Epigenetic Mechanisms in Diabetes Research
A topical collection in Epigenomes (ISSN 2075-4655).
Viewed by 22784
Share This Topical Collection
Editor
 Prof. Dr. Assam El-Osta
Prof. Dr. Assam El-Osta
 Prof. Dr. Assam El-Osta
Prof. Dr. Assam El-Osta
E-Mail
Website
Collection Editor
Baker Heart and Diabetes Institute, Melbourne, VIC 3004, Australia
Interests: diabetes; clinical and molecular epigenetics; chromatin; DNA and RNA methylation; histones; transcriptional regulation; computational epigenomics; metabolic disease
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The rising global epidemic of obesity, cardiovascular disease and diabetes underscores the current challenge of characterizing the metabolically responsive epigenome. Recent studies investigating the molecular mechanisms linking fat and glucose metabolism with the epigenome highlight important regulatory determinants that underpin the expression of genes implicated in disease yet remain poorly understood. Epigenomes is now accepting submissions for a collection on "Epigenetic Mechanisms in Diabetes Research". This Topical Collection is edited by Professor Sam El-Osta from Monash University and will accept original research results and reviews, as well as special interest topics related to methods and resources of exceptional interest on topics related to metabolic regulation by epigenetic mechanisms in health and disease. You can find all the information related to the new Topical Collection at the following link: https://www.mdpi.com/journal/epigenomes/special_issues/diabetes.
We kindly invite you to submit your unpublished work to this Epigenomes Topical Collection, "Epigenetic Mechanisms in Diabetes Research".
I do hope you will join me in this Topical Collection.
Prof. Dr. Assam El-Osta
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Epigenomes is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 1600 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (4 papers)
Open AccessArticle
Epigenome-Wide and Methylation Risk Score Analysis of Body Mass Index Among People with HIV
by
Nanzha Abi, Alexandra Young, Pradeep Tiwari, Junyu Chen, Chang Liu, Qin Hui, Kaku So-Armah, Matthew S. Freiberg, Amy C. Justice, Ke Xu, Marta Gwinn, Vincent C. Marconi and Yan V. Sun
Viewed by 1997
Abstract
Background/Objectives: People with HIV (PWH) on antiretroviral therapy (ART) often gain weight, which increases their risk of type 2 diabetes and cardiovascular disease. The role of DNA methylation (DNAm) markers in obesity among PWH is understudied. This research explores the relationship between body
[...] Read more.
Background/Objectives: People with HIV (PWH) on antiretroviral therapy (ART) often gain weight, which increases their risk of type 2 diabetes and cardiovascular disease. The role of DNA methylation (DNAm) markers in obesity among PWH is understudied. This research explores the relationship between body mass index (BMI) and epigenetic patterns to better understand and manage obesity-related risks in PWH.
Methods: We conducted an epigenome-wide association study (EWAS) on 892 African American male PWH from the Veterans Aging Cohort Study, examining BMI associations with DNAm using linear mixed models, adjusting for covariates, including soluble CD14. We compared our results with BMI-associated DNAm markers from non-HIV individuals and developed a methylation risk score (MRS) for BMI using machine learning and a cross-validation approach.
Results: We identified four epigenome-wide significant CpG sites, including one in the
RAP1B gene, indicating shared and unique BMI-related epigenetic markers between PWH and non-HIV individuals. The constructed BMI MRS explained approximately 19% of the BMI variance in PWH.
Conclusions: DNAm markers and MRS are significantly linked to BMI in PWH, suggesting shared and distinct molecular mechanisms with non-HIV populations. These insights could lead to targeted interventions to reduce cardiometabolic disease risks in PWH under ART.
Full article
►▼
Show Figures
Open AccessReview
Retrotransposons and Diabetes Mellitus
by
Andromachi Katsanou, Charilaos Kostoulas, Evangelos Liberopoulos, Agathocles Tsatsoulis, Ioannis Georgiou and Stelios Tigas
Cited by 3 | Viewed by 2887
Abstract
Retrotransposons are invasive genetic elements, which replicate by copying and pasting themselves throughout the genome in a process called retrotransposition. The most abundant retrotransposons by number in the human genome are Alu and LINE-1 elements, which comprise approximately 40% of the human genome.
[...] Read more.
Retrotransposons are invasive genetic elements, which replicate by copying and pasting themselves throughout the genome in a process called retrotransposition. The most abundant retrotransposons by number in the human genome are Alu and LINE-1 elements, which comprise approximately 40% of the human genome. The ability of retrotransposons to expand and colonize eukaryotic genomes has rendered them evolutionarily successful and is responsible for creating genetic alterations leading to significant impacts on their hosts. Previous research suggested that hypomethylation of Alu and LINE-1 elements is associated with global hypomethylation and genomic instability in several types of cancer and diseases, such as neurodegenerative diseases, obesity, osteoporosis, and diabetes mellitus (DM). With the advancement of sequencing technologies and computational tools, the study of the retrotransposon’s association with physiology and diseases is becoming a hot topic among researchers. Quantifying Alu and LINE-1 methylation is thought to serve as a surrogate measurement of global DNA methylation level. Although Alu and LINE-1 hypomethylation appears to serve as a cellular senescence biomarker promoting genomic instability, there is sparse information available regarding their potential functional and biological significance in DM. This review article summarizes the current knowledge on the involvement of the main epigenetic alterations in the methylation status of Alu and LINE-1 retrotransposons and their potential role as epigenetic markers of global DNA methylation in the pathogenesis of DM.
Full article
►▼
Show Figures
Open AccessArticle
Exploring Transcriptional Regulation of Beta Cell SASP by Brd4-Associated Proteins and Cell Cycle Control Protein p21
by
Jasmine Manji, Jasmine Pipella, Gabriel Brawerman and Peter J. Thompson
Cited by 2 | Viewed by 3778
Abstract
Type 1 diabetes (T1D) is a metabolic disease resulting from progressive autoimmune destruction of insulin-producing pancreatic beta cells. Although the majority of beta cells are lost in T1D, a small subset undergoes senescence, a stress response involving growth arrest, DNA damage response, and
[...] Read more.
Type 1 diabetes (T1D) is a metabolic disease resulting from progressive autoimmune destruction of insulin-producing pancreatic beta cells. Although the majority of beta cells are lost in T1D, a small subset undergoes senescence, a stress response involving growth arrest, DNA damage response, and activation of a senescence-associated secretory phenotype (SASP). SASP in beta cells of the nonobese diabetic (NOD) mouse model of T1D and primary human islets is regulated at the level of transcription by bromodomain extra-terminal (BET) proteins, but the mechanisms remain unclear. To explore how SASP is transcriptionally regulated in beta cells, we used the NOD beta cell line NIT-1 to model beta cell SASP and identified binding partners of BET protein Brd4 and explored the role of the cyclin-dependent kinase inhibitor p21. Brd4 interacted with a variety of proteins in senescent NIT-1 cells including subunits of the Ino80 chromatin remodeling complex, which was expressed in beta cells during T1D progression in NOD mice and in human beta cells of control, autoantibody-positive, and T1D donors as determined from single-cell RNA-seq data. RNAi knockdown of p21 during senescence in NIT-1 cells did not significantly impact viability or SASP. Taken together, these results suggest that Brd4 interacts with several protein partners during senescence in NIT-1 cells, some of which may play roles in SASP gene activation and that p21 is dispensable for the SASP in this beta cell model.
Full article
►▼
Show Figures
Open AccessReview
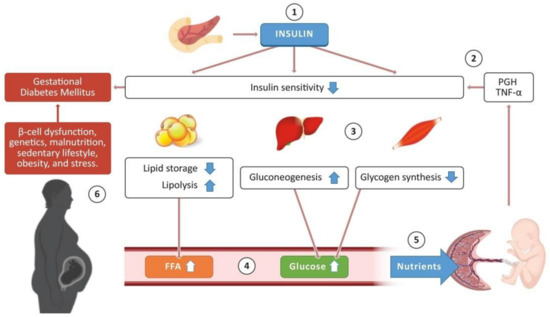
The Placenta as a Target of Epigenetic Alterations in Women with Gestational Diabetes Mellitus and Potential Implications for the Offspring
by
Dennise Lizárraga and Alejandra García-Gasca
Cited by 18 | Viewed by 11714
Abstract
Gestational diabetes mellitus (GDM) is a pregnancy complication first detected in the second or third trimester in women that did not show evident glucose intolerance or diabetes before gestation. In 2019, the International Diabetes Federation reported that 15.8% of live births were affected
[...] Read more.
Gestational diabetes mellitus (GDM) is a pregnancy complication first detected in the second or third trimester in women that did not show evident glucose intolerance or diabetes before gestation. In 2019, the International Diabetes Federation reported that 15.8% of live births were affected by hyperglycemia during pregnancy, of which 83.6% were due to gestational diabetes mellitus, 8.5% were due to diabetes first detected in pregnancy, and 7.9% were due to diabetes detected before pregnancy. GDM increases the susceptibility to developing chronic diseases for both the mother and the baby later in life. Under GDM conditions, the intrauterine environment becomes hyperglycemic, while also showing high concentrations of fatty acids and proinflammatory cytokines, producing morphological, structural, and molecular modifications in the placenta, affecting its function; these alterations may predispose the baby to disease in adult life. Molecular alterations include epigenetic mechanisms such as DNA and RNA methylation, chromatin remodeling, histone modifications, and expression of noncoding RNAs (ncRNAs). The placenta is a unique organ that originates only in pregnancy, and its main function is communication between the mother and the fetus, ensuring healthy development. Thus, this review provides up-to-date information regarding two of the best-documented (epigenetic) mechanisms (DNA methylation and miRNA expression) altered in the human placenta under GDM conditions, as well as potential implications for the offspring.
Full article
►▼
Show Figures