Abstract
Background: balloon cell melanoma represents less than 1% of all histological forms of malignant melanoma and represents a diagnostic challenge for the dermatopathologist. Methods: in this paper we present our cases of BCM found in our daily practice from 1 January 2008 to 31 December 2021, and we conduct a review of the literature relating to this entity in the period from the first description, 1970, to early 2022. Results: four cases of melanoma balloon cell have been extrapolated from our electronic database, while in the review of the literature we have identified 115 cases of patients with primary and/or metastatic BCM. Conclusions: we believe that future studies with numerous case series are essential not only to increase the knowledge of the pathophysiology of this neoplasm but also to correctly evaluate the response of BCM patients to new oncological therapies.
1. Introduction
Malignant melanoma poses an ongoing challenge for health systems across the globe, and incidence and prevalence rates continue to rise, making prevention a crucial issue []. It is known that the histological diagnosis has a fundamental significance in the correct nosographic classification, which supports decision making and planning of the different therapies []. However, the diagnosis is not always easy, and every day the dermatopathologist has to deal with complex pictures that require integration with immunohistochemical and molecular data. Furthermore, this neoplasm can arise at the level of other parts of the body, such as mucous sites including the oral cavity [], the vagina [] or intestine []. In this context, balloon cell melanoma (BCM), is a fairly rare, bizarre entity that can sometimes manifest not only in a context of melanoma metastases but also as a primary lesion []. Over time, different explanations have been proposed to justify the morphological changes, but ultimately, the best accepted view (also thanks to electron microscopy studies and acquisitions) is that an overproduction of swollen and defective melanosomes is at the origin of this morphotype []. In this paper, we present four cases of balloon cell melanoma, discuss their main differential diagnoses and perform an extensive review of the current literature in order to trace the state of the art and future prospects.
2. Materials and Methods
To carry out this work, the historical archive of our laboratory was consulted from 1 January 2008 to 31 December 2021, applying the term “Balloon Cell” for the search, so that only cases of malignant melanoma were extrapolated. Sections staining with Hematoxylin/Eosin (EE) and blocks were retrieved and re-analyzed by two pathologists with expertise in skin pathology (G.C. and A.C.). In the event that there was no agreement, a third dermatopathologist (C.A.) was included in the discussion. Clinical information was retrieved from fellow dermatologists and plastic surgeons, and, when not available, the patient or family members were contacted directly. In addition, a systematic review was elaborated following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A databases search of PubMed, Web of Science (WoS) and Scopus was performed for the period 1970–2021 using the following terms: balloon cell melanoma and melanoma with balloon cell in combination with each of the following: dermatopathology, skin. Only articles in English were selected. The last search was run on 31 December 2021. Eligible articles were assessed according to the Oxford Centre for Evidence-Based Medicine 2011 guidelines []. Review articles, meta-analyses, observational studies, case reports, survey snapshot studies, letters to the editor and comments to the letters were all included. Other potentially relevant articles were identified by manually checking the references of the included literature. An independent extraction of articles was performed by two investigators according to the inclusion criteria. Disagreement was resolved by discussion between the two review authors.
3. Results
Four cases of melanoma balloon cell have been extrapolated from our electronic database, the clinical-pathological characteristics of which are reported in Table 1.

Table 1.
Clinical features of patients with balloon cell melanoma.
Records of two male (50.0%) and two female patients (50.0%) were retrieved, with balloon cell melanoma localizations in four different body districts. In three of the four cases (75.0%) the clinical suspicion was malignant melanoma. Microscopically, all the lesions had the same characteristics, consisting of more than 50% of “balloon-shaped” melanocytes. (Figure 1A–C). These cells featured an abundant and finely vacuolized cytoplasm and hyperchromatic nuclei, generally located in the periphery of the cell, but not pycnotic (Figure 1D). Very rare mitoses were observed, and melanin was quantitatively reduced within the cell, with a “disordered” dispersion within the lesion itself and in the numerous melanophages (Figure 1D). Architecturally, in all four cases, the cells were organized in large pale masses that replaced the dermis and seemed to thin the epidermis (Figure 1B). These large solid sheets of “ballooniform” melanocytes were divided into irregular aggregates by thin collagenous septa. There were no clear signs of activity at the dermo-epidermal junction and/or pagetoid spreading. In the second case (Figure 1B), a component of “spindle cell” melanoma could be observed.
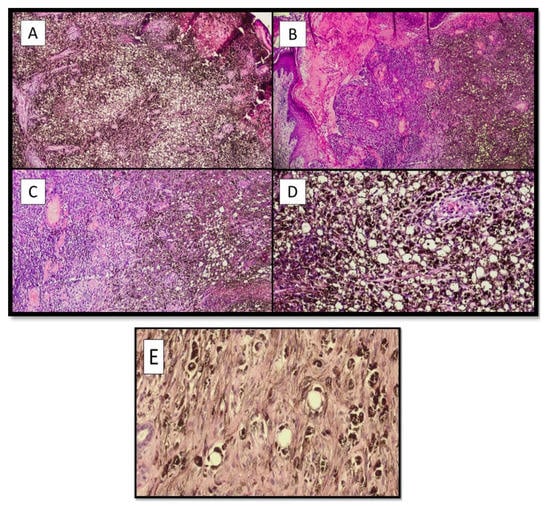
Figure 1.
(A) Photomicrograph of the first case (F, 76 years old), showing the pseudolipoblastic/balloon cell aspects of the melanocytes, characterized by a swollen histological appearance and the disintegration of disordered and abundant melanic pigment (Hematoxylin-Eosin, Original Magnification 4×). (B) Histological micrograph of patient number two (M, 75), showing two different neoplastic parts: on the right, the more properly “balloon cell” part, while in the center and on the left, there is a part with spindle cells of malignant melanoma. Additionally, in this case, there was an abundant and irregular presence of melanic pigment (Hematoxylin-Eosin, Original Magnification 4×). (C) Histological preparation of sections from the third patient (F, 36 years old) showed very similar morphological characteristics to those in case number two (Hematoxylin-Eosin, Original Magnification: 10×). (D) Balloon cell melanoma photomicrograph of the lesion in patient number four (M, 51 years old). Note the balloon-shaped appearance of melanocytes with histological characteristics that sometimes resemble pseudolipoblasts (Hematoxylin-Eosin, Original Magnification: 20×). (E) Histological micrograph showing the cytological detail of melanocytes with balloon cell characteristics of the cytoplasm (Hematoxylin-Eosin, Original Magnification: 40×).
Immunohistochemically, all four cases expressed S-100 protein and Melan-A (Figure 2A,B), as well as positivity for HMB-45 and SOX-10.
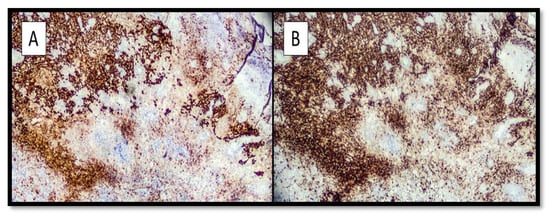
Figure 2.
(A) Immunohistochemical preparation with S-100 protein antibody: note the intense positivity of the marker at the level of the melanoma component with spindle cells and a more tenuous positivity at the level of the balloon cell component. (Immunohistochemistry, Original magnification: 10×). (B) Photomicrograph showing immunostaining with anti-Melan-A antibody: note that the positivity of staining is almost entirely comparable to the previous one. (Immunohistochemistry anti-Melan-A, Original Magnification: 10×).
In the review of the literature, a total of 137 records was initially identified, of which 33 were duplicates. After screening for eligibility and inclusion criteria, 70 publications were ultimately included (Figure 3). The authors and clinical/pathological characteristics are summarized in Table 2. Most of the publications were case reports (n = 51), followed by reviews (n = 10), case series (n = 6) and editorials (n = 3). All studies included were rated as evidence level 4 or 5 for clinical research, as detailed in the Oxford Centre for Evidence-Based Medicine 2011 guidelines []. In total, 115 patients with primitive or metastatic balloon cell melanoma were described.
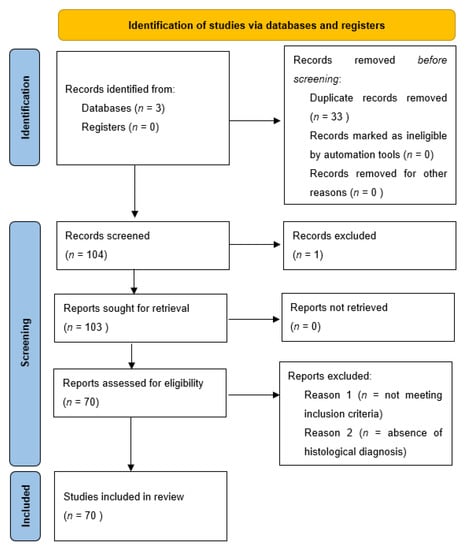
Figure 3.
PRISMA 2020 Flow chart utilized for this systematic review related to balloon cell melanoma.

Table 2.
Summary table of all cases searched in the literature and reported in this review.
Of these 115 patients, 36 (31.3%) had a primary lesion starting in the back (1 case starting in the left shoulder blade); 20 (17.4%) a lesion starting in the extremities (17 cases in the upper limbs and 3 cases in the lower limbs); 11 patients (9.6%) had a primary head/neck lesion; 9 patients (7.8%) had primary BCM of the choroid or ciliary body, while 2 patients (1.7%) had BCM originating in the conjunctiva. Metasases were present in 15 patients (13.0%) at the time of observation, while in 9 cases (7.8%), the site of the first melanoma was unknown. Finally, there were two cases (1.7%) of primary lesions originating in the orbit (one of which was a uveal melanoma), two cases (1.7%) originating in the chest and cases (6.9%) starting in the anal canal and another case in the urethra. The mean age was 54 years, and the dimensions ranged from 0.3 to 5 cm in maximum diameter. In almost 90% of the cases the immunohistochemistry described positivity for S-100 protein and HMB-45, with 7% of the cases positive for Neuron-Specific Enolase (NSE) and 23.5% of the lesions expressed the carcinoembryonic antigen.
In the vast majority of cases, the clinical suspicion was that of an atypical pigmented lesion, suggestive of malignant melanoma. In a small number of cases, amelanotic lesions were appreciated.
4. Discussion
Malignant melanoma continues to represent a very frequent malignant neoplasm, rapidly increasing worldwide, and this increase is occurring at a faster rate than that of any other cancer except lung cancer in women [,]. Histopathological diagnosis is still the gold standard for programming subsequent steps in the therapeutic diagnostic path of the affected patient [], and a correct morphological and immunohistochemical recognition is the basis for improving the outcome of patients (in fact, the five-year relative survival rate for patients with stage 0 melanoma is 97%, compared with about 10% for those with stage IV disease) [,,,,]. Among the best known different histological patterns, there are unusual and bizarre forms of MM [] whose knowledge is important to reduce and avoid the risk of wrong diagnoses. In this view, BCM represents a very rare variant (<1% of all histological forms of melanoma), defined by at least the presence of 50% of melanocytes with balloniform histological appearance []. Over the years, there have been different reports of BCM since Gardner’s first report in 1970 [], which reported a case of BCM developed at the level of the back of an older patient. Since then, descriptions of this entity have multiplied [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]; there are up to about 115 patients described in the literature, according to our review conducted and presented in this paper. From the analysis of the studies included, the most represented primitive localizations turned out to be the back, the lower and upper extremities, the choroid and the district head/neck, with also two rare cases to depart from the conjunctiva. This heterogeneity of distribution with predominance of the back has been found also in our new four described cases (two cases to the back, one left leg case and one right flank case). As described in the literature, even in our presented cases, there were no distinctive clinical characteristics, being generally present the suspicion of MM. In this regard, in recent years, some authors [] have tried to look for suggestive and distinctive dermaoscopic criteria for the diagnosis of BCM. Resente F. et al., for example, have found that elements such as yellowish structureless areas, white lines, irregular hairpin-shaped and curved vessels can be suggestive of BCM. Regarding the prognosis, from the analyzed works it does not seem that there is a substantial difference compared to the conventional melanoma, always depending on the thickness of Breslow; therefore, the degree and depth of balloon cell changes do not affect the prognosis.
An aspect of great importance for the dermatopathologist is represented by the differential diagnostics with benign and/or malignant lesions to balloniform cells (such as the nevus, a balloon cell) or to other skin neoplasms to clear cells. The differential diagnosis between nevus and melanoma balloon cells can be very complex, as both of these entities may present very similarly [,,]: in this regard, it may be necessary to dissect the sample extensively in search of areas of possible conventional malignant melanoma that may orient the diagnosis in the right direction. On the contrary, in the case of rather mild melanocytes, without cytological features being atypical, we can think of the diagnosis of nevus as balloon cells. Consideration should also be given to the possibility of being faced with a Spitz a balloon cell nevus [,,] where the presence of certain histological details may help to orient oneself. In the case of large cells and epithelioids, with a ground glass cytoplasm and vesicular nucleus, in the absence of significant mitotic activity and with presence of epidermal hyperplasia, we can reasonably think of a Spitz balloon cell nevus, especially in the case of persons under 20 years of age [,]. BCM may also be confused with non-melanocytic entities, including a classic differential diagnosis of renal cell carcinoma, but also with lesions such as clear-cell sarcoma (malignant melanoma of soft parts), xanthoma, hibernoma and clear-cell carcinoma of the lung, ovary and endometrium. Regarding clear-cell melanoma, although some authors have proposed a distinction with BCM, we tend to avoid using this nosographic category, as it can be easily confused with clear-cell sarcoma []. We also remember entities such as clear-cell syringoma, granular cell tumor, malignant eccrine acrospiroma, sebaceous carcinoma, atypical fibroxanthoma and lepromatous leprosy. In all these cases, immunohistochemical investigations and essential integration with clinical-anamnestic information may help in the correct nosographic classification [,,,,,,,,,,,,,,,,,,,].
In recent years, some authors such as Chen Y. have described cases of BCM developing a mutation of BRAFV600E in the metastatic setting and, therefore, brought attention to how this entity, despite the peculiar morphological characteristics, is able to behave also from the molecular point of view as a conventional MM. This is already affecting the therapeutic side, as shown by recent papers [].
5. Conclusions
In this work we have presented four new cases of BCM, and covering a rather long period of time, we ended up dwelling on the latest molecular acquisitions also in the context of such a rare variant of MM. We believe that future studies with numerous case series are essential not only to increase the knowledge of the pathophysiology of this neoplasm but also to correctly evaluate the response of BCM patients to new oncological therapies.
Author Contributions
Conceptualization, G.C. (Gerardo Cazzato), P.P. and A.C. (Anna Colagrande); methodology, A.C. (Antonietta Cimmino); software, G.C. (Gerardo Cazzato) and M.D.; validation, G.C. (Gerardo Cazzato), E.C., F.A., V.L. and G.I.; formal analysis, G.C. (Gerardo Cazzato), G.C. (Gennaro Cormio) and A.D.; investigation, G.C. (Gerardo Cazzato), T.L., I.T., E.B. and P.R.; resources, G.C. (Gerardo Cazzato) and C.F.; data curation, A.C. (Antonietta Cimmino); writing—original draft preparation, G.C. (Gerardo Cazzato); writing—review and editing, G.C. (Gerardo Cazzato) and L.R.; visualization, L.L.; supervision, C.F. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable because this one is a retrospective study with cases diagnosed during routine pathological activity.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbas, O.; Miller, D.D.; Bhawan, J. Cutaneous malignant melanoma: Update on diagnostic and prognostic biomarkers. Am. J. Dermatopathol. 2014, 36, 363–379. [Google Scholar] [CrossRef]
- Kozovska, Z.; Gabrisova, V.; Kucerova, L. Malignant melanoma: Diagnosis, treatment and cancer stem cells. Neoplasma 2016, 63, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Limongelli, L.; Cascardi, E.; Capodiferro, S.; Favia, G.; Corsalini, M.; Tempesta, A.; Maiorano, E. Multifocal Amelanotic Melanoma of the Hard Palate: A Challenging Case. Diagnostics 2020, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Verginelli, F.; Pisacane, A.; Gambardella, G.; D’Ambrosio, A.; Candiello, E.; Ferrio, M.; Panero, M.; Casorzo, L.; Benvenuti, S.; Cascardi, E.; et al. Cancer of unknown primary stem-like cells model multi-organ metastasis and unveil liability to MEK inhibition. Nat. Commun. 2021, 12, 2498. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Pasculli, A.; Sgaramella, L.I.; Cazzato, G.; Macorano, E.; Piscitelli, D.; Gurrado, A.; Testini, M. Severe anemia in a patient with vulvar melanoma. Surgery 2020, 168, e21–e22. [Google Scholar] [CrossRef]
- Massi, G.; Leboit, E.P. Histological Diagnosis of Nevi and Melanoma, 2nd ed.; Spinger: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Maher, J.; Cameron, A.; Wallace, S.; Acosta-Rojas, R.; Weedon, D.; Rosendahl, C. Balloon cell melanoma: A case report with polarized and non-polarized dermatoscopy and dermatopathology. Dermatol. Pract. Concept. 2014, 4, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxford Centre for Evidence-Based Medicine. Levels of Evidence. 2011. Available online: http://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf (accessed on 31 December 2021).
- Gardner, W.A.; Vazquez, M.D. Balloon cell melanoma. Arch. Pathol. 1970, 89, 470–472. [Google Scholar]
- Ranchod, M. Metastatic melanoma with balloon cell changes. Cancer 1972, 30, 1006–1013. [Google Scholar] [CrossRef]
- Riley, F.C. Balloon cell melanoma of the choroid. Arch. Ophthalmol. 1974, 92, 131–133. [Google Scholar] [CrossRef]
- Rodrigues, M.M.; Shields, J.A. Malignant melanoma of the choroid with balloon cells a clinicopathologic study of three cases. Can. J. Ophthalmol. 1976, 11, 208–216. [Google Scholar]
- Gatteschi, B.; Lapertosa, G.; Quaglia, A.C. Diagnosis of a case of partly clear-cell melanoblastoma (balloon cell melanoma) with metastases. Pathologica 1978, 70, 221–226. [Google Scholar] [PubMed]
- Jakobiec, F.A.; Shields, J.A.; Desjardins, L.; Iwamoto, T. Balloon cell melanomas of the ciliary body. Arch. Ophthalmol. 1979, 97, 1687–1692. [Google Scholar] [CrossRef]
- Søndergaard, K.; Henschel, A.; Hou-Jensen, K. Metastatic melanoma with balloon cell changes: An electron microscopic study. Ultrastruct. Pathol. 1980, 1, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, R.; Manetto, V.; Minghetti, G.; Lanzanova, G. Cerebellar balloon-cell metastasis of a melanoma. Tumori 1982, 68, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Rao, U.; Fox, S. The cytology of metastatic balloon cell melanoma. Acta Cytol. 1982, 26, 39–43. [Google Scholar]
- Khalil, M.K. Balloon cell malignant melanoma of the choroid: Ultrastructural studies. Br. J. Ophthalmol. 1983, 67, 579–584. [Google Scholar] [CrossRef]
- Horton, J.J.; MacDonald, D.M. Balloon cell melanoma: A case report. Br. J. Dermatol. 1983, 108, 617–619. [Google Scholar] [CrossRef]
- Fievez, M. Balloon cell melanoma of the skin. Review of the literature. Apropos of 1 case. Ann. Pathol. 1984, 4, 231–235. [Google Scholar]
- Peters, M.S.; Su, W.P. Balloon cell malignant melanoma. J. Am. Acad. Dermatol. 1985, 13 Pt 2, 351–354. [Google Scholar] [CrossRef]
- Driot, J.Y.; Liotet, S. Ultrastructural study of a choroidal balloon-cell melanoma. Bull. De La Soc. Belg. Ophtalmol. 1986, 218, 103–113. [Google Scholar]
- Da, J.P. Primary anorectal malignant melanoma--report of 7 cases and review of literature. Chin. J. Oncol. 1987, 9, 372–374, 17. [Google Scholar]
- Driot, J.Y.; Rault, J.; Bonnin, P.; Liotet, S. Electron microscopy of three cases of choroid malignant melanomas. Int. Ophthalmol. 1987, 10, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Aloi, F.G.; Coverlizza, S.; Pippione, M. Balloon cell melanoma: A report of two cases. J. Cutan. Pathol. 1988, 15, 230–233. [Google Scholar] [CrossRef]
- Margo, C.E. Conjunctival melanoma with balloon cell transformation. Case report. Arch. Ophthalmol. 1988, 106, 1653–1654. [Google Scholar] [CrossRef]
- Napoli, P. Balloon cell malignant melanoma. Pathologica 1988, 80, 379–385. [Google Scholar] [PubMed]
- Heid, E. A case for diagnosis: Primary melanoma or metastasis of a melanoma with balloon-like cells. Ann. Dermatol. Venereol. 1988, 115, 489–490. [Google Scholar]
- Akslen, L.A.; Myking, A.O. Balloon cell melanoma mimicking clear cell carcinoma. Pathol. Res. Pract. 1989, 184, 548–550. [Google Scholar] [CrossRef]
- Martinez, F.; Merenda, G.; Bedrossian, C.W. Lipid-rich metastatic balloon-cell melanoma: Diagnosis by a multimodal approach to aspiration biopsy cytology. Diagn. Cytopathol. 1990, 6, 427–433. [Google Scholar] [CrossRef]
- Kao, G.F.; Helwig, E.B.; Graham, J.H. Balloon cell malignant melanoma of the skin. A clinicopathologic study of 34 cases with histochemical, immunohistochemical, and ultrastructural observations. Cancer 1992, 69, 2942–2952. [Google Scholar] [CrossRef]
- Messmer, E.; Bornfeld, N.; Foerster, M.; Schilling, H.; Wessing, A. Histopathologic findings in eyes treated with a ruthenium plaque for uveal melanoma. Graefes. Arch. Clin. Exp. Ophthalmol. 1992, 230, 391–396. [Google Scholar] [CrossRef]
- Cardesi, E.; Cassia, A.; Cera, G. A case of anatomo-clinical diagnosis of balloon cell melanoma metastasis. Minerva Med. 1993, 84, 709–712. [Google Scholar] [PubMed]
- Megahed, M.; Hofmann, U.; Scharffetter-Kochanek, K.; Ruzicka, T. Amelanotic polypoid malignant melanoma of the balloon cell type. Der Pathol. 1994, 15, 350–353. [Google Scholar]
- Mowat, A.; Reid, R.; Mackie, R. Balloon cell metastatic melanoma: An important differential in the diagnosis of clear cell tumours. Histopathology 1994, 24, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Adamek, D.; Kaluza, J.; Stachura, K. Primary balloon cell malignant melanoma of the right temporo-parietal region arising from meningeal naevus. Clin. Neuropathol. 1995, 14, 29–32. [Google Scholar]
- Kawamura, T.; Ohtake, N.; Takayama, O.; Furue, M.; Tamaki, K. Balloon cell melanoma cells in metastatic lesions from pedunculated malignant melanoma. J. Dermatol. 1995, 22, 527–529. [Google Scholar] [CrossRef]
- Kiene, P.; Petres-Dunsche, C.; Funke, G.; Christophers, E. Nodular balloon cell component in a cutaneous melanoma of the superficial spreading type. Dermatology 1996, 192, 274–276. [Google Scholar] [CrossRef]
- Gregel, C.; Wolter, M.; Kaufmann, R. Coincidence of balloon cell melanoma with balloon cells in a dermal nevus. Der Pathol. 1998, 19, 151–153. [Google Scholar]
- Terayama, K.; Hirokawa, M.; Shimizu, M.; Mikami, Y.; Kanahara, T.; Manabe, T. Balloon melanoma cells mimicking foamy histiocytes. Acta Cytol. 1999, 43, 325–326. [Google Scholar]
- Requena, L.; de la Cruz, A.; Moreno, C.; Sangüeza, O.; Requena, C. Animal type melanoma: A report of a case with balloon-cell change and sentinel lymph node metastasis. Am. J. Dermatopathol. 2001, 23, 341–346. [Google Scholar] [CrossRef]
- August, C.; Baba, H.A.; Heinig, J.; Nashan, D.; Höhn, P.; Holzhausen, H.J.; Metze, D.; Böcker, W. Endometrial metastasis of a “balloon” cell melanoma mimicking a “xanthomatous endometritis”. Der Pathol. 2001, 22, 145–150. [Google Scholar]
- Baehner, F.L.; Ng, B.; Sudilovsky, D. Metastatic balloon cell melanoma: A case report. Acta Cytol. 2005, 49, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Hoque, S.R.; Cliff, S.H. Balloon cell melanoma: A rare form of malignant melanoma in three patients. Br. J. Dermatol. 2005, 153, 13–14. [Google Scholar]
- McGowan, J.W.; Smith, M.K.; Ryan, M.; Hood, A.F. Proliferative nodules with balloon-cell change in a large congenital melanocytic nevus. J. Cutan. Pathol. 2006, 33, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Plaza, J.A.; Torres-Cabala, C.; Evans, H.; Diwan, H.A.; Suster, S.; Prieto, V.G. Cutaneous metastases of malignant melanoma: A clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am. J. Dermatopathol. 2010, 32, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Zhou, F.; Simms, A.; Wieczorek, R.; Fang, Y.; Subietas-Mayol, A.; Wang, B.; Heller, P.; Huang, H.; Pei, Z.; et al. Metastatic balloon cell malignant melanoma: A case report and literature review. Int. J. Clin. Exp. Pathol. 2011, 4, 315–321. [Google Scholar]
- Gessi, M.; Fischer, H.P.; Rösseler, L.; Urbach, H.; Pietsch, T.; van Landeghem, F.K. Unusual balloon cell features in melanoma brain metastasis: A potential diagnostic pitfall in surgical neuropathology. Clin. Neuropathol. 2011, 30, 86–88. [Google Scholar] [CrossRef]
- Richardson, M.D.; Somerset, H.; Kleinschmidt-DeMastersm, B.K.; Waziri, A. 76-year-old man with a cerebellar lesion. Brain Pathol. 2012, 22, 861–864. [Google Scholar] [CrossRef]
- Inskip, M.; Magee, J.; Barksdale, S.; Weedon, D.; Rosendahl, C. Balloon cell melanoma in primary care practice: A case report. Dermatol. Pract. Concept. 2013, 3, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Bal, M.M.; Ramadwar, M.; Deodhar, K. Balloon cell melanoma of the anal canal: A wolf in sheep’s clothing? J. Cancer Res. Ther. 2013, 9, 706–708. [Google Scholar] [CrossRef]
- WHO. Classification of Skin Tumours. In WHO Classification of Tumours, 4th ed.; WHO: Genevan, Switzerland, 2018; Volume 11. [Google Scholar]
- Bures, N.; Monaco, S.E.; Palekar, A.; Pantanowitz, L. Cytomorphology of metastatic balloon cell melanoma. Diagn. Cytopathol. 2015, 43, 485–487. [Google Scholar] [CrossRef]
- Han, J.S.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C. Primary cutaneous balloon cell melanoma: A very rare variant. Int J. Dermatol. 2014, 53, e535–e536. [Google Scholar] [CrossRef] [PubMed]
- Duman, N.; Şahin, S.; Özaygen, G.E.; Gököz, Ö. Dermoscopy of satellite metastasis of balloon cell melanoma. J. Am. Acad. Dermatol. 2014, 71, e11–e12. [Google Scholar] [CrossRef] [PubMed]
- McComiskey, M.; Iavazzo, C.; Datta, M.; Slade, R.; Winter-Roach, B.; Lambe, G.; Sangar, V.K.; Smith, M. Balloon Cell Urethral Melanoma: Differential Diagnosis and Management. Case Rep. Obstet. Gynecol. 2015, 2015, 919584. [Google Scholar] [CrossRef] [PubMed]
- Seabra Resende, F.S.; Conforti, C.; Giuffrida, R.; Corneli, P.; Fagotti, S.; Custrin, A.; Shaffiei, V.; Zalaudek, I.; Di Meo, N. Balloon Cell Primary Nodular Melanoma: Dermoscopy Evidence. Dermatol. Pract. Concept. 2019, 9, 155–156. [Google Scholar] [CrossRef] [Green Version]
- Inskip, M.; James, N.; Magee, J.; Rosendahl, C. Pigmented primary cutaneous balloon cell melanoma demonstrating balloon cells in the dermoepidermal junction: A brief case report with dermatoscopy and histopathology. Int J. Dermatol. 2016, 55, e110–e112. [Google Scholar] [CrossRef]
- Hattori, Y.; Sentani, K.; Hattori, T.; Matsuo, Y.; Kawai, M.; Shindo, H.; Tanaka, M.; Hide, M.; Yasui, W. Balloon Cell Malignant Melanoma in a Young Female: A Case Report and Review of the Literature. Case Rep. Oncol. 2016, 9, 262–266. [Google Scholar] [CrossRef]
- Chavez-Alvarez, S.; Villarreal-Martinez, A.; Miranda-Maldonado, I.; Ocampo-Candiani, J.; Garza-Rodriguez, V. Balloon Cell Melanoma and Its Metastasis, a Rare Entity. Am. J. Dermatopathol. 2017, 39, 404–411. [Google Scholar] [CrossRef]
- Iliadis, A.; Zaraboukas, T.; Selviaridis, P.; Chatzisotiriou, A. Balloon cell melanoma metastasis to the temporal lobe. Indian J. Pathol. Microbiol. 2017, 60, 622–623. [Google Scholar] [CrossRef]
- Saharti, S.; Isaila, B.; Mudaliar, K.; Wojcik, E.M.; Pambuccian, S.E. Balloon cells in metastatic melanoma. Diagn. Cytopathol. 2017, 45, 828–831. [Google Scholar] [CrossRef]
- Friedman, B.J.; Stoner, R.; Sahu, J.; Lee, J.B. Association of Clinical, Dermoscopic, and Histopathologic Findings with Gene Expression in Patients with Balloon Cell Melanoma. JAMA Dermatol. 2018, 154, 77–81. [Google Scholar] [CrossRef]
- Farah, M.; Nagarajan, P.; Torres-Cabala, C.A.; Curry, J.L.; Amaria, R.N.; Wargo, J.; Tawbi, H.; Ivan, D.; Prieto, V.G.; Tetzlaff, M.T.; et al. Metastatic melanoma with balloon/histiocytoid cytomorphology after treatment with immunotherapy: A histologic mimic and diagnostic pitfall. J. Cutan. Pathol. 2018, 45, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, G.M.; Baraldi, C.; Dika, E.; Fanti, P.A.; Misciali, C. An Ulcerated Reddish Nodule of the Chest: When You See, Think …. Dermatopathology 2018, 5, 117–120. [Google Scholar] [CrossRef]
- Caltabiano, R.; Broggi, G.; Garro, R.; Terranova, M.; Argenziano, G. Balloon cell melanoma: An uncommon entity representing a diagnostic pitfall in dermatopathology. Ital. J. Dermatol. Venereol. 2021, 156, 82–88. [Google Scholar] [CrossRef]
- Goto, H.; Shimauchi, T.; Fukuchi, K.; Yokota, N.; Koizumi, S.; Aoshima, M.; Endo, Y.; Masuda, Y.; Miyazawa, H.; Kasuya, A.; et al. Therapeutic Effectiveness of Immunoradiotherapy on Brain-metastatic BRAF/MEK Inhibitor-resistant Melanoma with Balloon Cell Change. Acta Derm. Venereol. 2019, 99, 612–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Y.; Lan, C.E.; Yen, M.C.; Cheng, S.T. Balloon cell melanoma possessed the ability to develop BRAF V600E mutation in cancer cells. Kaohsiung J. Med. Sci. 2021, 37, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Hennessy, K.; Kevin Heard, L.; Gaudi, S.; Mhaskar, R.; Patel, R.R.; Bennett, A.E. Balloon cell melanoma: A systematic review. Int J. Dermatol. 2021, 61, 266–277. [Google Scholar] [CrossRef]
- García-Piqueras, P.; Avilés-Izquierdo, J.A.; Almodóvar-Real, A.; Nieto-Benito, L.M.; Rodríguez Lomba, E. Clues in dermoscopy: White and yellow structures in balloon cell melanoma. Eur. J. Dermatol. 2021, 31, 676–677. [Google Scholar] [CrossRef]
- Laforga, J.B. Fine-needle aspiration cytological findings in three cases of metastatic amelanotic melanoma to the parotid gland with divergent differentiation. Diagn. Cytopathol. 2021, 24. [Google Scholar] [CrossRef]
- Macák, J.; Krc, I.; Elleder, M.; Lukás, Z.; Nádasdy, T.; Güttnerová, J. Balloon cell melanoma of the skin. Part I: Histology, immunohistology and histochemistry. Acta Univ. Palacki. Olomuc. Fac. Med. 1990, 126, 71–82. [Google Scholar]
- Macák, J.; Krc, I. Balloon cell melanoma of the skin. Part II: An electron-microscopic study. Acta Univ. Palacki. Olomuc. Fac. Med. 1990, 126, 83–92. [Google Scholar]
- Barnhill, R.L. Pathology and prognostic factors. Curr. Opin. Oncol. 1993, 5, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.A.; Fatteh, S.M.; Campbell, T.E. Glycogen-rich malignant melanomas and glycogen-rich balloon cell malignant melanomas: Frequency and pattern of PAS positivity in primary and metastatic melanomas. Arch. Pathol. Lab. Med. 1998, 122, 353–360. [Google Scholar] [PubMed]
- McCarthy, S.W.; Scolyer, R.A. Melanocytic lesions of the face: Diagnostic pitfalls. Ann. Acad. Med. Singap. 2004, 33, 3–14. [Google Scholar] [PubMed]
- Larre Borges, A.; Zalaudek, I.; Longo, C.; Dufrechou, L.; Argenziano, G.; Lallas, A.; Piana, S.; Moscarella, E. Melanocytic nevi with special features: Clinical-dermoscopic and reflectance confocal microscopic-findings. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 833–845. [Google Scholar] [CrossRef]
- Kazlouskaya, V.; Guo, Y.; Maia-Cohen, S.; Mones, J. Clear-cell melanocytic lesions with balloon-cell and sebocyte-like melanocytes: A unifying concept. Am. J. Dermatopathol. 2014, 36, 380–386. [Google Scholar] [CrossRef]
- Valdivielso-Ramos, M.; Burdaspal, A.; Conde, E.; de la Cueva, P. Balloon-cell variant of the Spitz nevus. J. Eur. Acad. Dermatol Venereol. 2016, 30, 1621–1622. [Google Scholar] [CrossRef]
- Borsa, S.; Toonstra, J.; van der Putte, S.C.; van Vloten, W.A. Balloon-cell variant of the Spitz nevus. Hautarzt 1991, 42, 707–708. [Google Scholar]
- Budde, M.; Schlötzer-Schrehardt, U.; Hofmann-Rummelt, C.; Holbach, L.M. Conjunctival balloon cell nevi--light- and electronmicroscopic findings in 2 patients. Klin. Monbl. Augenheilkd. 2001, 218, 269–272. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).