studies in meta-analysis due to differences in diagnostic methods, evaluation, and reporting of intervention outcomes. Approximately half of the studies provided sufficient data to compute them. Fourth, our search strategy may have resulted in a bias for positive results because null or negative results are less likely to be published. Finally, the full text of certain studies was not available.
5. Conclusions
The systematic review includes twenty-five studies that were discussed about mobile health intervention for pre-diabetes among middle-aged and elderly patients. A few studies showed that each mobile health intervention promised an effective and acceptable method to deliver advice and support towards lifestyle modification to prevent diabetes. Although, evidence of the effectiveness of mobile health as an intervention for prediabetes was inconclusive.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192013638/s1, Supplementary File S1: PRISMA 2020 statement checklist. Supplementary File S2: Screening Forms. Supplementary File S3: Data Extraction Forms.
Author Contributions
Y.A.J. was responsible for the formulation of research question, design the main conceptual ideas; drafting the manuscript, analysis and interpretation of data; R.N.A., R.N. and D.D.E. assisted with the formulation of the research question, wrote the manuscript in consultation with Y.A.J.; R.N.A., R.N. and D.D.E. performed the research, analysis and interpretation of data; R.N.A., L.L. and H.K. commented on manuscript drafts. All authors have read and agreed to the published version of the manuscript.
Funding
The authors have no funding source to declare.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its Supplementary Information Files].
Acknowledgments
The authors acknowledge the contributions of Siti Solichatul Makkiyyah from the Medical Faculty, Islamic University of Indonesia for reviewing and editing the manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
ADA: American Diabetes Association; BMI: Body Mass Index; CKD: Chronic Kidney Disease; DPP: Diabetes Prevention Program; FG: Fasting Glucose; HbA1c: Hemoglobin A1c; IFG: Impaired Fasting Glucose; IGT: Impaired Glucose Tolerance; OGTT: Oral Glucose Tolerance Test PRISMA: Preferred Reporting Items for Systematic Reviews and Meta Analyses; RCTs: Randomized Controlled Trials; SMS: Short Text Message; TD2M: Type02 Diabetes Mellitus; WHO: World Health Organization.
References
- Introduction: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S1–S2. [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Richter, B.; Hemmingsen, B.; Metzendorf, M.-I.; Takwoingi, Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst. Rev. 2018, 10, CD012661. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.R.; Rawlings, A.M.; Pankow, J.S.; Echouffo Tcheugui, J.B.; Coresh, J.; Sharrett, A.R.; Selvin, E. Risk of Progression to Diabetes Among Older Adults With Prediabetes. JAMA Intern. Med. 2021, 181, 511–519. [Google Scholar] [CrossRef]
- Anothaisintawee, T.; Sansanayudh, N.; Thamakaison, S.; Lertrattananon, D.; Thakkinstian, A. Neck Circumference as an Anthropometric Indicator of Central Obesity in Patients with Prediabetes: A Cross-Sectional Study. BioMed Res. Int. 2019, 2019, 4808541. [Google Scholar] [CrossRef]
- Grundy, S.M. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 2012, 59, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, B.; Sonne, D.P.; Metzendorf, M.-I.; Richter, B. Insulin secretagogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2016, 10, CD012151. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Guo, X.; Zhang, Z. The efficacy of mobile phone apps for lifestyle modification in diabetes: Systematic review and meta-analysis. JMIR mHealth uHealth 2019, 7, e12297. [Google Scholar] [CrossRef] [PubMed]
- Center, P.R. Older Adults and Technology Use. PEW Research Center. 2014. Available online: http://www.pewinternet.org/2014/04/03/older-adults-andtechnology-%0Ause/%0A (accessed on 25 September 2022).
- Carroll, J.K.; Moorhead, A.; Bond, R.; LeBlanc, W.G.; Petrella, R.J.; Fiscella, K. Who uses mobile phone health apps and does use matter? A secondary data analytics approach. J. Med. Internet Res. 2017, 19, e125. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Carter, B.; Hewitt, J.; Francisa, T.; Mayor, S. Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care 2016, 39, 2089–2095. [Google Scholar] [CrossRef]
- Ang, S.M.; Chen, J.; Liew, J.H.; Johal, J.; Dan, Y.Y.; Allman-Farinelli, M.; Lim, S.L. Efficacy of interventions that incorporate mobile apps in facilitating weight loss and health behavior change in the asian population: Systematic review and meta-analysis. J. Med. Internet Res. 2021, 23, e28185. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, Q.; Yang, X.; Cao, J.; Chen, J.; Mo, X.; Huang, J.; Wang, L.; Gu, D. Effect of mobile phone intervention for diabetes on glycaemic control: A meta-analysis. Diabet. Med. 2011, 28, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Fakih El Khoury, C.; Karavetian, M.; Halfens, R.J.G.; Crutzen, R.; Khoja, L.; Schols, J.M.G.A. The Effects of Dietary Mobile Apps on Nutritional Outcomes in Adults with Chronic Diseases: A Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2019, 119, 626–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Min, J.; Khuri, J.; Xue, H.; Xie, B.; Kaminsky, L.A.; Cheskin, L.J. Effectiveness of Mobile Health Interventions on Diabetes and Obesity Treatment and Management: Systematic Review of Systematic Reviews. JMIR mHealth uHealth 2020, 8, e15400. [Google Scholar] [CrossRef] [PubMed]
- Kitsiou, S.; Paré, G.; Jaana, M.; Gerber, B. Effectiveness of mHealth interventions for patients with diabetes: An overview of systematic reviews. PLoS ONE 2017, 12, e0173160. [Google Scholar] [CrossRef] [PubMed]
- Villinger, K.; Wahl, D.R.; Boeing, H.; Schupp, H.T.; Renner, B. The effectiveness of app-based mobile interventions on nutrition behaviours and nutrition-related health outcomes: A systematic review and meta-analysis. Obes. Rev. 2019, 20, 1465–1484. [Google Scholar] [CrossRef] [PubMed]
- Schoeppe, S.; Alley, S.; Van Lippevelde, W.; Bray, N.A.; Williams, S.L.; Duncan, M.J.; Vandelanotte, C. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Dounavi, K.; Tsoumani, O. Mobile Health Applications in Weight Management: A Systematic Literature Review. Am. J. Prev. Med. 2019, 56, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shu, W.; Du, J.; Du, M.; Wang, P.; Xue, M.; Zheng, H.; Jiang, Y.; Yin, S.; Liang, D.; et al. Mobile health in the management of type 1 diabetes: A systematic review and meta-analysis. BMC Endocr. Disord. 2019, 19, 21. [Google Scholar] [CrossRef]
- Dobson, R.; Whittaker, R.; Pfaeffli Dale, L.; Maddison, R. The effectiveness of text message-based self-management interventions for poorly-controlled diabetes: A systematic review. Digit. Health 2017, 3, 2055207617740315. [Google Scholar] [CrossRef]
- Marcolino, M.S.; Oliveira, J.A.Q.; D’Agostino, M.; Ribeiro, A.L.; Alkmim, M.B.M.; Novillo-Ortiz, D. The Impact of mHealth Interventions: Systematic Review of Systematic Reviews. JMIR mHealth uHealth 2018, 6, e23. [Google Scholar] [CrossRef] [PubMed]
- Cotter, A.P.; Durant, N.; Agne, A.A.; Cherrington, A.L. Internet interventions to support lifestyle modification for diabetes management: A systematic review of the evidence. J. Diabetes Complicat. 2014, 28, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Lorig, K.; Ritter, P.L.; Laurent, D.D.; Plant, K.; Green, M.; Jernigan, V.B.B.; Case, S. Online diabetes self-management program: A randomized study. Diabetes Care 2010, 33, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.R.; Mehari, K.S.; McIntyre, L.G.; Janney, A.W.; Fortlage, L.A.; Sen, A.; Strecher, V.J.; Piette, J.D. A randomized trial comparing structured and lifestyle goals in an internet-mediated walking program for people with type 2 diabetes. Int. J. Behav. Nutr. Phys. Act. 2007, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.; Spruijt-Metz, D.; Wen, C.K.F.; Hingle, M.D. Prevention and treatment of pediatric obesity using mobile and wireless technologies: A systematic review. Pediatr. Obes. 2015, 10, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.; McBain, H.; Newman, S. The impact of mobile monitoring technologies on glycosylated hemoglobin in diabetes: A systematic review. J. Diabetes Sci. Technol. 2012, 6, 1185–1196. [Google Scholar] [CrossRef]
- Bloomfield, G.S.; Vedanthan, R.; Vasudevan, L.; Kithei, A.; Were, M.; Velazquez, E.J. Mobile health for non-communicable diseases in Sub-Saharan Africa: A systematic review of the literature and strategic framework for research. Global. Health 2014, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Barengo, N.C.; Diaz Valencia, P.A.; Apolina, L.M.; Estrada Cruz, N.A.; Fernández Garate, J.E.; Correa González, R.A.; Cinco Gonzalez, C.A.; Gómez Rodriguez, J.A.; González, N.C. Mobile Health Technology in the Primary Prevention of Type 2 Diabetes: A Systematic Review. Curr. Diabetes Rep. 2022, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Rhoon, L.; Byrne, M.; Morrissey, E.; Murphy, J.; McSharry, J. A systematic review of the behaviour change techniques and digital features in technology-driven type 2 diabetes prevention interventions. Digit. Health 2020, 6, 2055207620914427. [Google Scholar] [CrossRef]
- Elavsky, S.; Knapova, L.; Klocek, A.; Smahel, D. Mobile health interventions for physical activity, sedentary behavior, and sleep in adults aged 50 years and older: A systematic literature review. J. Aging Phys. Act. 2019, 27, 565–593. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Macaskill, P.; Gatsonis, C.; Deeks, J.; Harbord, R.; Takwoingi, Y. Chapter 10 Analysing and Presenting Results. In Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy; 2010; pp. 1–61. Available online: http://srdta.cochrane.org/handbook-dta-reviews (accessed on 25 September 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA 2009 Flow Diagram. Prism. Statement 2009, 6, 1000097. [Google Scholar]
- Ramachandran, A.; Snehalatha, C.; Ram, J.; Selvam, S.; Simon, M.; Nanditha, A.; Shetty, A.S.; Godsland, I.F.; Chaturvedi, N.; Majeed, A.; et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: A prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013, 1, 191–198. [Google Scholar] [CrossRef]
- Block, G.; Azar, K.M.J.; Romanelli, R.J.; Block, T.J.; Hopkins, D.; Carpenter, H.A.; Dolginsky, M.S.; Hudes, M.L.; Palaniappan, L.P.; Block, C.H. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: A randomized controlled trial among persons with prediabetes. J. Med. Internet Res. 2015, 17, e240. [Google Scholar] [CrossRef]
- Xu, Z.; Geng, J.; Zhang, S.; Zhang, K.; Yang, L.; Li, J.; Li, J. A Mobile-Based Intervention for Dietary Behavior and Physical Activity Change in Individuals at High Risk for Type 2 Diabetes Mellitus: Randomized Controlled Trial. JMIR mHealth uHealth 2020, 8, e19869. [Google Scholar] [CrossRef]
- Staite, E.; Bayley, A.; Al-Ozairi, E.; Stewart, K.; Hopkins, D.; Rundle, J.; Basudev, N.; Mohamedali, Z.; Ismail, K. A Wearable Technology Delivering a Web-Based Diabetes Prevention Program to People at High Risk of Type 2 Diabetes: Randomized Controlled Trial. JMIR mHealth uHealth 2020, 8, e15448. [Google Scholar] [CrossRef]
- Francis, S.L.; Simmering, J.E.; Polgreen, L.A.; Evans, N.J.; Hosteng, K.R.; Carr, L.J.; Cremer, J.F.; Coe, S.; Cavanaugh, J.E.; Segre, A.M.; et al. Gamifying accelerometer use increases physical activity levels of individuals pre-disposed to type II diabetes. Prev. Med. Rep. 2021, 23, 101426. [Google Scholar] [CrossRef]
- Al-Hamdan, R.; Avery, A.; Al-Disi, D.; Sabico, S.; Al-Daghri, N.M.; McCullough, F. Efficacy of lifestyle intervention program for Arab women with prediabetes using social media as an alternative platform of delivery. J. Diabetes Investig. 2021, 12, 1872–1880. [Google Scholar] [CrossRef]
- Khunti, K.; Griffin, S.; Brennan, A.; Dallosso, H.; Davies, M.; Eborall, H.; Edwardson, C.; Gray, L.; Hardeman, W.; Heathcote, L.; et al. Behavioural interventions to promote physical activity in a multiethnic population at high risk of diabetes: PROPELS three-arm RCT. Health Technol. Assess. 2021, 25, 1–190. [Google Scholar] [CrossRef]
- Katula, J.A.; Dressler, E.V.; Kittel, C.A.; Harvin, L.N.; Almeida, F.A.; Wilson, K.E.; Michaud, T.L.; Porter, G.C.; Brito, F.A.; Goessl, C.L.; et al. Effects of a Digital Diabetes Prevention Program: An RCT. Am. J. Prev. Med. 2022, 62, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Ong, K.W.; Johal, J.; Han, C.Y.; Yap, Q.V.; Chan, Y.H.; Zhang, Z.P.; Chandra, C.C.; Thiagarajah, A.G.; Khoo, C.M. A Smartphone App-Based Lifestyle Change Program for Prediabetes (D’LITE Study) in a Multiethnic Asian Population: A Randomized Controlled Trial. Front. Nutr. 2021, 8, 780567. [Google Scholar] [CrossRef] [PubMed]
- Moravcová, K.; Karbanová, M.; Bretschneider, M.P.; Sovová, M.; Ožana, J.; Sovová, E. Comparing Digital Therapeutic Intervention with an Intensive Obesity Management Program: Randomized Controlled Trial. Nutrients 2022, 14, 2005. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Okitsu, T.; Waki, K.; Yamauchi, T.; Nangaku, M.; Ohe, K. Effect of Information and Communication Technology-Based Self-management System DialBeticsLite on Treating Abdominal Obesity in the Specific Health Guidance in Japan: Randomized Controlled Trial. JMIR Form. Res. 2022, 6, e33852. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, Y.; Gay, C.L.; Joiner, K.L.; Vittinghoff, E. A Novel Diabetes Prevention Intervention Using a Mobile App: A Randomized Controlled Trial with Overweight Adults at Risk. Am. J. Prev. Med. 2015, 49, 223–237. [Google Scholar] [CrossRef]
- Fischer, H.H.; Fischer, I.P.; Pereira, R.I.; Furniss, A.L.; Rozwadowski, J.M.; Moore, S.L.; Durfee, M.J.; Raghunath, S.G.; Tsai, A.G.; Havranek, E.P. Text Message Support for Weight Loss in Patients with Prediabetes: A Randomized Clinical Trial. Diabetes Care 2016, 39, 1364–1370. [Google Scholar] [CrossRef]
- Bender, M.S.; Cooper, B.A.; Flowers, E.; Ma, R.; Arai, S. Filipinos Fit and Trim—A feasible and efficacious DPP-based intervention trial. Contemp. Clin. Trials Commun. 2018, 12, 76–84. [Google Scholar] [CrossRef]
- Griauzde, D.; Kullgren, J.T.; Liestenfeltz, B.; Ansari, T.; Johnson, E.H.; Fedewa, A.; Saslow, L.R.; Richardson, C.; Heisler, M. A Mobile Phone-Based Program to Promote Healthy Behaviors Among Adults with Prediabetes Who Declined Participation in Free Diabetes Prevention Programs: Mixed-Methods Pilot Randomized Controlled Trial. JMIR mHealth uHealth 2019, 7, e11267. [Google Scholar] [CrossRef]
- Toro-Ramos, T.; Michaelides, A.; Anton, M.; Karim, Z.; Kang-Oh, L.; Argyrou, C.; Loukaidou, E.; Charitou, M.M.; Sze, W.; Miller, J.D. Mobile Delivery of the Diabetes Prevention Program in People with Prediabetes: Randomized Controlled Trial. JMIR mHealth uHealth 2020, 8, e17842. [Google Scholar] [CrossRef]
- Nanditha, A.; Thomson, H.; Susairaj, P.; Srivanichakorn, W.; Oliver, N.; Godsland, I.F.; Majeed, A.; Darzi, A.; Satheesh, K.; Simon, M.; et al. A pragmatic and scalable strategy using mobile technology to promote sustained lifestyle changes to prevent type 2 diabetes in India and the UK: A randomised controlled trial. Diabetologia 2020, 63, 486–496. [Google Scholar] [CrossRef]
- McLeod, M.; Stanley, J.; Signal, V.; Stairmand, J.; Thompson, D.; Henderson, K.; Davies, C.; Krebs, J.; Dowell, A.; Grainger, R.; et al. Impact of a comprehensive digital health programme on HbA1c and weight after 12 months for people with diabetes and prediabetes: A randomised controlled trial. Diabetologia 2020, 63, 2559–2570. [Google Scholar] [CrossRef]
- Muralidharan, S.; Ranjani, H.; Anjana, R.M.; Gupta, Y.; Ambekar, S.; Koppikar, V.; Jagannathan, N.; Jena, S.; Tandon, N.; Allender, S.; et al. Change in cardiometabolic risk factors among Asian Indian adults recruited in a mHealth-based diabetes prevention trial. Digit. Health 2021, 7, 20552076211039030. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chai, J.; Cheng, J.; Li, K.; Xie, S.; Liang, H.; Shen, X.; Feng, R.; Wang, D. A smart web aid for preventing diabetes in rural China: Preliminary findings and lessons. J. Med. Internet Res. 2014, 16, e98. [Google Scholar] [CrossRef] [PubMed]
- Sharit, J.; Idrees, T.; Andrade, A.D.; Anam, R.; Karanam, C.; Valencia, W.; Florez, H.; Ruiz, J.G. Use of an online personal health record’s Track Health function to promote positive lifestyle behaviors in Veterans with prediabetes. J. Health Psychol. 2018, 23, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.H.; Durfee, M.J.; Raghunath, S.G.; Ritchie, N.D. Short message service text message support for weight loss in patients with prediabetes: Pragmatic trial. JMIR Diabetes 2019, 4, e12985. [Google Scholar] [CrossRef]
- Summers, C.; Tobin, S.; Unwin, D. Evaluation of the Low Carb Program Digital Intervention for the Self-Management of Type 2 Diabetes and Prediabetes in an NHS England General Practice: Single-Arm Prospective Study. JMIR Diabetes 2021, 6, e25751. [Google Scholar] [CrossRef]
- Stewart, J.L.; Hatzigeorgiou, C.; Davis, C.L.; Ledford, C.J.W. DPPFit: Developing and Testing a Technology-Based Adaptation of the Diabetes Prevention Program (DPP) to Address Prediabetes in a Primary Care Setting. J. Am. Board Fam. Med. 2022, 35, 548–558. [Google Scholar] [CrossRef]
- Sevilla-Gonzalez, M.D.R.; Bourguet-Ramirez, B.; Lazaro-Carrera, L.S.; Martagon-Rosado, A.J.; Gomez-Velasco, D.V.; Viveros-Ruiz, T.L. Evaluation of a Web Platform to Record Lifestyle Habits in Subjects at Risk of Developing Type 2 Diabetes in a Middle-Income Population: Prospective Interventional Study. JMIR Diabetes 2022, 7, e25105. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Gillies, C.L.; Taub, N.A.; Mostafa, S.A.; Hiles, S.L.; Abrams, K.R.; Davies, M.J. A comparison of cost per case detected of screening strategies for Type 2 diabetes and impaired glucose regulation: Modelling study. Diabetes Res. Clin. Pract. 2012, 97, 505–513. [Google Scholar] [CrossRef]
- Berger, V.W.; Alperson, S.Y. A general framework for the evaluation of clinical trial quality. Rev. Recent Clin. Trials 2009, 4, 79–88. [Google Scholar] [CrossRef]
- Dany, F.; Kusumawardani, N.; Pradono, J.; Kristianto, Y.; Delima, D. Faktor Risiko Prediabetes: Isolated Impaired Fasting Glucose (i-IFG), Isolated Impaired Glucose Tolerance (i-IGT) dan Kombinasi IFG-IGT (Analisis Lanjut Riskesdas 2013). Bul. Penelit. Kesehat. 2017, 45, 113–124. [Google Scholar] [CrossRef]
- Xiao, L.; Yank, V.; Wilson, S.R.; Lavori, P.W.; Ma, J. Two-year weight-loss maintenance in primary care-based Diabetes Prevention Program lifestyle interventions. Nutr. Diabetes 2013, 3, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, S.; Ranjani, H.; Anjana, R.; Allender, S.; Mohan, V. Mobile health technology in the prevention and management of Type 2 diabetes. Indian J. Endocrinol. Metab. 2017, 21, 334–340. [Google Scholar] [CrossRef]
- Lee, J. Hype or hope for diabetes mobile health applications? Diabetes Res. Clin. Pract. 2014, 106, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Gossain, V.V.; Aldasouqi, S. The challenge of undiagnosed pre-diabetes, diabetes and associated cardiovascular disease. Int. J. Diabetes Mellit. 2010, 2, 43–46. [Google Scholar] [CrossRef]
- Seo, D.C.; Niu, J. Evaluation of internet-based interventions on waist circumference reduction: A meta-analysis. J. Med. Internet Res. 2015, 17, e181. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Menon, A.; Brashear, M.; Johnson, W.D. Prediabetes: Prevalence, pathogenesis, and recognition of enhanced risk. In Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 15–32. ISBN 9780128120194. [Google Scholar]
- Anderson, K.; Burford, O.; Emmerton, L. Mobile health apps to facilitate self-care: A qualitative study of user experiences. PLoS ONE 2016, 11, e0156164. [Google Scholar] [CrossRef]
- Kannisto, K.A.; Koivunen, M.H.; Välimäki, M.A. Use of mobile phone text message reminders in health care services: A narrative literature review. J. Med. Internet Res. 2014, 16, e222. [Google Scholar] [CrossRef] [PubMed]
- Strandbygaard, U.; Thomsen, S.F.; Backer, V. A daily SMS reminder increases adherence to asthma treatment: A three-month follow-up study. Respir. Med. 2010, 104, 166–171. [Google Scholar] [CrossRef]
- Prochaska, J.J.; Coughlin, S.S.; Lyons, E.J. Social media and mobile technology for cancer prevention and treatment. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 128–137. [Google Scholar] [CrossRef]
- Lupton, D. Apps as Artefacts: Towards a Critical Perspective on Mobile Health and Medical Apps. Societies 2014, 4, 606–622. [Google Scholar] [CrossRef]
- MacPherson, M.; Merry, K.; Locke, S.; Jung, M. Developing Mobile Health Interventions with Implementation in Mind: Application of the Multiphase Optimization Strategy (MOST) Preparation Phase to Diabetes Prevention Programming. JMIR Form. Res. 2022, 6, e36143. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).