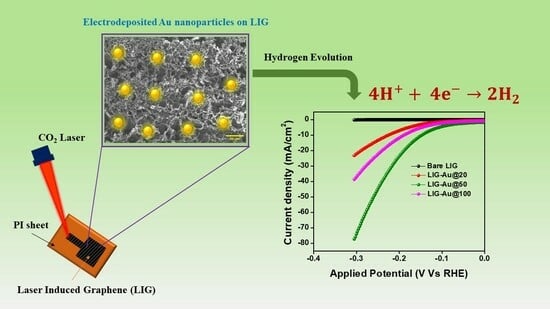
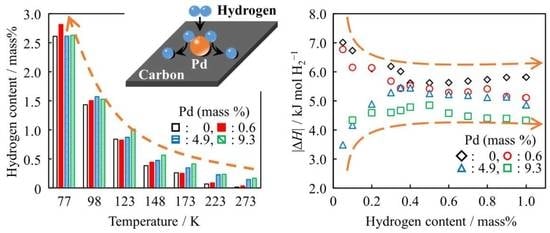
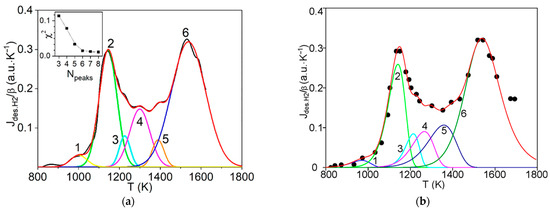
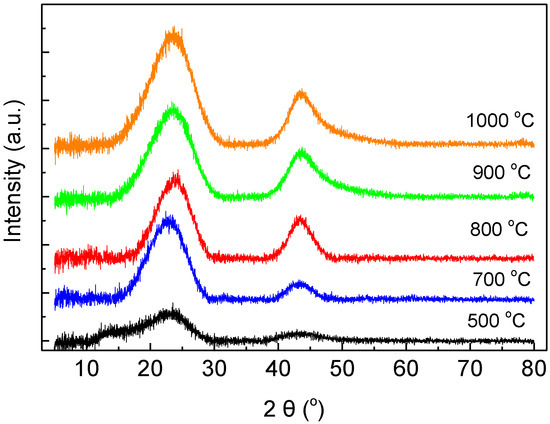
Carbon-Based Materials for Hydrogen Production, Storage and Conversion
A topical collection in C (ISSN 2311-5629). This collection belongs to the section "Carbon Materials and Carbon Allotropes".
Viewed by 94484Editors
Interests: nanomaterials; nanoporous materials; functionalized carbons; metal-decorated carbons; nanocomposites; nanoparticles; carbon nanostructures; few-layer graphene; carbon nanotubes; graphene oxide foams; activated carbons; boron nitride nanostructures; plasma treatment; gas adsorption; hydrogen storage; selective gas separation; electrochemical energy storage; supercapacitors; water splitting; water purification; detection of chemical and biological substances; surface enhanced Raman spectroscopy
Special Issues, Collections and Topics in MDPI journals
Interests: materials and surface engineering; thin films and coatings; reactive/energetic materials; energy and environmental science
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
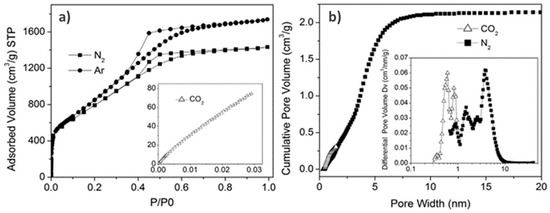
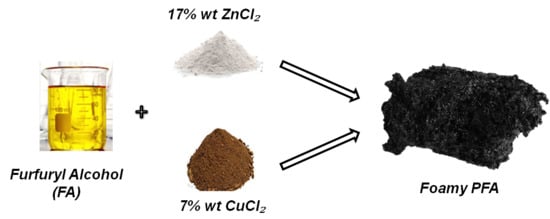
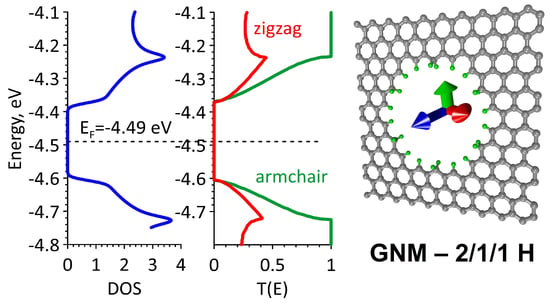
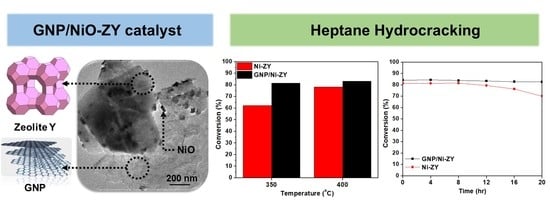
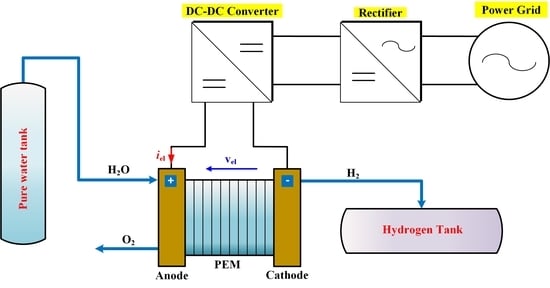
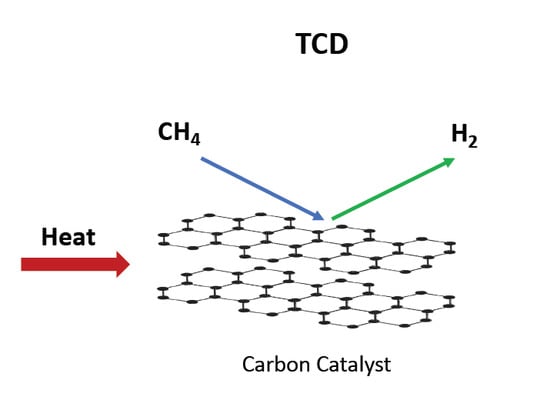
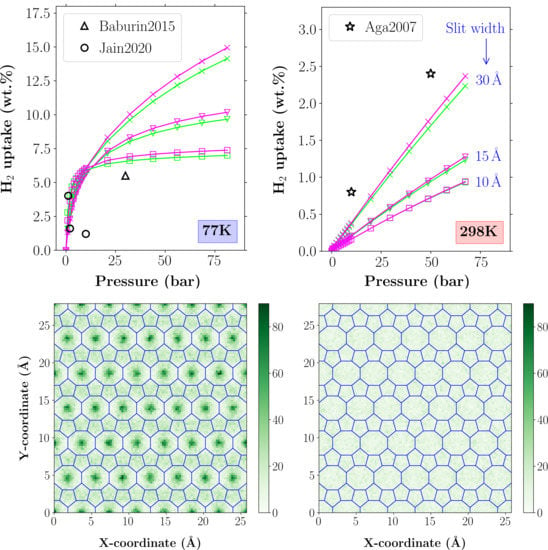
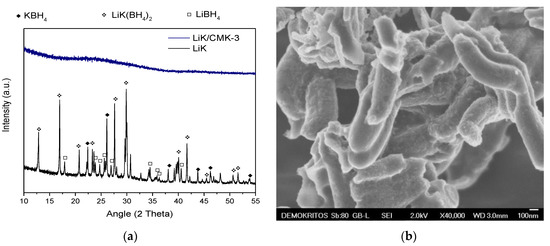
The aim of this Special Issue is to present the state-of-the-art research progress in the emerging field of carbon-based nanoporous materials for hydrogen (H2) production, storage, and conversion purposes. H2 is considered to be the ideal carbon-free energy carrier for stationary, mobile, and portable applications, in addition to being the most promising alternative to fossil fuel combustion. Nanoporous carbons and novel composites thereof could play a key role in the development of H2 technologies and provide practical solutions for open issues and on-going challenges. Carbon-based materials (e.g., activated carbons, fullerenes, carbon nanotubes, fibers, foams, and ordered mesoporous carbons) have attracted significant attention in the past four decades due to their large surface areas and pore volumes, lightweight nature, plethora of macroscopic forms and nanostructures, mass-scale availability, excellent recyclability, and low manufacturing cost. Even more attractive and promising carbonaceous materials have emerged in recent years, including 0D (e.g., carbon dots), 1D (e.g., carbon nanowires), 2D (e.g., graphene flakes), and 3D (e.g., graphene/graphene oxide foams) nanostructures and novel nanocomposites (e.g., carbons decorated with nanoparticles, doped with heteroatoms, etc.). This Special Issue will highlight the implementation of different carbons and composite structures produced in various forms (e.g., powders, monoliths, cloths, membranes, electrodes, thin films, coatings, etc.) for advanced applications related to H2 generation (e.g., electrolyzers), solid-state H2 storage (e.g., physical or chemical adsorption), and H2 conversion (e.g., fuel cells). Emphasis will be given to the effect of the porosity-related properties (e.g., surface area, pore volume, pore size distribution, average pore size, etc.) and surface chemistry characteristics (e.g., functionalities, dopants, etc.) on the respective performance. Both experimental and theoretical studies are welcome for submission in this Special Issue.
Dr. Nikolaos Kostoglou
Dr. Claus Rebholz
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. C is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- nanoporous carbons
- novel composites
- hydrogen production
- hydrogen storage
- physical adsorption
- chemical adsorption
- hydrogen conversion
- fuel cells