Abstract
Background: Alzheimer’s Disease (AD) is a global problem affecting 58 million people, expected to reach a prevalence of 88 million people by 2050. The disease affects the brain, memory, cognition, language, and motor movement. Many interventions have sought to improve memory and cognition. mHealth and virtual reality (VR) are two such interventions. Objectives: To analyze studies from the last 10 years with older adults with AD to ascertain the effectiveness of telehealth techniques such as mHealth and VR for memory care. Methods: In accordance with the Kruse Protocol and reported in accordance with PRISMA 2020, five reviewers searched four research databases (PubMed, CINAHL, Web of Science, and ScienceDirect) on 3 August 2022 for studies with strong methodologies that fit the objective statement. Results: Twenty-two studies from 13 countries were analyzed for trends. Four interventions (mHealth/eHealth, VR, mHealth + VR, game console, and telephone) used RCT, quasi-experimental, pre-post, observational, and mixed methods. These interventions improved cognition, memory, brain activity, language, depression, attention, vitality, quality of life, cortical atrophy, cerebral blood flow, neuro plasticity, and mental health. Only three interventions reported either no improvements or no statistically significant improvements. Cost, time, training, and low reimbursement were barriers to the adoption of these interventions. Conclusion: mHealth and VR offer interventions with positive effectiveness for memory care for AD. The long-term effect of this improvement is unclear. Additional research is needed in this area to establish clinical practice guidelines.
1. Introduction
1.1. Rationale
Alzheimer’s Disease (AD) is a growing condition around the word. As we approached the COVID-19 pandemic, AD was the largest killer of older adults: it kills more people than breast cancer and prostate cancer [1]. The prevalence of the disease was calculated in 2021 to be 58 million people, but it is predicted to exceed 88 million by 2050 [1]. Of the dementia population, AD accounts for about 2/3 s [1]. There is currently no cure for AD, and there are only about 10 pharmaceuticals approved to manage the condition. The disease creates plaque on the brain (tau) that eventually affects the communication of 100 billion neurons in the brain, degrading and ultimately destroying these neurons [2]. Early stages of AD is seen as simple forgetfulness of recently learned facts, but late stages of AD affects speech, motor skills, and long-term memory [1]. Researchers and practitioners do not fully understand the etiology and pathogenesis of AD: we can treat the symptoms, but we cannot prevent or cure the disease [3,4,5]. Researchers have searched for decades for interventions to improve symptoms of cognitive decline, and one of these is cognitive training through telemedicine.
Many tests are used to assess impairment and symptoms associated with AD. AD affects cognition, which is a complex process in the brain that involves memory, abstraction and iconic concepts, mental operations, consciousness, search strategies, problem solving, and social context [6]. One common method to measure cognition is the mini-mental state examination (MMSE), which estimates a severity of cognitive impairment through a series of questions organized into seven categories: orientation to time, orientation to place, registration of three words, attention to calculation, recall of three words, language, and visual construction [7]. Given over time, the MMSE can identify rate of decline or document improvement.
Telemedicine is defined as healing from a distance using information communication technology to overcome geographical boundaries and increase health outcomes [8]. mHealth is a subset of telemedicine that leverages mobile technology to deliver some sort of intervention or interaction with a provider. mHealth interventions with patients who have AD suffer from barriers such as cognition, perception, physical ability, frame of mind, speech and language [9]. mHealth design must break steps into very simple, easy to understand modules, must often repeat instructions to keep the attention of the users, and use simple memory tests to avoid overwhelming the user [10]. mHealth has been coupled with other interventions such as transcranial alternating current during cognitive training, but results are not conclusive [11]. Virtual reality (VR) has also entered the area of AD research, specifically in the area of cognitive training. The reason is that VR exercises multiple perception components of psychophysics (visual, tactile, and kinesthetic perceptual sensations) [12]. The proponents of VR like its immersive and adaptable environment. It has been used in the areas of brain damage, poststroke intervention, musculoskeletal recovery, and in cognitive training for AD. This review will focus on the telemedicine-related interventions (mHealth, VR, and serious games) in the area of memory for AD patients. Multiple systematic literature reviews have examined this interaction. Many conclude that telemedicine can assess cognition, monitor activity, and improve communication with provider teams [13]. Telemedicine can positively affect mood, function, and quality of life, but its effect on cognition is unclear [14].
A systematic literature review and meta-analysis was published in 2022 that analyzed 16 Randomized Controlled Trials (RCTs) [15]. The meta-analysis focused on a smaller set of studies. It found that serious games are as effective as no intervention or passive interventions at improving executive functions. It concluded that conventional exercises were just as effective. The reviewers felt their group for analysis was too small for final conclusions.
A systematic literature review was published in 2022 that analyzed 28 studies over 10 years [9]. It evaluated several aspects of mHealth. It found positive perceptions of the users of mHealth (both AD patients and their caregivers). The caregivers attributed positive effect of mHealth interventions on their physical and mental health; however, effectiveness was not evaluated.
1.2. Objectives
The purpose of this review is to analyze the effectiveness of telemedicine-related interventions (mHealth, VR, and serious games) to improve cognition for older adults suffering from Alzheimer’s Disease or mild cognitive impairment (MCI) using published literature from the last 10 years. Secondary outcomes will be memory, language, mood, vitality, attention, brain waves, and other conditions measured and reported in the literature. Our review will be different from previous reviews. We will use a larger group of articles for analysis than the former review [15], and it will analyze effectiveness, different from the latter review [9].
2. Methods
2.1. Eligibility Criteria
Articles eligible for this review required older adults (>50) with early-stage Alzheimer’s Disease or MCI as participants, published in the last ten years, published in peer-reviewed journals, and used strong methods such as RCT or true experiments. Other methods were accepted such as quasi-experimental, mixed method, quantitative, and qualitative.
2.2. Information Sources
We searched in four well-known databases: PubMed (MEDLINE), Complete Index of Nursing and Allied Health Literature (CINAHL), Web of Science, and Embase’s ScienceDirect. We conducted the search on 3 August 2022. We also performed a journal-specific search of Healthcare. MEDLINE was excluded from all but PubMed. We eliminated reviews from our search to not confound the results. We used only published literature to ensure it was peer reviewed.
2.3. Search Strategy
We visited the U.S. Library of Medicine’s website to use the Medical Subject Heading’s (MeSH) indexing database. Using MeSH, we created a Boolean search string to combine key terms. We used the same search sting in all databases: (mhealth OR telemedicine OR “virtual reality” OR “serious games”) AND (“Alzheimer disease” OR dementia) AND memory. Due to differences in filter options in each database, we could not use the exact same filters, but we used similar filter strategies. In CINAHL, we filtered by date, full-text, humans, English language, academic journals, excluded MEDLINE, and excluded reviews. In ScienceDirect, we filtered by date, excluded MEDLINE, and excluded reviews and conference proceedings. In Web of Science, we filtered by date, excluded reviews, and excluded MEDLINE. This practice eliminated most duplicates.
2.4. Selection Process
In accordance with the Kruse Protocol, we searched key terms in all databases, filtered results, and screened abstracts for applicability [16]. At least two reviewers screened each abstract, and at least two reviewers analyzed each article for data extraction and thematic analysis.
2.5. Data Collection Process
The Kruse Protocol standardized an Excel spreadsheet for data extraction and analysis. We used a series of three consensus meetings to finalize the group of articles for analysis, identify themes in the literature, and perform additional analysis on the data extracted.
2.6. Data Items
In accordance with the Kruse Protocol, we collected the following fields of data: database source, date of publication, authors, title of study, participant population, experimental intervention, results (compared to a control), medical outcomes, sample size, bias within study, effect size (Cohen’s d), sensitivity, specificity, F1, country of origin, statistics used, patient satisfaction, effectiveness, barriers to adoption, strength of evidence, and quality of evidence. Results were reported in comparison to a control group. Outcomes and effectiveness are highly similar fields, but they are designed for different audiences (providers and administrators). A provider might not be as concerned as length of stay or cost savings as much as direct medical outcomes (e.g., improvement in cognition), but the administrator is.
The primary outcome for this study is cognition, as measured by the MMSE or similar tool such as Addenbrooke’s Cognitive Examination-Revised (ACE-R), Cognitive Failures Questionnaire (CFQ), Wechsler Adult Intelligence Scale (WAIS), or Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog). Secondary outcomes are reported by studies through a range of measurement tools such as story recall, Hamilton Depression Rating Scale (HAMD), Wechsler Memory Scale 3rd edition (WMS-III), Rey-Osterrieth Complex Figure (ROCF), Controlled Oral Words Association Test (COWAT), Symbol Digit Modalities Test (SDMT), Bayer Activities of Daily Living, etc.
2.7. Study Risk of Bias Assessment and Reporting Bias Assessment
Not only did reviewers note observations of bias in each study, but we also assessed the strength and quality of each study using the Johns Hopkins Nursing Evidence Based Practice tool (JHNEBP) [17]. The overall ratings of quality from the JHNEDP provided us with an assessment of the applicability of the cumulative evidence.We considered the instances of bias in how to interpret the results because bias can limit external validity [18].
2.8. Effect Measures
Because we accepted mixed methods and qualitative studies, we were unable to standardize summary measures, as would be performed in a meta-analysis. Measures of effect are summarized in tables for those studies in which it was reported. Measures of effect can be reported as Cohen’s d, Wald’s W, Eta2, sensitivity, or specificity. Effects vary based on the statistic used, but they usually follow small (0.0–0.2), medium (0.21–0.79), large (0.8 or higher). An average effect size (ES) can be calculated through a weighted average by using the sample size.
2.9. Synthesis Methods
We performed a thematic analysis of the data combining observations (observed multiple times) into themes [19]. We calculated the frequency of occurrences and reported the findings in a series of affinity matrices. This frequency reporting states the probability of finding that theme in the group for analysis, and it provides confidence in the data analyzed. Although thematic analyses are usually reserved for qualitative studies, there is a pattern in the literature for systematic literature reviews to utilize this technique to help synthesize data extracted [20,21,22].
2.10. Additional Analyses and Certainty Assessment
Using the standardized spreadsheet, we sorted by intervention and theme to identify interactions. Some interventions appear more effective than others. Sensitivity and specificity were tabulated where reported.
3. Results
3.1. Study Selection
Figure 1 illustrates our study selection process. Four databases and one focused journal search were conducted with a standardized Boolean search string. The initial 1096 results were filtered to remove duplicates. At the end of the filtering exercise, 869 records were screened using filters on each database. This exercise removed 812 articles. The resulting 57 were retrieved for a full analysis for eligibility. Several more were filtered out (protocols, conference papers, and those that were not germane to our research objective). The remaining group for analysis was 22.
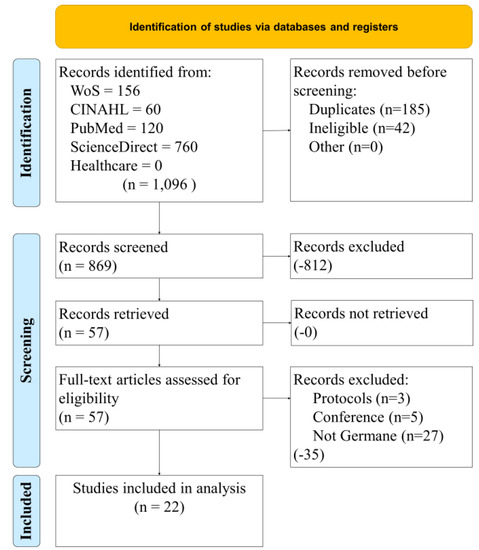
Figure 1.
Study selection process.
3.2. Study Characteristics
Following the PRISMA 2020 checklist, characteristics for each study were systematically extracted and tabulated to include the following data fields: participants, intervention, comparison (to control or other group), observation, study design (PICOS). The standard PICOS table summarizes study characteristics in a manner commensurate with the literature (See Table 1). Of the 22 studies analyzed over the 10-year period, 0 were from 2012, 1 was from 2013 [23], 3 were from 2014 [24,25,26], 2 were from 2015 [27,28], 4 were from 2016 [29,30,31,32], 2 were from 2017 [33,34], 2 were from 2018 [35,36], 3 were from 2019 [37,38,39], 3 were from 2020 [40,41,42], 2 were from 2021 [43,44], and 0 were from 2022. All studies involved older adults mostly above 50 years except one study where participants with MCI were above 42 years. The interventions were heavily loaded with mHealth and eHealth (13/22, 59%), while 6/22 (27%) were VR, and 3 were a combination of telephone, mHealth + VR, and a game console. About 73% (16/22) of the studies were RCTs, 2 were either quasi-experimental or pre-post (using a control), and one each for observational and mixed-methods. Of the 16 RCTs, only 5 provided effect sizes (ES). The weighted average ES was 1.48. Studies originated in 13 different countries, but half were from Korea, the United States, and Italy.

Table 1.
PICOS.
3.3. Risk of Bias in and across Studies
Reviewers exercised the JHNEBP quality assessment tool to identify strength and quality of evidence. Reviewers also made notes of other observations of bias throughout the data extraction. The JHNEBP tool identified 16/22 (73%) of Strength I due to the use of strong methodologies such as RCT and true experiment. Four others (18%) were identified as Strength II due to either quasi-experimental or a pre-post with a control group. Only 2/22 (9%) were identified as Strength III because of the use of observational or mixed methods methodologies. The JHNEBP tool also identified 16/22 (73%) as Quality A due to the use of adequate control groups and sample sizes, and for reporting consistent results. Only 6/22 (27%) were identified as Quality B. No studies were identified as less than Strength III or Quality B.
Reviewers also identified other incidents of bias. [18] There were 22 observations of selection bias, which threatens the internal validity of the studies. These observations stemmed from limiting the population to one region or one country. Reviewers also noted four observations of sample bias, which threatens the external validity of the studies. These observations were noted where the population was a majority of one race or gender. There were two observations of design bias, which threatens the internal validity of the study. These were noted when there seemed to be a significant flaw in the methodology (e.g., short intervention time).
3.4. Results of Individual Studies
Table 2 summarized the results of individual studies. This table shows the themes identified in the literature. In multiple occasions, there were multiple observations of the same theme identified in the same study. This was an artifact of collapsing observations of a similar nature into one theme. An observation-to-theme match can be found in Appendix A. Other observations incident to the data extraction can be found in Appendix B (sample size, bias, effect size, country of origin, statistics used, patient satisfaction, and the JHNEBP strength and quality of evidence).

Table 2.
Summary of analysis, sorted chronologically.
3.5. Results of Syntheses, Additional Analysis, and Certainty of Evidence
We conducted a thematic analysis of the literature to make sense of the data extracted. Through this process, observations noted multiple times became themes. Not all observations were fit into themes: Some remained as individual observations. These themes and observations are reported by category in affinity matrices with frequency distributions. Frequencies do not imply importance—instead they identify the probability the theme was identified in the group of articles analyzed.
3.5.1. Patient Satisfaction
Observations of patient satisfaction can be found in Appendix C. This appendix tabulates the. Only two themes and two individual observations were made. Patients commented their appreciation and how they valued the technology inherent to the interventions. This theme appeared in 11/32 (34%) of the observations [23,26,28,29,30,31,32,33,34,35,36]. The interventions had a positive effect on the patient experience. This appeared in 10/30 (32%) of the observations [23,24,26,27,28,29,30,31,33,34]. The intervention improved cognitive function in one study [25], and the technology frustrated patients in another study [37].
3.5.2. Results to the Adoption of mHealth and VR for Memory Care for AD Patients
Table 3 summarizes the results incident to the intervention of mHealth and VR for memory care. Six themes and seven individual observations were identified by the reviewers for a total of 41 occurrences in the literature. Nine interventions improved cognition, as measured by the MMSE, ADAS-Cog, or WAIS tests [24,25,26,29,32,34,35,38,43]. Seven interventions improved memory [23,28,30,31,34,36,40]. Five interventions improved language [23,24,25,31,34]. Four interventions improved brain activity, as measured by EEG [33,38,40,42]. Four interventions improved attention [31,34,36,41], and three improved vitality [31,36,40]. One intervention improved cortical atrophy [23]. One intervention improved resistance training through a combination of resistance and cognitive training protocol [25]. One intervention improved both quality of life and mental health [36]. One intervention improved both cerebral blood flow and neuro plasticity [38]. One intervention improved depression [27]. Only three interventions showed either no improvements or no significant improvements [37,39,44].

Table 3.
Results to the adoption of mHealth and VR for memory care.
3.5.3. Medical Outcome Commensurate with the Adoption of mHealth and VR for Memory Care
Table 4 summarizes the medical outcomes observed. Six themes and seven individual observations were recorded commensurate with the adoption of mHealth and VR for memory care for patients with AD, for a total of 41 occurrences. The results and medical outcomes are highly similar.

Table 4.
Medical outcomes commensurate with the adoption of mHealth and VR.
3.5.4. Effectiveness Themes and Observations
Table 5 summarizes the medical outcomes observed. Six themes and seven individual observations were recorded commensurate with the adoption of mHealth and VR for memory care for patients with AD, for a total of 41 occurrences. The medical outcomes and Effectiveness themes are highly similar. The only difference was that two interventions noted a time savings by using the intervention [34,35].

Table 5.
Effectiveness of mHealth and VR for memory care for patients with AD.
3.5.5. Barriers to the Adoption of mHealth and VR for Memory Care for Patients with AD
Table 6 summarizes the barriers to the adoption of mHealth and VR for memory care for patients with AD. Four themes and one individual observation was recorded commensurate with the adoption of the interventions, for a total of 88 occurrences. The most common barriers, which occurred together in many of the studies, was time of providers (to manage the intervention and administer tests) [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44], training (providers, staff, and patients) [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44], cost (of technology and tests) [23,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44], low reimbursement (which is highly correlated with cost) [23,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44], and dexterity limitations of older adults [33].

Table 6.
Barriers to the adoption of mHealth and VR for memory care.
3.5.6. Interactions between Observations
About 60% of the interventions were mHealth, eHealth. This intervention was associated with improvements in cognition [25,29,32,34,35], memory [23,30,31,34,36], language [23,25,31,34], attention [31,34,36,41], brain activity [33], cortical atrophy [23], resistance training [25], and depression [27]. Only one study that used this intervention reported no improvement [37]. The VR interventions reported improved cognition [26,43], brain activity [40,42], memory [40], and vitality [40]. Two VR studies reported either no improvement or no statistically significant improvements [39,44]. The mHealth + VR intervention reported improved memory [28]. The game console intervention reported improved cognition, brain activity, cerebral blood flow, and neuro plasticity [38]. The telephone intervention reported an increase in cognition and language [24].
4. Discussion
4.1. Summary of Evidence
This systematic literature review analyzed 22 studies from 13 countries published over 10 years to analyze the effectiveness of mHealth and VR for memory care for patients with AD. Five interventions were identified; however, the dominant intervention was mHealth, eHealth. The lines between mHealth and eHealth are significantly blurred due to the capabilities of mobile devices. This intervention comprised 13/22 (59%) of the studies. Virtual Reality was the most often cited intervention, appearing in 6/22 (27%) studies. Methodologies were very strong in the studies analyzed. About 73% of the studies used RCT as the study design [23,25,26,28,29,30,31,34,35,36,37,38,39,41,42,43]. The strong study designs resulted in a low rate of bias within and among studies because the studies used adequate sample sizes and controls, and they reported consistent results. Very small observations of internal and external bias were observed in all studies. There were 9 instances of an improvement of cognition [24,25,26,29,32,34,35,38,43], 7 instances of an improvement in memory [23,28,30,31,34,36,40], 5 instances of an improvement in language [23,24,25,31,34], four improvements in EEG scores [33,38,40,42], four improvements in attention [31,34,36,41] three improvements in vitality [31,36,40], and several individual improvements in cortical atrophy, resistance training, quality of life, mental health, cerebral blood flow, depression, and neuro plasticity [25,27,36,38].
This review highlights are large diversity of results from these five interventions. The mHealth and eHealth interventions consistently showed the largest improvements in cognition [25,29,32,34,35], memory [23,30,31,34,36], language [23,25,31,34], attention [31,34,36,41], brain activity [33], cortical atrophy [23], resistance training [25], and depression [27]. The game console intervention reported improvements in several areas: cognition, brain activity, cerebral blood flow, and neuro plasticity [38]. The VR interventions did not report as many improvements: cognition [26,43], brain activity [40,42], memory [40], and vitality [40]. The telephone intervention reported improvements in two areas: cognition and language [24]. The mHealth + VR intervention only improved memory [28].
Future research should focus on the improvements in cognition, memory, and brain waves to identify the duration of the improvements. The studies analyzed did not imply the results would be long term. Both mHealth and VR offer some good interventions to provide temporal relief and improvement of AD symptoms. Only three studies identified no improvement or no statically significant improvement [37,39,44]. The rest identified improvements in at least one area. Future considerations should focus on the interventions with the largest reported improvements. In this review, those would be mHealth, eHealth.
The results of this review should provide options for providers and care givers who want to see an improvement in one area or another. The results of these studies are positive. However, providers do face several barriers to the adoption of these interventions. The cost to acquire the equipment would not currently be reimbursed with current treatment codes. It would help to codify some of these interventions into critical practice guidelines. An existing CPG would have a better chance of being reimbursed. After acquiring the equipment, the provider would need to train the staff and the users of the equipment for each intervention. The provider and staff would need additional time to operate the equipment, administer and analyze the measurement tests like the MMSE, and EEG. These barriers are not compelling, but they present significant stumbling blocks to universal adoption.
4.2. Limitations
To control for sample bias, we queried four well-known databases, and we used every article that emerged from the abstract screening step. We chose only four databases, but others may have identified additional studies with additional interventions. We also limited the search to published articles that had been peer reviewed. This publication bias may have prevented us from identifying other interventions with various margins of success. To control for confirmation bias, we had multiple reviewers participate in every step: screening, data extraction, and analysis. To control for design bias, we stuck with a published protocol aligned with more than 40 published systematic literature reviews.
4.3. Conclusions
mHealth and VR offer promising interventions to help memory and cognition for those who suffer from AD. Several interventions show temporary improvement in cognition, memory, and brain activity. The mHealth and eHealth interventions seem to affect a larger scope of measurable criteria, and they may be easier to implement without complicated VR apparatus. Several barriers stand in the way of universal adoption. Additional reimbursement mechanisms would enable providers to adopt these interventions or test them under different circumstances. The AD patients and their caregivers look for answers and an improvement in the AD symptoms. With additional development, mHealth and VR might provide some viable solutions.
Author Contributions
Conceptualization, methodology, and editor C.S.K.; All authors participated in abstract screening, data extraction, and interpretation of results; writing C.S.K. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Protocol and Registration
This review was conducted in accordance with the Kruse Protocol for writing a systematic review. It was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). This review is registered with PROSPERO: CRD42021266730.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from this study can be obtained by asking the lead author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Observation-to-theme conversion (Intervention, Results, and Medical Outcomes).
Table A1.
Observation-to-theme conversion (Intervention, Results, and Medical Outcomes).
| Authors | Experimental Intervention | Intervention Themes | Results (Compared to Control Group) | Results Themes | Medical Outcomes Reported | Outcome Themes |
|---|---|---|---|---|---|---|
| Zhuang et al. | mHealth, eHealth cognitive training program | mHealth, eHealth | Intervention group with global cortical atrophy (GCA) showed improvement (p < 0.05). No change with baseline cognitive exam. | Improvement in cortical atrophy | Improvement in memory, language, and visuospatial abilities | Improvement in cortical atrophy |
| Improved memory | Improved memory | |||||
| Improved language | Improved language | |||||
| Jelcic et al. | Telephone-based | Telephone | The mean Mini Mental State Examination (MMSE) score improved significantly in Telecommunication technology (LSS-tele) and LSS-direct treatments | Improved MMSE scores (cognition) | Improvement in working memory and semantic fluency | Improved MMSE scores (cognition) |
| Improved language | Improved language | |||||
| Singh et al. | mHealth, eHealth multidomain cognitive training | mHealth, eHealth | Resistance training was 74% higher for Executive Domain compared with combined training, cognition, and verbal memory | Improved resistance training | Improvement in global cognition, executive function and verbal/constructional memory | Improved resistance training |
| Improved MMSE scores (cognition) | Improved MMSE scores (cognition) | |||||
| Improved language | Improved language | |||||
| Tarnanas et al. | Virtual Reality (VR), and Augmented Reality (AR) | Virtual Reality (VR) | improvements of specific cognitive functions and working memory | Improved MMSE scores (cognition) | improves untrained cognitive functions in MCI | Improved MMSE scores (cognition) |
| Burdea et al. | mHealth (BrightBrainer) app | mHealth, eHealth | statistically significant improvement in decision making, with trend improvements in depression. Non-statistically significant results found in processing speed and auditory attention. | Improved depression | Improvements in decision making and depression | Improved depression |
| Finn et al. | mHealth, VR, Telemedicine | mHealth + VR | (p < 0.05). Improved on the task itself over the course of training. | Improved memory | repetition-lag training (RLT), a form of recognition memory training reported | Improved memory |
| Callan et al. | mHealth cognitive training task (APVSAT) | mHealth, eHealth | Improved task performance, in terms of speed, by nearly 50% | Improved MMSE scores (cognition) | Reported as useful approach for incorporating device usage into daily routines. | Improved MMSE scores (cognition) |
| Cavallo et al. | structured rehabilitative software | mHealth, eHealth | (p < 0.05). Improvement in the intervention group greater than the control. | Improved memory | Improvement in memory | Improved memory |
| Hagovska et al. | Training battery prog- Cogni-Plus, SCHUHFRIED GmbH Austria, Dynamic balance training | mHealth, eHealth | (p < 0.05). improvement in postural reactions, attention, memory and language ability in the intervention group | Improved attention | improvement in postural reactions, attention, memory and language | Improved attention |
| Improved memory | Improved memory | |||||
| Improved language | Improved language | |||||
| Improved vitality | Improved vitality | |||||
| Hyer et al. | Cogmed or a Sham computer program. For Repeatable Battery for Neuropsychological Status and the Clinical Dementia Rating | mHealth, eHealth | The Cogmed group demonstrated better performance on the Functional Activities Questionnaire (FAQ), a measure of adjustment and far transfer, at follow-up. | Improved MMSE scores (cognition) | Both groups, especially Cogmed, enjoyed the intervention. Cognitive stimulation activities improved mental skills | Improved MMSE scores (cognition) |
| Boyd et al. | Trials to use Apps-evaluation of EnCare diagnostics (ECD) and the brain fit plan (BFP) in healthy older adults | mHealth, eHealth | No control group. Improved brain waves | Improved EEG scores (brain waves) | ECD is highly acceptable in both healthy older adults and those with early stage dementia when given the shorter versions to accommodate their diagnosis. | Improved EEG scores (brain waves) |
| Yang et al. | 24 sessions of computer-based cognitive training, over a 12 week period. | mHealth, eHealth | Computer-based cognitive treatment resulting in self-training and self-learning of a patient | Improved MMSE scores (cognition) | Improvement in language, attention, calculation, verbal memory, and frontal function for the experimental group | Improved MMSE scores (cognition) |
| Improved memory | Improved memory | |||||
| Improved language | Improved language | |||||
| Lee et al. | 12 sessions of a computerized cognitive rehabilitation program for three weeks | mHealth, eHealth | “No control group”. Two treatment groups only | Improved MMSE scores (cognition) | Improvement in subjects who underwent computerized cognitive rehabilitation using Bettercog. | Improved MMSE scores (cognition) |
| Park et al. | NCT group showed improvement in vitality, role-emotional, and mental health compared with the CCT group | mHealth, eHealth | Cognitive function (attention, memory, and visual spatial ability) showed a significant increase in both groups (p < 0.05), as did the mental components of health-related quality of life (p < 0.05) | Improved attention | Regarding health-related quality of life, the NCT group showed more improvement in vitality, role-emotional, and mental health compared with the CCT group | Improved attention |
| Improved memory | Improved memory | |||||
| Improved vitality | Improved vitality | |||||
| Improved mental health | Improved mental health | |||||
| Improved quality of life | Improved quality of life | |||||
| Flak et al. | mHealth memory training app | mHealth, eHealth | Adaptive training group did not show significantly greater improvement on the main outcome of working memory performance at 1 and 4 months after training | No improvement | no improvement | None reported |
| Kahn | Game console with cognitive games | Game console | Theta, delta waves and complexity of EEG significantly improved | Improved EEG scores (brain waves) | Xbox 360 Kinect cognitive games improved EEG indicators and cognitive functions probably through multiple mechanisms, such as, cognition improvement, 15–17 increasing cerebral blood flow, 59 neural plasticity, 60 activation of arousal system, 61 neurotransmitters modulation | Improved EEG scores (brain waves) |
| Improved MMSE scores (cognition) | Improved MMSE scores (cognition) | |||||
| Improved cerebral blood flow | Improved cerebral blood flow | |||||
| Improved neuro plasticity | Improved neuro plasticity | |||||
| Park | culture based virtual reality | Virtual Reality (VR) | VR-based training group exhibited no significant differences following the three-month VR program | No significant differences | no significant improvements noted | None reported |
| Park et al. | VR | Virtual Reality (VR) | No control group. improvement in physical, memory and brain stimulation, but the participants have a low focus on decision making | Improved vitality | Improvement in physical outcomes, memory and brain stimulation | Improved vitality |
| Improved memory | Improved memory | |||||
| Improved EEG scores (brain waves) | Improved EEG scores (brain waves) | |||||
| Robert et al. | mHealth app (MeMo) | mHealth, eHealth | Significant differences in two attention tests | Improved attention | Improvement in attention tests | Improved attention |
| Thapa et al. | VR | Virtual Reality (VR) | Intervention group exhibited a significantly improved executive function and brain function at the resting state | Improved EEG scores (brain waves) | Intervention group exhibited a significantly improved executive function and brain function at the resting state | Improved EEG scores (brain waves) |
| Oliveria et al. | VR | Virtual Reality (VR) | an improvement in overall cognitive function in the experimental group | Improved MMSE scores (cognition) | an improvement in overall cognitive function in the experimental group | Improved MMSE scores (cognition) |
| Seredakis et al. | VR | Virtual Reality (VR) | No group interaction | No improvement | No group interaction | None reported |
Appendix B

Table A2.
Observation-to-theme conversion (Effectiveness and Barriers to adoption).
Table A2.
Observation-to-theme conversion (Effectiveness and Barriers to adoption).
| Authors | Effectiveness | Effectiveness Themes | Barriers to Adoption | Barrier Themes |
|---|---|---|---|---|
| Zhuang et al. | pts value technology, improvement in memory, language, and visuospatial abilities | Improvement in cortical atrophy | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Improved memory | Training | |||
| Improved language | Low reimbursement | |||
| Time of providers | ||||
| Jelcic et al. | Improvement in memory, phonemic fluency, semantic fluency, stabilizing delayed/working memory | Improved MMSE scores (cognition) | Time of providers/staff on phone, training of staff, time to administer tests | Time of providers |
| Improved language | Training | |||
| Time of providers | ||||
| Singh et al. | trials of isolated moderate-high intensity resistance training had significant effects on memory, cognition, and language | Improved resistance training | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Improved MMSE scores (cognition) | Training | |||
| Improved language | Low reimbursement | |||
| Time of providers | ||||
| Tarnanas et al. | improves untrained cognitive functions in MCI | Improved MMSE scores (cognition) | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Burdea et al. | Improvements in decision making and depression | Improved depression | Cost to acquire equipment, staff training, low reimbursement | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Finn et al. | repetition-lag training (RLT), a form of recognition memory training reported | Improved memory | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Callan et al. | Improved task performance, in terms of speed, by nearly 50% | Improved MMSE scores (cognition) | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Cavallo et al. | Improvement in memory | Improved memory | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Hagovska et al. | improvement in postural reactions, attention, memory and language | Improved attention | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Improved memory | Training | |||
| Improved attention | Low reimbursement | |||
| Improved language | Time of providers | |||
| Hyer et al. | improvement in mental sharpness | Improved MMSE scores (cognition) | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Boyd et al. | Improved brain waves | Improved EEG scores (brain waves) | dexterity limitations, use of touch screen and accidental screen presses, cost to acquire equipment, staff training, low reimbursement, time to administer benchmark tests | Dexterity limitations of older adults |
| Cost | ||||
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Yang et al. | Improvement in language, attention, calculation, verbal memory, and frontal function for the experimental group, convenience, savings in time | Improved MMSE scores (cognition) | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Improved memory | Training | |||
| Improved language | Low reimbursement | |||
| Savings in time | Time of providers | |||
| Lee et al. | convenience, savings in time, improved cognition | Savings in time | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Improved MMSE scores (cognition) | Training | |||
| Low reimbursement | ||||
| Time of providers | ||||
| Park et al. | Regarding health-related quality of life, the NCT group showed more improvement in vitality, role-emotional, and mental health compared with the CCT group | Improved attention | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Improved memory | Training | |||
| Improved vitality | Low reimbursement | |||
| Improved mental health | Time of providers | |||
| Improved quality of life | ||||
| Flak et al. | none | None reported | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Kahn | Increase in brain waves, increase in cognition, incresae in cerebral blood flow, improved neuro plasticity | Improved EEG scores (brain waves) | Cost to acquire equipment, staff training, low reimbursement, time to administer tests | Cost |
| Improved MMSE scores (cognition) | Training | |||
| Improved cerebral blood flow | Low reimbursement | |||
| Improved neuro plasticity | Time of providers | |||
| Park | none | None reported | Cost to acquire equipment, staff training, low reimbursement | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Park et al. | Improvement in physical outcomes, memory and brain stimulation | Improved vitality | Cost to acquire equipment, staff training, low reimbursement | Cost |
| Improved memory | Training | |||
| Improved EEG scores (brain waves) | Low reimbursement | |||
| Time of providers | ||||
| Robert et al. | significant differences in two attention tests | Improved attention | Cost to acquire equipment, staff training, low reimbursement | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Thapa et al. | Intervention group exhibited a significantly improved executive function and brain function at the resting state | Improved EEG scores (brain waves) | Cost to acquire equipment, staff training, low reimbursement | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Oliveria et al. | an improvement in overall cognitive function in the experimental group | Improved MMSE scores (cognition) | Cost to acquire equipment, staff training, low reimbursement | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers | ||||
| Seredakis et al. | No group interaction | None reported | Cost to acquire equipment, staff training, low reimbursement | Cost |
| Training | ||||
| Low reimbursement | ||||
| Time of providers |
Appendix C

Table A3.
Other observations incident to review.
Table A3.
Other observations incident to review.
| Authors | Sample Size (#s only) | Bias within Study Selection Bias, Sample Bias, etc. | Effect Size | Country of Origin (Where the Study Was Conducted) | Statistics Used | Patient Satisfaction | Strength of Evidence | Quality of Evidence |
|---|---|---|---|---|---|---|---|---|
| Zhuang et al. | 33 | China only (selection bias), Mostly female (sample bias) | Not reported | China | Measures of central tendency, MANOVA, ANOVA, Wilk’s lambda | Positive effect on patient experience | I | A |
| Short intervention period (design bias) | Pts value technology | |||||||
| Jelcic et al. | 27 | Venice only (selection bias), Mostly female and Caucasian (sample bias) | not reported | Venice | Measures of central tendency, Kruskal–Wallis ANOVA, Mann–Whitney U-test | Positive effect on patient experience | II | B |
| Singh et al. | 100 | Australia and New Zealand only (selection bias) | small (0.2) | Australia and New Zealand | Measures of central tendency, Odds ratio | improved global cognitive function | I | A |
| Tarnanas et al. | 114 | Greece only (selection bias) | sensitivity 80.4%, specificity 94.3%Large effect (3.91) | Greece | Measures of central tendency, ANOVA | Positive effect on patient experience, | I | A |
| pts value technology | ||||||||
| Burdea et al. | 10 | one country (selection bias), majority male (sample bias) | not reported | USA | paired t-test | high rates of satisfaction | II | B |
| Finn et al. | 31 | Sydney, Australia only (selection bias) | small (0.17) | Australia | Measures of central tendency, ANOVA, t-test | Positive effect on patient experience, | I | A |
| pts value technology | ||||||||
| Callan et al. | 27 | Pittsburg, USA only (selection bias) | not reported | USA | Measures of central tendency, paired t-test, Fisher’s exact test | Positive effect on patient experience, | I | B |
| pts value technology | ||||||||
| Cavallo et al. | 80 | Moncalieri, Italy (selection bias) | not reported | Italy | Measures of central tendency, repeated measures GLM, t-tests | Positive effect on patient experience, | I | A |
| pts value technology | ||||||||
| Hagovska et al. | 80 | Kosice, Slovak Republic only (selection bias) | medium (0.64) | Slovakia | Measures of central tendency, ANOVA, t-tests, Shapiro–Wilk test, D’Agostino-Pearson test | Positive effect on patient experience, | I | A |
| pts value technology | ||||||||
| Hyer et al. | 68 | US only (selection bias) | medium | USA | Measures of central tendency, ANOVA | pts value technology | II | A |
| Boyd et al. | 19 | Northern Ireland only (selection bias) | not reported | Ireland | Measures of central tendency, t-tests | Positive effect on patient experience, | III | B |
| pts value technology | ||||||||
| Yang et al. | 20 | Namyangju, south Korea only (selection bias) | not reported | Korea | Measures of central tendency, Mann–Whitney U-test, t-tests | Positive effect on motivation and mood | I | B |
| pts value technology | ||||||||
| Lee et al. | 20 | Chungbuk National University Hospital, Korea only (selection bias) | not reported | Korea | Measures of central tendency, independent t-test, Mann–Whitney U-test | not reported | I | B |
| limited number of treatment sessions (design bias) | pts value technology | |||||||
| Park et al. | 78 | one country (selection bias) | not reported | Korea | Measures of central tendency | not reported | I | A |
| pts value technology | ||||||||
| Flak et al. | 68 | Norway only (selection bias), majority male (sample bias) | Not reported | Norway | Linear mixed models | patients experienced frustration | I | A |
| Kahn | 38 | Pakistan only (selection bias) | not reported | Pakistan | ANOVA with Scheffe post hoc analysis, paired t-test | not reported | I | A |
| Park | 21 | Korea only (selection bias) | not reported | Korea | ANOVA with Shapiro–Wilks test, student’s t-test | not reported | I | A |
| Park et al. | 45 | One country (selection bias) | not reported | Korea | GLM | not reported | III | A |
| Robert et al. | 46 | One country (selection bias) | not reported | France | Student t-test, Wilcoxon-Mann–Whitney, Chi-square, Fisher’s exact, and Wilcoxon | not reported | I | A |
| Thapa et al. | 66 | One country (selection bias) | not reported | Korea | ANOVA, Shapiro–Wilk | not reported | I | A |
| Oliveria et al. | 34 | One country (selection bias) | large | Portugal | ANOVA with Bonferroni correction | not reported | I | A |
| Seredakis et al. | 43 | One country (selection bias) | medium | Australia | Chi-square, Shapiro–Wilk, Wilcoxon signed rank test, Mann–Whitney U test | not reported | II | A |
References
- Alzheimer’s Association. 2022 Alzheimer’s Disease Facts and Figures; Alzheimer’s Association: Chicago, IL, USA, 2022; pp. 131–168. [Google Scholar]
- Patterson, C. World Alzheimer Report 2018; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- Marcello, E.; Gardoni, F.; Di Luca, M. Alzheimer’s disease and modern lifestyle: What is the role of stress? J. Neurochem. 2015, 134, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; He, M.; Xiong, M.; Zhang, X.; Nie, S.; Xiong, J.; Hu, D.; Zhang, Z.; Mao, L.; Zhang, Z. 2′,3′-Dideoxycytidine, a DNA polymerase-β inhibitor, reverses memory deficits in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 67, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Harding, J.W. The brain renin–angiotensin system: A diversity of functions and implications for CNS diseases. Pflüg. Arch.-Eur. J. Physiol. 2013, 465, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I. Cognition: An Introduction; Scott, Foresman & Co.: Dallas, TX, USA, 1973. [Google Scholar]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- World Health Organization. Telemedicine: Opportunities and Developments in Member States. Report on the Second Global Survey on eHealth; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Garnett, A.; Northwood, M.; Ting, J.; Sangrar, R. mHealth Interventions to Support Caregivers of Older Adults: Equity-Focused Systematic Review. JMIR Aging 2022, 5, e33085. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Brown, L.J.; Antrobus, S.; Brough, D.; Drake, R.J.; Jury, F.; Leroi, I.; Parry-Jones, A.R.; Machin, M. Co-design of a Smartphone App for People Living with Dementia by Applying Agile, Iterative Co-design Principles: Development and Usability Study. JMIR Mhealth Uhealth 2022, 10, e24483. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, N.; Lithgow, B.; Jafari Jozani, M.; Moussavi, Z. The Effect of Transcranial Alternating Current Stimulation with Cognitive Training on Executive Brain Function in Individuals with Dementia: Protocol for a Crossover Randomized Controlled Trial. JMIR Res. Protoc. 2022, 11, e37282. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Betances, R.I.; Jiménez-Mixco, V.; Arredondo, M.T.; Cabrera-Umpiérrez, M.F. Using virtual reality for cognitive training of the elderly. Am. J. Alzheimer’s Dis. Other Dement. 2015, 30, 49–54. [Google Scholar] [CrossRef]
- Bateman, D.R.; Srinivas, B.; Emmett, T.W.; Schleyer, T.K.; Holden, R.J.; Hendrie, H.C.; Callahan, C.M. Categorizing health outcomes and efficacy of mHealth apps for persons with cognitive impairment: A systematic review. J. Med. Internet Res. 2017, 19, e7814. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.T.; Mowszowski, L.; Naismith, S.L.; Chadwick, V.L.; Valenzuela, M.; Lampit, A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: A systematic review and meta-analysis. Am. J. Psychiatry 2017, 174, 329–340. [Google Scholar] [CrossRef]
- Abd-alrazaq, A.; Alhuwail, D.; Ahmed, A.; Househ, M. Effectiveness of Serious Games for Improving Executive Functions Among Older Adults with Cognitive Impairment: Systematic Review and Meta-analysis. JMIR Serious Games 2022, 10, e36123. [Google Scholar] [CrossRef]
- Kruse, C.S. Writing a Systematic Review for Publication in a Health-Related Degree Program. JMIR Res. Protoc. 2019, 8, e15490. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, R.; Dearholt, S.; Poe, S.; Pugh, L.; White, K. The Johns Hopkins Nursing Evidence-Based Practice Rating Scale; The Johns Hopkins Hospital: Baltimore, MD, USA, 2005. [Google Scholar]
- Pannucci, C.J.; Wilkins, E.G. Identifying and avoiding bias in research. Plast. Reconstr. Surg. 2010, 126, 619. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Kruse, C.; Betancourt, J.; Ortiz, S.; Luna, S.M.V.; Bamrah, I.K.; Segovia, N. Barriers to the use of mobile health in improving health outcomes in developing countries: Systematic review. J. Med. Internet Res. 2019, 21, e13263. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.; Heinemann, K. Facilitators and Barriers to the Adoption of Telemedicine during the First Year of COVID-19: Systematic Review. J. Med. Internet Res. 2022, 24, e31752. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.S.; Lee, K.; Watson, J.B.; Lobo, L.G.; Stoppelmoor, A.G.; Oyibo, S.E. Measures of effectiveness, efficiency, and quality of telemedicine in the management of alcohol abuse, addiction, and rehabilitation: Systematic review. J. Med. Internet Res. 2020, 22, e13252. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.-P.; Fang, R.; Feng, X.; Xu, X.-H.; Liu, L.-H.; Bai, Q.-K.; Tang, H.-D.; Zhao, Z.-G.; Chen, S.-D. The impact of human-computer interaction-based comprehensive training on the cognitive functions of cognitive impairment elderly individuals in a nursing home. J. Alzheimer’s Dis. 2013, 36, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Jelcic, N.; Agostini, M.; Meneghello, F.; Bussè, C.; Parise, S.; Galano, A.; Tonin, P.; Dam, M.; Cagnin, A. Feasibility and efficacy of cognitive telerehabilitation in early Alzheimer’s disease: A pilot study. Clin. Interv. Aging 2014, 9, 1605. [Google Scholar]
- Singh, M.A.F.; Gates, N.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C. The Study of Mental and Resistance Training (SMART) study—resistance training and/or cognitive training in mild cognitive impairment: A randomized, double-blind, double-sham controlled trial. J. Am. Med. Dir. Assoc. 2014, 15, 873–880. [Google Scholar] [CrossRef]
- Tarnanas, I.; Tsolakis, A.; Tsolaki, M. Assessing virtual reality environments as cognitive stimulation method for patients with MCI. In Technologies of Inclusive Well-Bein; Springer: New York, NY, USA, 2014; pp. 39–74. [Google Scholar]
- Burdea, G.; Polistico, K.; Krishnamoorthy, A.; House, G.; Rethage, D.; Hundal, J.; Damiani, F.; Pollack, S. Feasibility study of the BrightBrainer™ integrative cognitive rehabilitation system for elderly with dementia. Disabil. Rehabil. Assist. Technol. 2015, 10, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Finn, M.; McDonald, S. Repetition-lag training to improve recollection memory in older people with amnestic mild cognitive impairment. A randomized controlled trial. Aging Neuropsychol. Cogn. 2015, 22, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Callan, J.A.; Siegle, G.J.; Abebe, K.; Black, B.; Martire, L.; Schulz, R.; Reynolds, C., III; Hall, M.H. Feasibility of a pocket-PC based cognitive control intervention in dementia spousal caregivers. Aging Ment. Health 2016, 20, 575–582. [Google Scholar] [CrossRef]
- Cavallo, M.; Hunter, E.M.; van der Hiele, K.; Angilletta, C. Computerized structured cognitive training in patients affected by early-stage Alzheimer’s disease is feasible and effective: A randomized controlled study. Arch. Clin. Neuropsychol. 2016, 31, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Hagovska, M.; Takáč, P.; Dzvonik, O. Effect of a combining cognitive and balanced training on the cognitive, postural and functional status of seniors with a mild cognitive deficit in a randomized, controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 101–109. [Google Scholar]
- Hyer, L.; Scott, C.; Atkinson, M.M.; Mullen, C.M.; Lee, A.; Johnson, A.; Mckenzie, L.C. Cognitive training program to improve working memory in older adults with MCI. Clin. Gerontol. 2016, 39, 410–427. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Synnott, J.; Nugent, C.; Elliott, D.; Kelly, J. Community-based trials of mobile solutions for the detection and management of cognitive decline. Healthc. Technol. Lett. 2017, 4, 93–96. [Google Scholar] [CrossRef]
- Yang, Y.; Kwak, Y.T. Improvement of cognitive function after computer-based cognitive training in early stage of Alzheimer’s dementia. Dement. Neurocogn. Disord. 2017, 16, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Bang, H.J.; Lee, K.M.; Kong, H.H.; Seo, H.S.; Oh, M.; Bang, M. A comparison of the effects between 2 computerized cognitive training programs, Bettercog and COMCOG, on elderly patients with MCI and mild dementia: A single-blind randomized controlled study. Medicine 2018, 97, e13007. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, J.-H. Does cognition-specific computer training have better clinical outcomes than non-specific computer training? A single-blind, randomized controlled trial. Clin. Rehabil. 2018, 32, 213–222. [Google Scholar] [CrossRef]
- Flak, M.M.; Hol, H.R.; Hernes, S.S.; Chang, L.; Engvig, A.; Bjuland, K.J.; Pripp, A.; Madsen, B.-O.; Knapskog, A.-B.; Ulstein, I. Adaptive computerized working memory training in patients with mild cognitive impairment. A randomized double-blind active controlled trial. Front. Psychol. 2019, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Khan, N. Xbox 360 Kinect cognitive games improve slowness, complexity of EEG, and cognitive functions in subjects with mild cognitive impairment: A randomized control trial. Games Health J. 2019, 8, 144–152. [Google Scholar]
- Park, S.-J. Brain Stimulation of Elderly with Dementia Using Virtual Reality Home. J. Inf. Technol. Appl. Manag. 2019, 26, 1–18. [Google Scholar]
- Park, J.-H.; Liao, Y.; Kim, D.-R.; Song, S.; Lim, J.H.; Park, H.; Lee, Y.; Park, K.W. Feasibility and tolerability of a culture-based virtual reality (VR) training program in patients with mild cognitive impairment: A randomized controlled pilot study. Int. J. Environ. Res. Public Health 2020, 17, 3030. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Manera, V.; Derreumaux, A.; Montesino, M.F.Y.; Leone, E.; Fabre, R.; Bourgeois, J. Efficacy of a web app for cognitive training (MeMo) regarding cognitive and behavioral performance in people with neurocognitive disorders: Randomized controlled trial. J. Med. Internet Res. 2020, 22, e17167. [Google Scholar] [CrossRef] [PubMed]
- Thapa, N.; Park, H.J.; Yang, J.-G.; Son, H.; Jang, M.; Lee, J.; Kang, S.W.; Park, K.W.; Park, H. The effect of a virtual reality-based intervention program on cognition in older adults with mild cognitive impairment: A randomized control trial. J. Clin. Med. 2020, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Gamito, P.; Souto, T.; Conde, R.; Ferreira, M.; Corotnean, T.; Fernandes, A.; Silva, H.; Neto, T. Virtual Reality-Based Cognitive Stimulation on People with Mild to Moderate Dementia due to Alzheimer’s Disease: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 5290. [Google Scholar] [CrossRef] [PubMed]
- Saredakis, D.; Keage, H.A.; Corlis, M.; Ghezzi, E.S.; Loffler, H.; Loetscher, T. The effect of reminiscence therapy using virtual reality on apathy in residential aged care: Multisite nonrandomized controlled trial. J. Med. Internet Res. 2021, 23, e29210. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).