Abstract
(1) Introduction: In the last two decades, telemedicine has been increasingly applied to telemonitoring (TM) of patients with pacemakers; however, presently, its growth has significantly accelerated because of the COVID-19 pandemic, which has pushed patients and healthcare workers alike to seek new ways to stay healthy with minimal physical contact. Therefore, the main objective of this study was to update the current knowledge on the differences in the medium-and long-term effectiveness of TM and conventional monitoring (CM) in relation to costs and health outcomes. (2) Methods: Three databases and one scientific registry were searched (PubMed, EMBASE, Scopus, and Google Scholar), with no restrictions on language or year of publication. Studies published until July 2021 were included. The inclusion criteria were: (a) experimental or observational design, (b) complete economic evaluation, (c) patients with implanted pacemakers, and (d) comparison of TM with CM. Measurements of study characteristics (author, study duration, sample size, age, sex, major indication for implantation, and pacemaker used), analysis, significant results of the variables (analysis performed, primary endpoints, secondary endpoints, health outcomes, and cost outcomes), and further miscellaneous measurements (methodological quality, variables coded, instrument development, coder training, and intercoder reliability, etc.) were included. (3) Results: 11 studies met the inclusion criteria, consisting of 3372 enrolled patients; 1773 (52.58%) of them were part of randomized clinical trials. The mean age was 72 years, and the atrioventricular block was established as the main indication for device implantation. TM was significantly effective in detecting the presence or absence of pacemaker problems, leading to a reduction in the number of unscheduled hospital visits (8.34–55.55%). The cost of TM was up to 87% lower than that of CM. There were no significant differences in health-related quality of life (HRQoL) and the number of cardiovascular events. (4) Conclusions: Most of the studies included in this systematic review confirm that in the TM group of patients with pacemakers, cardiovascular events are detected and treated earlier, and the number of unscheduled visits to the hospital is significantly reduced, without affecting the HRQoL of patients. In addition, with TM modality, both formal and informal costs are significantly reduced in the medium and long term.
1. Introduction
Telemedicine is the delivery of healthcare services with the help of information and telecommunication technology. Because of the enormous progress made in these technological fields, more and more hospitals have adopted electronic health records, leading to an exponential growth in the use of telemedicine [1,2]. Conventionally, telemedicine has been used to encourage self-care through remote and chronic disease monitoring, to provide consultations to patients who are unable to attend in-person (face-to-face) appointments, and to improve patient care within hospitals and clinics. A key advantage of telemedicine is its ability to increase access to health care by offering patients the opportunity to receive care in their homes and communities [2,3,4]. This becomes more important in the present COVID-19 pandemic, as both patients and health care workers are adopting methods with minimal physical contact [2].
Cardiovascular diseases affect heart and blood vessels and are the leading cause of death globally. A pacemaker is a device that is widely used in cardiac patients to restore normal heart rates, and patients with implanted pacemakers must be followed up regularly. Telemonitoring (TM) systems or remote monitoring of pacemakers provide a convenient means for regular assessment of device-related parameters, such as lead impedance and battery status, which may allow early detection of device and lead malfunctions [5,6,7,8,9,10]. Based on this, if required, changes in medication can be prescribed without consuming time and medical resources [5,11,12,13,14,15] and causing discomfort to patients and their caregivers [16]. Research indicates that clinically actionable events are detected sooner with telemonitoring than with standard in-office follow-ups [5,16], thereby allowing clinicians to act on these issues before they cause increased morbidity, hospitalizations, and costs [5]. Several studies have shown that TM represents an effective and cost-saving way in which to significantly reduce in-office follow-up visits and lower the burden for both hospitals and patients and their caregivers [17,18,19,20,21,22,23,24,25,26,27,28] without compromising safety [5,28,29,30]. Besides, TM has been associated with high patient acceptance, satisfaction, and quality of life as it entails less travel time, time off work, and interruption of patient activities, as well as increased adherence to programmed follow-up [5,31,32,33]. However, in spite of this, TM of users with pacemakers has not been universally adopted [34,35,36] and even hospitals that have incorporated this technology into routine clinical practice for other Cardiac Implantable Electronic Devices (CIEDs) do not routinely use it for pacemakers [37].
The recent strong growth in the number of patients with remotely monitored pacemakers has generated the need for studies comparing TM to conventional in-hospital monitoring (CM). Therefore, the main objective of this study was to conduct a systematic review analyzing the current scientific literature to evaluate the effectiveness and costs of both monitoring modalities
2. Methods
This systematic review has followed PRISMA guidelines, and the study has been registered in PROSPERO (PROVISIONAL ID number: 290,328).
2.1. Search Strategy
A structured review of the following databases was conducted: Medline via PubMed, EMBASE, Scopus, and Google Scholar. The Boolean operators used were AND OR. The following English search terms were used: “Pacemaker”, “Telemedicine”, and “Cost-Benefit Analysis.” These terms were searched in all the selected databases and in complete articles, including the title, summary, text, and keywords. The inclusion criteria for studies were (a) experimental or observational design; (b) studies based on complete economic evaluations, i.e., studies comparing health outcomes and costs, with no exclusions for analysis method (cost-effectiveness, cost-utility, cost-benefit, and cost-minimization); (c) patients with pacemakers, and (d) comparison of TM with CM. The search was conducted between 13 and 21 July 2021, with no restrictions on language or year of publication. In addition to the above-mentioned databases and registers, bibliographic references of interest, including systematic reviews and meta-analyses, were hand-searched.
2.2. Data Extraction
The extraction and reading of all the titles and abstracts of the selected studies (Figure 1) were carried out independently by two researchers (M.P.-H. and I.V.-T.) in the first week of August 2021. As stated in the study aims, articles that could potentially meet the inclusion criteria were preselected. In the second half of August, the same two investigators read the full texts of the previously screened articles. In case of any disagreement between the two investigators regarding the inclusion or exclusion of an article, a third investigator (D.C.-M.) mediated. The variables included in the data analysis were (a) study characteristics (author, year of publication, country, study duration, sample size, age, sex, main indication for implantation, and pacemaker used) and (b) analysis and significant results of the variables (analysis performed, primary endpoints, secondary endpoints, health outcomes, and cost outcomes). Two researchers (C.L.-C. and D.C.-M.) independently evaluated the methodological quality of the selected articles using the checklist of López-Bastida et al. [38] as an assessment tool.
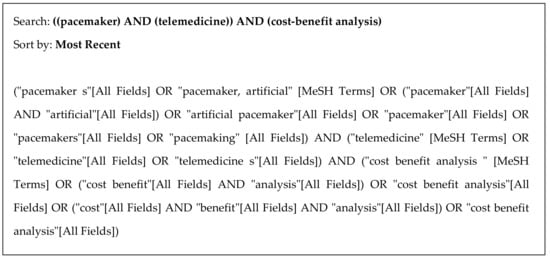
Figure 1.
Search strategy used in MEDLINE (via PubMed).
2.3. Variables Coded, Instrument Development, Coder Training, and Intercoder Reliability
Two researchers (A.L.-V. and M.P.-H.) tested the initial draft of the coding instrument informally by independently coding 10 print papers from the list of 1438 studies initially screened (title, abstract, and text), 6 articles from PubMed, and 4 from Google Scholar. Based on this, any issues and disagreements related to coding were discussed, and the form was revised. This protocol was repeated three times until the instrument was considered reliable, then a reliability pilot test was formally conducted using the below-mentioned methods. To establish the intercoder reliability, both researchers coded 88 (70.97%) papers from the list of 124 full-text revised studies. In addition, each researcher coded half of the remaining 36 articles. To build the final database, the papers used in the reliability analysis were divided randomly into two different groups, and the decisions of each coder were randomly selected. To assess intercoder reliability for each variable, percent agreement, Scott’s pi, Cohen’s kappa, and Krippendorff’s alpha were utilized [39], and ReCal (“Reliability Calculator”) software was used to calculate these variables [40,41]. In the case of two coders evaluating the same variable, Holsti’s method was not included because it is identical to Scott’s pi. Besides, to consider the coding of a variable reliable, either a Krippendorff’s alpha of ≥ 0.70 or a percent agreement of ≥ 0.90 is needed.
3. Results
The literature search identified 1438 articles. After the first screening, the full texts of 124 relevant studies were reviewed. Out of these 11 articles [7,42,43,44,45,46,47,48,49,50,51], corresponding to 10 different studies (references [49,51] belong to the same study), met the selection criteria (Figure 2) and were included in the subsequent synthesis of evidence. The references from the 113 excluded articles are available in the Supplementary Material (File S1). Because of the substantial heterogeneity of the selected manuscripts, a meta-analysis could not be carried out.
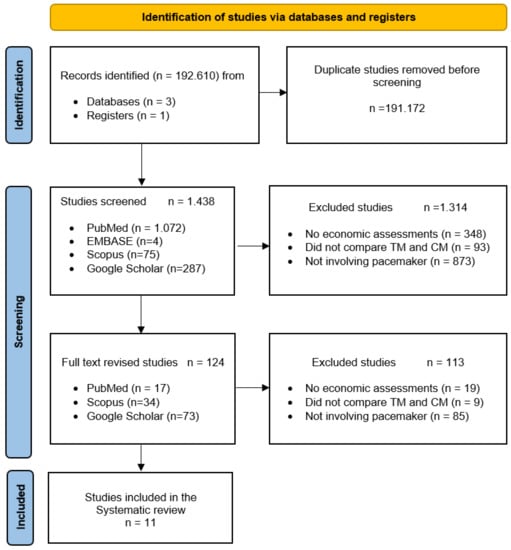
Figure 2.
PRISMA flow diagram [52] of the selection process of studies for the systematic review of economic evaluations of remote monitoring systems and follow-up of patients with pacemakers. CM—Conventional monitoring; TM—Telemonitoring.
3.1. Characteristics of the Selected Studies
This review included seven experimental [7,42,47,48,49,50,51] and four descriptive/observational [43,44,45,46] studies and aimed to evaluate the results on quality of life, effectiveness, safety, reliability, and costs of TM of pacemakers compared with CM [7,42,43,44,45,46,47,48,49,50,51].
The main characteristics of the studies are summarized in Table 1. The selected publications represent a total of 3372 enrolled patients. Out of them, 1773 (52.58%) were part of randomized clinical trials. The sample sizes of the studies varied (50–802 patients). The mean age of the patients in 10 of the publications [7,43,44,45,46,47,48,49,50,51] was 71.85 ± 22.09 years (minimum age 12; maximum age 88). The major indication for pacemaker implantation was atrioventricular block [7,44,45,46,47,48,49,51]. The study period ranged from 4 weeks [7] to 372 months [48]. All of the selected studies used the same pacemaker model in both follow-up arms, with the exception of the studies by Folino et al. [45,46] and Lopez-Villegas et al. [50], who used two different pacemaker models in the CM group. None of the selected studies stated if monitoring systems were previously being used for all pacemakers followed up by the hospital. A cost-utility analysis was performed in five of the publications [7,48,49,50,51].

Table 1.
Study characteristics.
3.2. Health Variables Analysis
Table 2 contains the primary and secondary endpoints analyzed in each of the studies, as well as the most significant results. Only the studies of Folino et al. [45,46] included the number of pacemaker replacements (ranging from 7 to 123), and the reported device longevity ranged from 6.7 years to 8.3 years [7,44,45,46]. Only two studies [7,48] specified the mean hospital stay, which was 34% to 73.2% shorter in the TM group. Besides, two studies [7,48] administered the SF-36 questionnaire, and the other three studies [49,50,51] used the EuroQol–5D (EQ–5D) questionnaire to evaluate the health-related quality of life (HRQoL). The results indicated no significant differences between the two alternatives of follow-up.

Table 2.
Analysis of main outcomes, inputs, and conclusions.
Out of the eight studies that included information on adverse events per year [7,42,43,44,45,46,47,48], six of them reported a higher percentage of events in the TM group [7,42,43,44,45,46]; the study by Folino et al. [46] reported the highest percentage (52%). In contrast, only one study [48] reported a higher percentage of events in the CM group (35.40%) as compared to the TM group (21.70%). The percentage of patients in the active group, who had to visit the hospital so that their pacemaker could be reprogrammed [46], ranged from 0.6 to 1.9% per year. In seven of the 11 studies included in this systematic review (63.64%), the annual mortality rate [7,42,44,45,46,50,51] ranged from 0 to 11.7%.
3.3. Cost Analysis
The costs of implementing TM in patients with pacemakers were not included in any of the studies selected in this systematic review (Table 2). Three of the papers stated that the costs of the “home monitoring system” (remote option) were paid by the hospital [43,49,50]. In contrast, in the articles written by Folino et al. [45,46], the costs of implementing TM systems were borne by the pacemaker manufacturers.
In order to facilitate the economic comparison of the different studies selected in this systematic review, the cumulative annual inflation was estimated from the year following the publication of the article to December 2020. Then, direct conversion of each currency to euros (€) based on the price on 12 August 2021, was made. The total costs of all the studies included in this review are lower in the TM group compared to that of the CM group, except in the results presented by Lopez-Villegas [50] (Table 2). In the WEST-SCOTLAND [42] study, the replacement of the CM modality of follow-up by the TM modality resulted in a saving of €14,669 per year (associated with ambulance transport) for the Scottish National Health System. A study carried out with a pediatric population [43] indicates that there would have been a saving of €18,611 over the 3 years of study period if the 96 participants had been able to substitute visits to the emergency room in the hospital with the data transmission system. The economic saving in the TM modality is evident in almost 82% (n = 9) of the selected studies [7,43,44,45,46,47,48,49,51], with the costs associated with the TM group being 9% to 86.69% lower than that of the CM group. In five (45.45%) of the selected studies [7,46,47,49,51], the patients in the TM group reported a reduced number of hospital visits (8.34–55.55%) compared to the patients in the CM group. The informal costs associated with each modality of follow-up (costs of transport, productivity, accompanying person, etc.) were estimated in five of the studies included in this systematic review [45,48,49,50,51], which indicates that in the remote modality of follow-up, cost savings of up to 56.70% per patient/year can be achieved [49]. Table 3 shows the costs of the follow-up alternatives included in this systematic review.

Table 3.
Costs evaluated in both modalities of follow-up.
3.4. Methodological Quality Assessment
The variables evaluated were scored based on the presence or absence (yes/no answers) of the criterion analyzed (Table 4). If, on the final review of the article, a parameter was not found, a response of “no” was recorded in the table, i.e., the study did not include that parameter.

Table 4.
Checklist for analyzing methodological quality of the studies.
The study by Bautista-Mesa et al. [51] obtained the highest overall score for methodological quality, with 24 out of a possible 25 points, whereas the lowest score of 7 was obtained in the study by Shaw [42]. The publications evaluated had a mean score of 15.55 ± 5.07 points (minimum 7; maximum 24). The main findings were as follows:
- (a)
- Five manuscripts [45,47,49,50,51] included results with both social and financial perspectives (NHS);
- (b)
- Five studies [7,48,49,50,51] have used social assessment scales for evaluating HRQoL, which were validated on a representative sample of the population;
- (c)
- Except for one study [51], none of the studies applied modeling techniques or discounts for costs and benefits or conducted a sensitivity analysis;
- (d)
- The results obtained from eight of the selected studies [7,44,45,47,48,49,50,51] could draw conclusions about the transferability or extrapolation of results to other contexts;
- (e)
- The results of all the included studies are presented with an incremental analysis; however, the results of three studies are disaggregated (costs and results of the alternatives) [49,50,51];
- (f)
- Five of the studies [7,42,43,44,49,50,51] have clearly indicated the financial source of the study.
3.5. Intercoder Reliability
The results for each variable are shown in Table 5. Mean and standard deviation values were not calculated because the variables included in this study were categorical. The study by Vincent et al. [43] obtained the lowest percentage agreement of 84%, with other parameters being Scott’s pi −0.087, Cohen’s kappa −0.087, and Krippendorff’s alpha −0.065; the highest percentage agreement of 100% was obtained in four studies [44,45,46,51].

Table 5.
Intercoder reliability and percentages.
4. Discussion
The results of this review indicate no significant differences in HRQoL and the number of cardiovascular events between TM and CM modalities of the follow-up. The results show that TM was significantly effective in detecting the presence or absence of pacemaker problems, leading to a reduction in the number of unscheduled hospital visits. In addition, follow-up costs in the remote modality are significantly lower than that of the CM modality. The economic impact of each of the monitoring alternatives is significantly influenced by the costs associated with staff salaries, transport, informal care, and absences from work.
4.1. Effectiveness and Clinical Safety of TM Systems
Four of the 11 studies analyzed in this systematic review (36.37%) included the data regarding the number of hospital visits made by patients in both follow-up alternatives [7,47,49,51]. The main finding was that in the TM group, there was a significant reduction of 8.34–55.55% in the number of hospital visits. These results were similar (in the upper range) to those previously published in the COMPAS study [19], which reported that patients of the TM group made 55% fewer hospital visits compared to patients included in the CM group. In contrast, three of the articles (27.27%) [43,44,45] reported results pertaining to the TM group only.
The development and expansion of remote pacemaker monitoring systems have proven that this is a safe and reliable technology [7,42,43,44,45,46,47,48,49,50,51]. The steady increase in the transmission of information from the patient’s home monitor to the service provider’s platform has enabled quick and efficient treatment of cardiac patients on an ongoing basis. It is also noted that in the medium to long term, there is a significant reduction in the number of unscheduled visits and/or hospitalizations. In four of the publications included in this review [7,46,47,48], there were no significant differences between the two follow-up modalities in relation to the number of adverse events detected, which is in accordance with two previously published studies [19,22]. In a previous study on pacemakers [16], it is reported that cardiovascular events were detected around two months earlier in the TM group (5.7 vs. 7.7). In two subsequent studies carried out on patients with implantable cardioverter-defibrillator and cardiac resynchronization therapy, the response time to these episodes was 22–36 days in the case of the CM group; however, in the TM group, it was reduced to 2–4.6 days [22,53].
Five of the selected studies analyzed HRQoL of the included patients (45.46%) [7,48,49,50,51]. The SF-36 questionnaire was used in two studies [7,48] and the EQ-5D was used by Lopez-Villegas et al. [49,50] and Bautista-Mesa et al. [51]. The results indicate no significant differences between the two follow-up modalities in all patients. These results coincide with those found in the COMPAS [19] and ECOST [22] trials, which used the SF-36 questionnaire, and with the PONIENTE [20] study, which used the EQ-5D questionnaire.
The analysis of the methodological quality of the manuscripts included in this study exhibited significant variability among them, with higher scores obtained by the most recent studies [49,50,51]. The results presented in this systematic review, which coincide with a previous study [54] published in 2016, indicate how difficult it is to assess the methodological quality of studies published in the last two decades based on the current criteria [42,43]. However, different inputs are included in all the selected studies, such as the establishment of an objective and research question, comparison of both follow-up modalities, adjustment of the costs collected to the perspective of the selected analysis, and adaptation of the time horizon to the study objectives. Additionally, they coincide, except for the study by Bautista-Mesa et al. [51], in not implementing modeling techniques, discounting costs, performing sensitivity analysis, justifying key parameters and statistical distribution of the variables, performing equity analysis, and including cost-effectiveness and cost-benefit ratios.
4.2. Cost Analysis
The results presented in this systematic review confirm that TM of pacemakers can significantly decrease the length of hospital stays [7,48] reaching in some cases a reduction of up to 80.49%. One of the most significant findings is the substantial reduction of 9% to 86.69% [7,42,43,44,45,46,47,48,49,50,51] in the costs of TM with respect to that of CM.
In addition, the results of most of the studies included in this review indicate that TM systems significantly reduce direct costs, such as for staff and health administration, as well as indirect costs related to monitoring, such as transport costs, maintenance of consultations, and waiting rooms, etc. The results found in this systematic review are similar to those obtained in previous studies that were carried out on different types of cardiovascular electronic devices [2,15,26,27,55], and on remote follow-up performed in other pathologies, such as rheumatoid arthritis [56], mental health [57], teleglaucoma [58], teledermatology, and tele radiology [59,60,61]. In a study published in 2009 by Raatikainen, it was reported that a lower number of hospital visits resulted in up to 41% reduction in costs per patient [27]. A study published by Elsner [62] reported a 61% increase in savings due to a reduction of 63% in the number of visits and the transport costs associated with them. Finally, and coinciding with the results obtained in this review, Crossley published a study reporting that reducing the number of days spent in hospitals can achieve savings of almost $1700 per patient per year [16].
4.3. Study Limitations
Although the results presented in this systematic review are highly relevant in relation to the effectiveness of TM in patients with pacemakers, the analysis carried out presents several limitations that should be taken into account.
First, the number of included studies (n = 11) and enrolled participants (n = 3427) were less, mainly due to the limited use of TM technology compared to CM. The second limitation is the variability in the methodological quality of the selected studies; except for one study [51], none of them used modeling techniques and discounts in costs and results. Apart from this, the key parameters of the study and the statistical distribution of the variables examined in the sensitivity analysis were not explained properly. The third limitation is the small number of studies [48,51] analyzing the medium-and long-term effectiveness of remote monitoring, as TM is a relatively new technology. The fourth limitation is the large time span of 39 years between the first and the last published study [42,51], during which, exponential changes in these technologies have occurred. Furthermore, in this study, the differences and similarities between both monitoring modalities have been verified in different spatiotemporal contexts. Finally, cost-effectiveness studies were less generalizable compared to effectiveness studies since they depend on both the duration and the context in which the studies are carried out; yet their importance is enormous since they facilitate decision-making by the different professionals involved [54]. This systematic review presents the significant results of studies carried out in the last 40 years (1981–2020), mainly focusing on the health outcomes and costs associated with TM of patients with pacemakers. Therefore, the findings of this systematic review have led to an update in scientific knowledge in this area, and the results can be further utilized to facilitate decision-making and the implementation of new health policies. The authors of this study advise future researchers to focus on economic evaluations, comparing both follow-up modalities, including the cost-effectiveness ratios and the informal costs associated with the follow-up. In addition, the time horizon should be medium and long term.
The results presented in this study can be used by both healthcare managers and cardiology unit professionals to promote the sustainability of current healthcare systems.
5. Conclusions
Most of the studies included in this systematic review confirm that in the TM of patients with pacemakers, there is a reduction in cardiovascular events and hospital visits without affecting the HRQoL of patients. In addition, both formal and informal costs are significantly reduced in the medium and long term.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph182212120/s1, File S1: References not included in the systematic review.
Author Contributions
Conceptualization, D.C.-M. and A.L.-V.; methodology, D.C.-M. and C.L.-C.; formal analysis, M.P.-H. and I.V.-T.; investigation, A.L.-V. and C.L.-C.; resources, A.L.-V.; data curation, A.L.-V.; writing—original draft preparation, A.L.-V. and D.C.-M.; writing—review and editing, D.C.-M., I.V.-T., M.P.-H. and C.L.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Instituto de Salud Carlos III through the project “No. PI17/02056” (Co-funded by European Regional Development Fund/European Social Fund “A way to make Europe”/“Investing in your future”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gruska, M.; Aigner, G.; Altenberger, J.; Burkart-Küttner, D.; Fiedler, L.; Gwechenberger, M.; Lercher, P.; Martinek, M.; Gruska, M.; Working Group Rhythmology of the Austrian Cardiological Society; et al. Recommendations on the utilization of telemedicine in cardiology. Wien. Klin. Wochenschr. 2020, 132, 782–800. [Google Scholar] [CrossRef]
- Eze, N.D.; Mateus, C.; Hashiguchi, T.C.O. Telemedicine in the OECD: An umbrella review of clinical and cost-effectiveness, patient experience and implementation. PLoS ONE 2020, 15, e0237585. [Google Scholar] [CrossRef] [PubMed]
- Delgoshaei, B.; Mobinizadeh, M.; Mojdekar, R.; Afzal, E.; Arabloo, J.; Mohamadi, E. Telemedicine: A systematic review of economic evaluations. Med. J. Islam. Repub. Iran 2017, 31, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.; Fenaughty, A. Technology and managed care: Patient benefits of telemedicine in a rural health care network. Health Econ. 2005, 14, 559–573. [Google Scholar] [CrossRef]
- Hummel, J.P.; Leipold, R.J.; Amorosi, S.L.; Bao, H.; Deger, K.A.; Jones, P.W.; Kansal, A.R.; Ott, L.S.; Stern, S.; Stein, K.; et al. Outcomes and costs of remote patient monitoring among patients with implanted cardiac defibrillators: An economic model based on the Predict RM database. J. Cardiovasc. Electrophysiol. 2019, 30, 1066–1077. [Google Scholar] [CrossRef]
- Abdelhadi, R.H.; Saba, S.F.; Ellis, C.R.; Mason, P.K.; Kramer, D.B.; Friedman, P.A.; Gura, M.T.; DiMarco, J.P.; Mugglin, A.S.; Reynolds, M.R.; et al. Independent multicenter study of Riata and Riata ST implantable cardioverter-defibrillator leads. Hear. Rhythm. 2013, 10, 361–365. [Google Scholar] [CrossRef]
- Halimi, F.; Clémenty, J.; Attuel, P.; Dessenne, X.; Amara, W.; on behalf of the OEDIPE trial Investigators. Optimized post-operative surveillance of permanent pacemakers by home monitoring: The OEDIPE trial. Europace 2008, 10, 1392–1399. [Google Scholar] [CrossRef]
- Hauser, R.G.; Kallinen, L.M.; Almquist, A.K.; Gornick, C.C.; Katsiyiannis, W.T. Early failure of a small-diameter high-voltage implantable cardio-verter-defibrillator lead. Heart Rhythm 2007, 4, 892–896. [Google Scholar] [CrossRef]
- Hauser, R.G.; Maron, B.J. Lessons From the Failure and Recall of an Implantable Cardioverter-Defibrillator. Circulation 2005, 112, 2040–2042. [Google Scholar] [CrossRef]
- Maisel, W.H. Semper Fidelis—Consumer Protection for Patients with Implanted Medical Devices. New Engl. J. Med. 2008, 358, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Klersy, C.; De Silvestri, A.; Gabutti, G.; Raisaro, A.; Curti, M.; Regoli, F.; Auricchio, A. Economic impact of remote patient monitoring: An integrated economic model derived from a me-ta-analysis of randomized controlled trials in heart failure. Eur. J. Heart Fail 2011, 13, 450–459. [Google Scholar] [CrossRef]
- Klersy, C.; De Silvestri, A.; Gabutti, G.; Regoli, F.; Auricchio, A. A Meta-Analysis of Remote Monitoring of Heart Failure Patients. J. Am. Coll. Cardiol. 2009, 54, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Landolina, M.; Perego, G.B.; Lunati, M.; Curnis, A.; Guenzati, G.; Vicentini, A.; Parati, G.; Borghi, G.; Zanaboni, P.; Valsecchi, S.; et al. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: The evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012, 125, 2985–2992. [Google Scholar] [CrossRef]
- Saxon, L.A.; Hayes, D.L.; Gilliam, F.R.; Heidenreich, P.A.; Day, J.; Seth, M.; Meyer, T.E.; Jones, P.W.; Boehmer, J.P. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: The ALTITUDE survival study. Circulation 2010, 122, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; Michalski, J.; Epstein, A.E.; Schweikert, R. Automatic remote monitoring of implantable cardioverter-defibrillator lead and generator performance: The Lumos-T Safely RedUceS RouTine Office Device Follow-Up (TRUST) trial. Circ. Arrhythmia Electrophysiol. 2010, 3, 428–436. [Google Scholar] [CrossRef]
- Crossley, G.H.; Boyle, A.; Vitense, H.; Chang, Y.; Mead, R.H. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) Trial: The Value of Wireless Remote Monitoring With Automatic Clinician Alerts. J. Am. Coll. Cardiol. 2011, 57, 1181–1189. [Google Scholar] [CrossRef]
- Lopez-Villegas, A.; Catalan-Matamoros, D.; Robles-Musso, E.; Peiró, S. Workload, time and costs of the informal cares in patients with tele-monitoring of pacemakers: The PONIENTE study. Clin. Res. Cardiol. 2016, 105, 307–313. [Google Scholar] [CrossRef]
- Cronin, E.M.; Varma, N. Remote monitoring of cardiovascular implanted electronic devices: A paradigm shift for the 21st century. Expert Rev. Med. Devices 2012, 9, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Mabo, P.; Victor, F.; Bazin, P.; Ahres, S.; Babuty, D.; Da Costa, A.; Binet, D.; Daubert, J.-C. A randomized trial of long-term remote monitoring of pacemaker recipients (The COMPAS trial). Eur. Hear. J. 2012, 33, 1105–1111. [Google Scholar] [CrossRef]
- López-Villegas, A.; Catalán-Matamoros, D.; Robles-Musso, E.; Peiró, S. Comparative effectiveness of remote monitoring of people with cardiac pacemaker versus conventional: Quality of life at the 6 months. Rev. Esp. Salud. Pública. 2015, 89, 149–158. [Google Scholar] [CrossRef][Green Version]
- López-Villegas, A.; Catalan-Matamoros, D.; Robles-Musso, E.; Peiró, S. Effectiveness of pacemaker tele-monitoring on quality of life, functional capacity, event detection and workload: The PONIENTE trial. Geriatr. Gerontol. Int. 2015, 16, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Guédon-Moreau, L.; Lacroix, D.; Sadoul, N.; Clémenty, J.; Kouakam, C.; Hermida, J.S.; Aliot, E.; Boursier, M.; Bizeau, O.; Kacet, S. ECOST trial investigators. A randomized study of re-mote follow-up of implantable cardioverter defibrillators: Safety and efficacy report of the ECOST trial. Eur. Heart J. 2013, 34, 605–614. [Google Scholar] [CrossRef]
- Ricci, R.P.; Morichelli, L.; Santini, M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: Impact on medical management and healthcare resource utilization. Europace 2008, 10, 164–170. [Google Scholar] [CrossRef]
- Zanaboni, P.; Landolina, M.E.; Marzegalli, M.; Lunati, M.; Perego, G.B.; Guenzati, G.; Curnis, A.; Valsecchi, S.; Borghetti, F.; Borghi, G.; et al. Cost-Utility Analysis of the EVOLVO Study on Remote Monitoring for Heart Failure Patients with Implantable Defibrillators: Randomized Controlled Trial. J. Med. Internet Res. 2013, 15, e106. [Google Scholar] [CrossRef] [PubMed]
- Bikou, O.; Licka, M.; Kathoefer, S.; Katus, A.H.; Bauer, A. Cost savings and safety of ICD remote control by telephone: A prospective, observational study. J. Telemed. Telecare 2010, 16, 403–408. [Google Scholar] [CrossRef]
- Halimi, F.; Cantù, F.; On behalf of the European Heart Rhythm Association (EHRA) Scientific Initiatives Committee (SIC). Remote monitoring for active cardiovascular implantable electronic devices: A European survey. Europace 2010, 12, 1778–1780. [Google Scholar] [CrossRef] [PubMed]
- Raatikainen, M.J.; Uusimaa, P.; van Ginneken, M.M.; Janssen, J.P.; Linnaluoto, M. Remote monitoring of implantable cardioverter defibrillator patients: A safe, time-saving, and cost-effective means for follow-up. Europace 2008, 10, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; Epstein, A.E.; Irimpen, A.; Schweikert, R.; Love, C.; Investigators, T. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: The Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010, 122, 325–332. [Google Scholar] [CrossRef]
- Hindricks, G.; Elsner, C.; Piorkowski, C.; Taborsky, M.; Geller, J.C.; Schumacher, B.; Bytesnik, J.; Kottkamp, H. Quarterly vs. yearly clinical follow-up of remotely monitored recipients of prophylactic implant-able cardioverter-defibrillators: Results of the REFORM trial. Eur. Heart J. 2014, 35, 98–105. [Google Scholar] [CrossRef]
- Boriani, G.; Da Costa, A.; Ricci, R.P.; Quesada, A.; Favale, S.; Iacopino, S.; Romeo, F.; Risi, A.; di S. Stefano, L.M.; Navarro, X.; et al. The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) randomized con-trolled trial: Phase 1 results on dynamics of early intervention with remote monitoring. J. Med. Internet Res 2013, 15, e167. [Google Scholar] [CrossRef]
- Gramegna, L.; Tomasi, C.; Gasparini, G.; Scaboro, G.; Zanon, F.; Boaretto, G.; Tomei, R.; Tomasi, L. In-hospital follow-up of implantable cardioverter defibrillator and pacemaker carriers: Patients’ inconvenience and points of view. A four-hospital Italian survey. Europace 2011, 14, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; Michalski, J.; Stambler, B.; Pavri, B.B. TRUST Investigators superiority of automatic remote monitoring compared with in-person evaluation for scheduled ICD follow-up in the TRUST trial—Testing execution of the recommendations. Eur. Heart J. 2014, 35, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.A.; Antonio, Ló. La Telesalud y la sociedad actual: Retos y oportunidades. Rev. Esp. Comun. Salud. 2016, 7, 336–345. [Google Scholar]
- Gillis, A.M. Remote Monitoring of Implantable Defibrillators: Reducing Hospitalizations and Saving Lives? Circ. Arrhythm. Electrophysiol. 2015, 8, 1010–1011. [Google Scholar] [CrossRef]
- Slotwiner, D.; Varma, N.; Akar, J.G.; Annas, G.; Beardsall, M.; Fogel, R.I.; Galizio, N.O.; Glotzer, T.V.; Leahy, R.A.; Love, C.J.; et al. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015, 12, e69–e100. [Google Scholar] [CrossRef]
- Varma, N.; Piccini, J.P.; Snell, J.; Fischer, A.; Dalal, N.; Mittal, S. The Relationship between Level of Adherence to Automatic Wireless Remote Monitoring and Survival in Pacemaker and Defibrillator Patients. J. Am. Coll. Cardiol. 2015, 65, 2601–2610. [Google Scholar] [CrossRef]
- Hernández-Madrid, A.; Lewalter, T.; Proclemer, A.; Pison, L.; Lip, G.Y.; Blomstrom-Lundqvist, C.; Scientific Initiatives Committee; European Heart Rhythm Association. Remote monitoring of cardiac implantable electronic devices in Europe: Results of the European Heart Rhythm Association survey. Europace 2014, 16, 129–132. [Google Scholar] [CrossRef]
- López-Bastida, J.; Oliva, J.; Antoñanzas, F.; García-Altés, A.; Gisbert, R.; Mar, J.; Puig-Junoy, J. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gac. Sanit. 2010, 24, 154–170. [Google Scholar] [CrossRef]
- Lombard, M.; Snyder-Duch, J.; Bracken, C.C. Content Analysis in Mass Communication: Assessment and Reporting of Intercoder Reliability. Hum. Commun. Res. 2002, 28, 587–604. [Google Scholar] [CrossRef]
- Freelon, D. ReCal: Intercoder reliability calculation as a web service. Int. J. Internet Sci. 2010, 5, 20–33. [Google Scholar]
- Freelon, D. ReCal OIR: Ordinal, interval, and ratio intercoder reliability as a web service. Int. J. Internet Sci. 2013, 8, 10–16. [Google Scholar]
- Shaw, G.B.; Evans, A.L.; Brewster, G.M.; Groden, B.M.; Murdoch, W.R. Telephone monitoring of patients with pacemakers in the west of Scot-land. Br. Med. J. 1981, 283, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.; Cavitt, D.; Karpawich, P. Diagnostic and Cost Effectiveness of Telemonitoring the Pediatric Pacemaker Patient. Pediatr. Cardiol. 1997, 18, 86–90. [Google Scholar] [CrossRef]
- Pang, H.W.; Campbell, D.; Hopman, W.M.; Brennan, F.J.; Abdollah, H.; Redfearn, D.P.; Simpson, C.S.; Baranchuk, A. Effectiveness and feasibility of a transtelephonic monitoring program: Implications for a time of crisis. Int. J. Cardiol. 2010, 145, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Folino, A.F.; Breda, R.; Calzavara, P.; Migliore, F.; Iliceto, S.; Buja, G. In-home controls of pacemakers in debilitated elderly patients. Geriatr. Gerontol. Int. 2011, 12, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Folino, A.F.; Breda, R.; Calzavara, P.; Borghetti, F.; Comisso, J.; Iliceto, S.; Buja, G. Remote follow-up of pacemakers in a selected population of debilitated elderly patients. Europace 2013, 15, 382–387. [Google Scholar] [CrossRef]
- Perl, S.; Stiegler, P.; Rotman, B.; Prenner, G.; Lercher, P.; Anelli-Monti, M.; Sereinigg, M.; Riegelnik, V.; Kvas, E.; Kos, C.; et al. Socio-economic effects and cost saving potential of remote patient monitoring (SAVE-HM trial). Int. J. Cardiol. 2013, 169, 402–407. [Google Scholar] [CrossRef]
- Parahuleva, M.S.; Soydan, N.; Divchev, D.; Lüsebrink, U.; Schieffer, B.; Erdogan, A. Home monitoring after ambulatory implanted primary cardiac implantable electronic devices: The home ambulance pilot study. Clin. Cardiol. 2017, 40, 1068–1075. [Google Scholar] [CrossRef]
- Lopez-Villegas, A.; Catalan-Matamoros, D.; Robles-Musso, E.; Bautista-Mesa, R.; Peiró, S. Cost-utility analysis on telemonitoring of users with pacemakers: The Poniente study. J. Telemed. Telecare 2019, 25, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Villegas, A.; Catalan, D.J.; Peiró, S.; Lappegard, K.T.; Lopez-Liria, R. Cost-utility analysis of telemonitoring versus conventional hospital-based follow-up of patients with pacemakers. The NORDLAND randomized clinical trial. PLoS ONE 2020, 15, e0226188. [Google Scholar] [CrossRef]
- Bautista-Mesa, R.J.; Lopez-Villegas, A.; Peiro, S.; Catalan-Matamoros, D.; Robles-Musso, E.; Lopez-Liria, R.; Leal-Costa, C. Long-term cost-utility analysis of remote monitoring of older patients with pacemakers: The PONIENTE study. BMC Geriatr. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Mabo, P.; Inserm, R.; Defaye, P. Remote Follow-up of Patients Implanted with an ICD: The Prospective randomized EVATEL study. ESC Congress report. In Proceedings of the European Society of Cardiology Congress 2011, París, France, 27–31 August 2011. [Google Scholar]
- López-Villegas, A.; Catalán-Matamoros, D.; Martín-Saborido, C.; Villegas-Tripiana, I.; Robles-Musso, E. A Systematic Review of Economic Evaluations of Pacemaker Telemonitoring Systems. Rev. Española Cardiol. 2016, 69, 125–133. [Google Scholar] [CrossRef]
- Fauchier, L.; Sadoul, N.; Kouakam, C.; Briand, F.; Chauvin, M.; Babuty, D.; Clementy, J. Potential cost savings by telemedicine-assited long-term care of implantable cardioverter defibrillator recipients. Pacing Clin. Electrophysiol. 2005, 28, 255–259. [Google Scholar] [CrossRef]
- McDougall, J.A.; Ferucci, E.D.; Glover, J.; Fraenkel, L. Telerheumatology: A Systematic Review. Arthritis Rheum. 2017, 69, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Musiat, P.; Tarrier, N. Collateral outcomes in e-mental health: A systematic review of the evidence for added benefits of computerized cognitive behavior therapy interventions for mental health. Psychol. Med. 2014, 44, 3137–3150. [Google Scholar] [CrossRef]
- Thomas, S.-M.; Jeyaraman, M.; Hodge, W.G.; Hutnik, C.; Costella, J.; Malvankar-Mehta, M.S. The Effectiveness of Teleglaucoma versus In-Patient Examination for Glaucoma Screening: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e113779. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Yoo, B.-K. A Systematic Review of the Economic Evaluation of Telemedicine in Japan. J. Prev. Med. Public Health 2016, 49, 183–196. [Google Scholar] [CrossRef]
- Iribarren, S.J.; Cato, K.; Falzon, L.; Stone, P.W. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS ONE 2017, 12, e0170581. [Google Scholar] [CrossRef]
- Snoswell, C.; Finnane, A.; Janda, M.; Soyer, H.P.; Whitty, J.A. Cost-effectiveness of Store-and-Forward Teledermatology. JAMA Dermatol. 2016, 152, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Elsner, C.H.; Sommer, P.; Piorkowski, C.; Taborsky, M.; Neuser, H.; Bytesnik, J.; Geller, J.C.; Kottkamp, H.; Wiesmeth, H.; Hindricks, G. A prospective multicenter comparison trial of home monitoring against regular follow-up in MADIT II patients: Additional visits and cost impact. Comput Cardiol. 2006, 33, 241–244. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).