Layered Double Hydroxides and Related Materials for Advanced Heterogeneous Catalytic Processes
A topical collection in Catalysts (ISSN 2073-4344). This collection belongs to the section "Catalytic Materials".
Viewed by 6118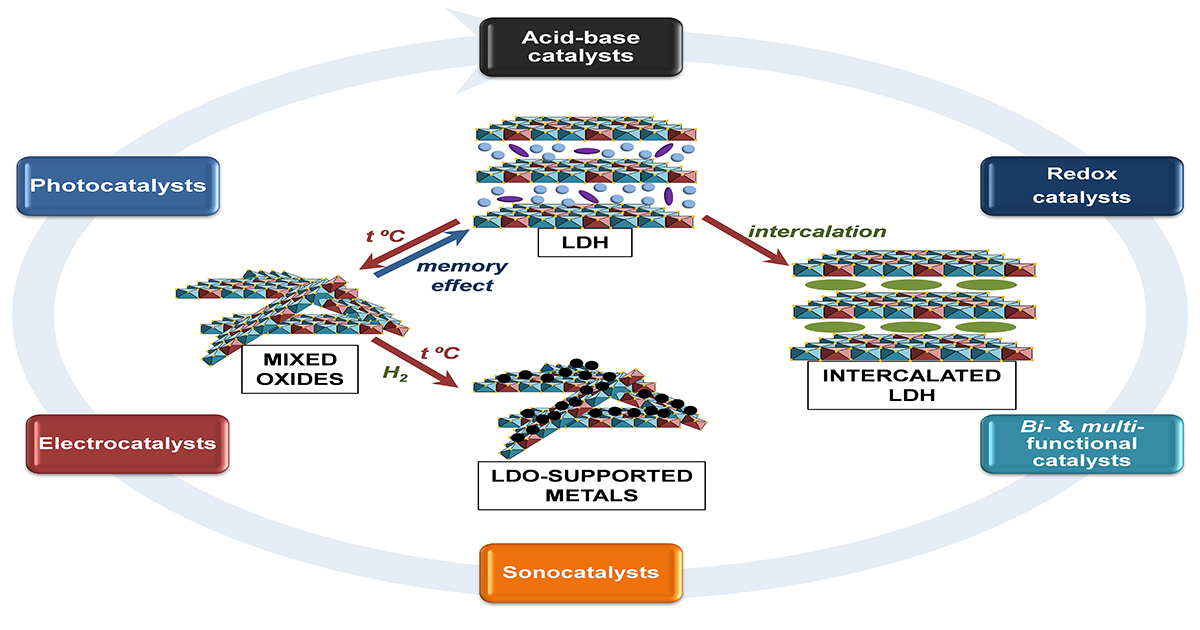
Editors
Interests: heterogeneous catalysis; catalysis by metal oxides; semiconducting metal oxides; layered double hydroxides and related materials; catalytic oxidation
Special Issues, Collections and Topics in MDPI journals
Interests: base catalysts; fine chemicals synthesis; ionic liquids
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Layered double hydroxides (LDHs) belong to the class of anionic clays and possess some specific properties, such as uniform distribution of cations/anions in the network, tailored textural characteristics, high surface area, both acid-base and redox properties, and memory effect, which make them peculiar catalytic materials, catalyst supports, and precursors for multicationic mixed oxides, supported metal catalysts, high-entropy oxides, and composite or hybrid materials (polymer/LDH nanocomposites, graphene oxide/LDH hybrids, core/shell multifunctional materials, thin films, etc.). They can act as acid-base, redox, bifunctional or multifunctional heterogeneous catalysts, photocatalysts, and electrocatalysts for different processes. Thus, the present topical collection is devoted to the investigation of the catalytic behavior of innovative LDH-based materials in a wide range of challenging processes, including, but not limited to, the synthesis of value-added chemicals and fuels, biomass conversion, energy production, pollution abatement, etc.
Prof. Dr. Ioan-Cezar Marcu
Dr. Octavian D. Pavel
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Catalysts is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2200 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- layered double hydroxides
- catalytic materials
- LDH-based composites and hybrids
- LDH and mixed oxide catalyst supports
- acid-base catalysts
- redox catalysts
- sustainable processes
- fine chemical synthesis
- photocatalysis
- electrocatalysis









