Hydrogen Sorption and Reversibility of the LiBH4-KBH4 Eutectic System Confined in a CMK-3 Type Carbon via Melt Infiltration
Abstract
1. Introduction
2. Materials and Methods
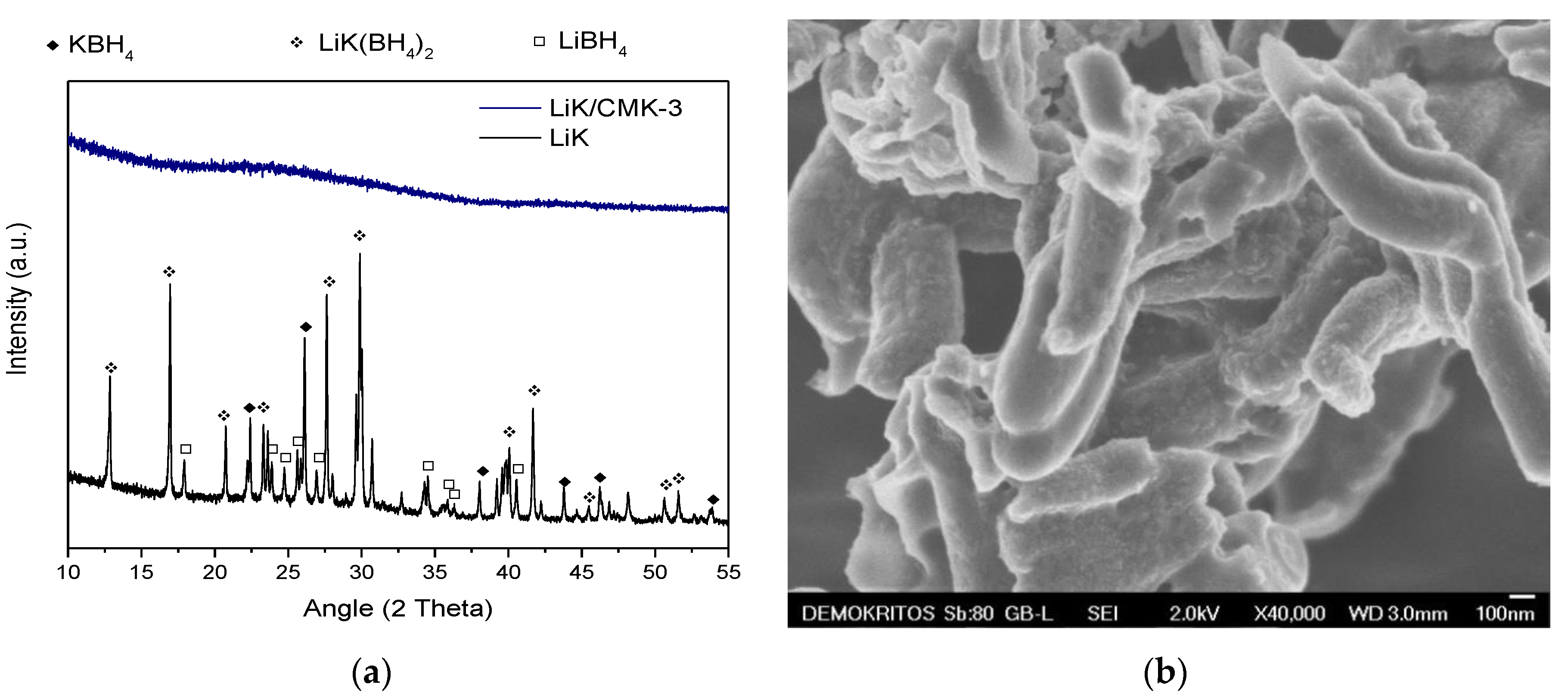
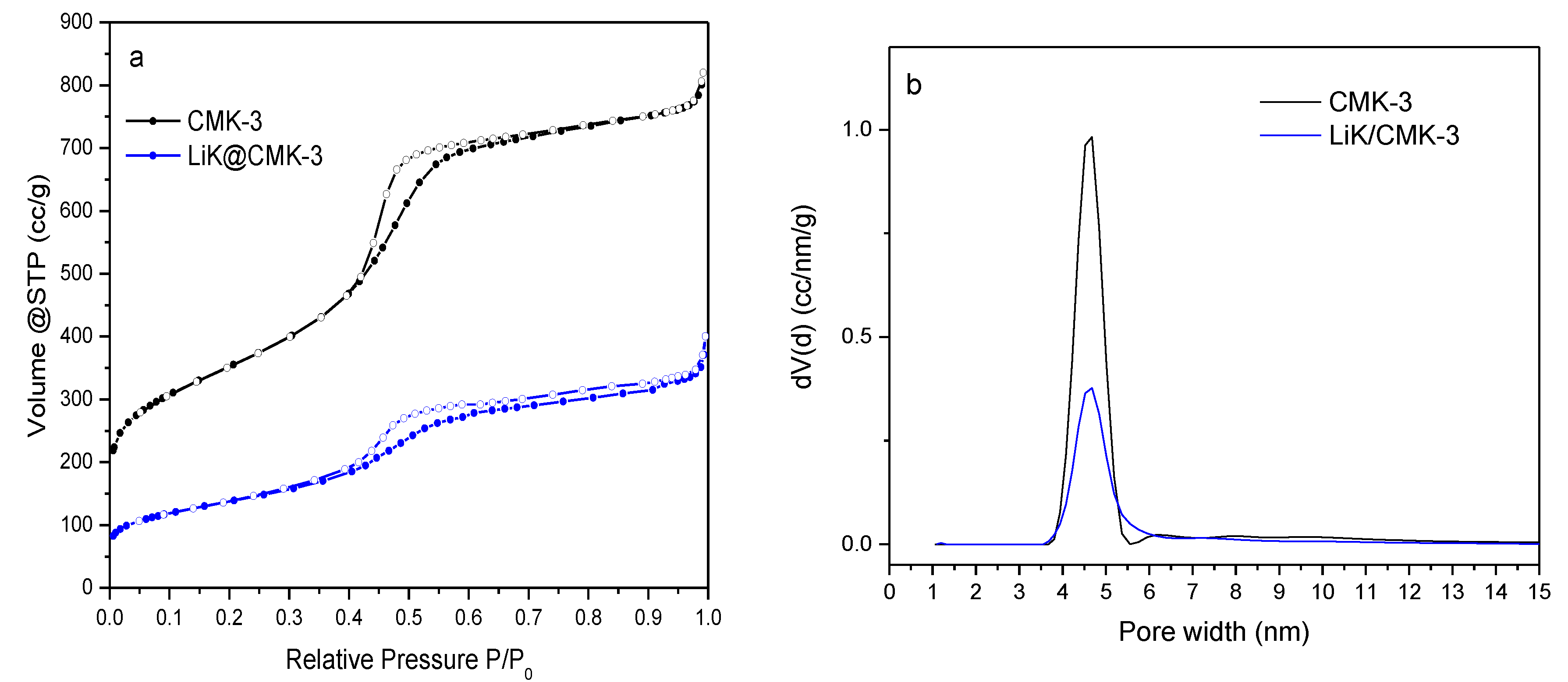
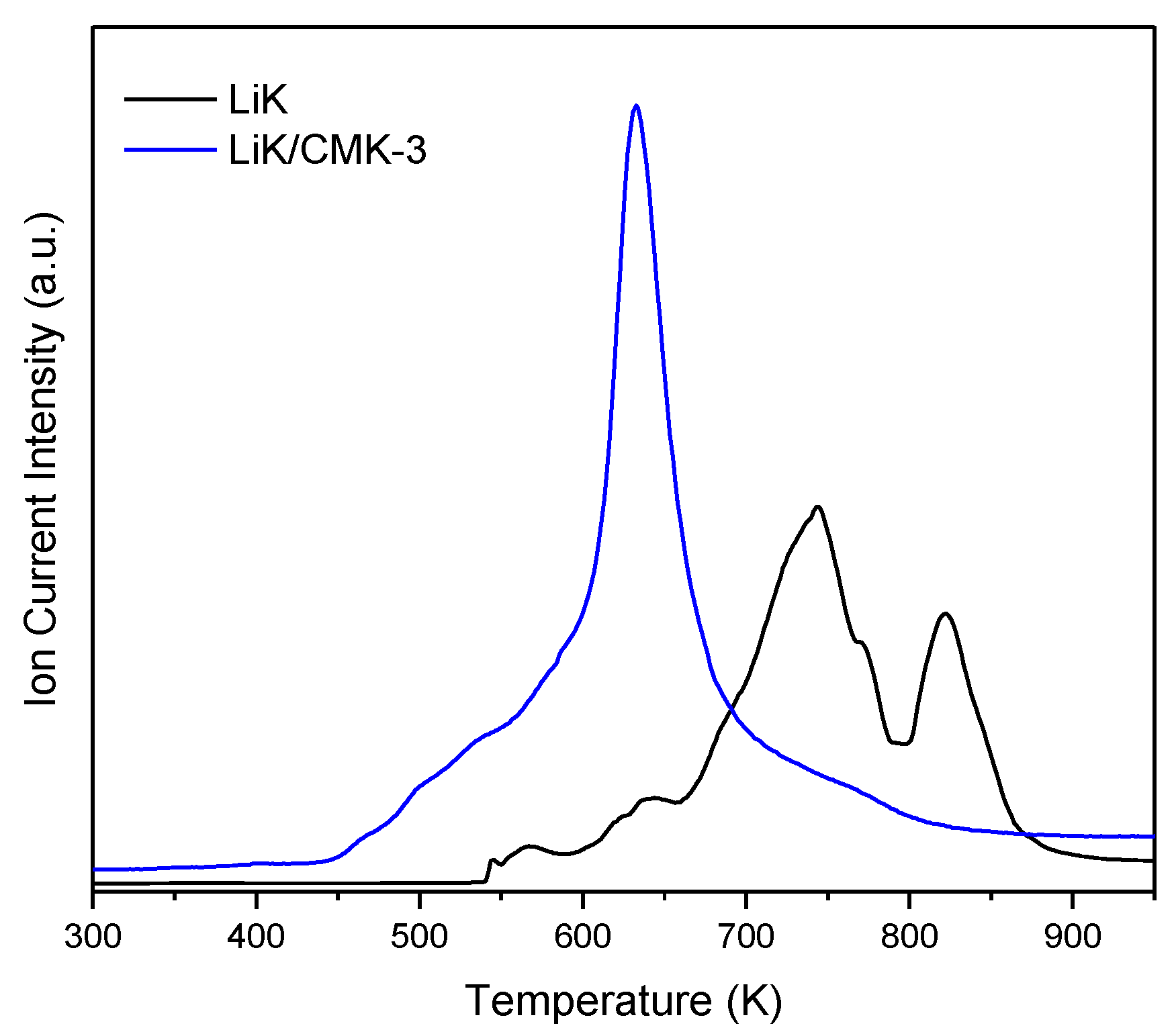
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sloth, M. 48 Hours To Build a Hydrogen Refuelling Station, 3 Minutes To Fuel: 10+ Years To Profit. Fuel Cells Bull. 2013, 5, 12–14. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen Storage Materials for Mobile and Stationary Applications: Current State of the Art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Ball, M.; Weeda, M. The hydrogen economy—Vision or reality? Int. J. Hydrog. Energy 2015, 40, 7903–7919. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta von Colbe, J.; Blanchard, D.; Bowman, R.C.J.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage—past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 1–39. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.; Ares, J.R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrog. Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Manickam, K.; Mistry, P.; Walker, G.; Grant, D.; Buckley, C.E.; Humphries, T.D.; Paskevicius, M.; Jensen, T.; Albert, R.; Peinecke, K.; et al. Future perspectives of thermal energy storage with metal hydrides. Int. J. Hydrog. Energy 2019, 44, 7738–7745. [Google Scholar] [CrossRef]
- Mazzucco, A.; Dornheim, M.; Sloth, M.; Jensen, T.R.; Oluf, J.; Rokni, M. Bed geometries, fueling strategies and optimization of heat exchanger designs in metal hydride storage systems for automotive applications: A review. Int. J. Hydrog. Energy 2014, 39, 17054–17074. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; Bellaosta von Colbe, J.M.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrog. Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Callini, E.; Aguey-Zinsou, K.F.; Ahuja, R.; Ares, J.R.; Bals, S.; Biliškov, N.; Chakraborty, S.; Charalambopoulou, G.; Chaudhary, A.L.; Cuevas, F.; et al. Nanostructured materials for solid-state hydrogen storage: A review of the achievement of COST Action MP1103. Int. J. Hydrog. Energy 2016, 41, 14404–14428. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.-w. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Hirscher, M. Handbook of Hydrogen Storage; Hirscher, M., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; ISBN 9783527629800. [Google Scholar]
- Zuttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Miwa, K.; Ohba, N.; Towata, S.I.; Nakamori, Y.; Orimo, S.I. First-principles study on lithium borohydride LiBH4. Phys. Rev. B Condens. Matter Mater. Phys. 2004, 69, 245120. [Google Scholar] [CrossRef]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.N.; Züttel, A. Stability and Reversibility of LiBH 4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Al, E. Complex hydrides for energy storage. Int. J. Hydrog. Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives-synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef]
- Callini, E.; Özlem Kocabas Atakli, Z.; Hauback, B.C.; Orimo, S.-I.; Jensen, C.; Dornheim, M.; Grant, D.; Cho, Y.W.; Chen, P.; Hjörvarsson, B.; et al. Complex and liquid hydrides for energy storage. Appl. Phys. A 2016, 122, 1–22. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.; Jensen, T.R. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Nickels, E.A.; Jones, M.O.; David, W.I.F.; Johnson, S.R.; Lowton, R.L.; Sommariva, M.; Edwards, P.P. Tuning the decomposition temperature in complex hydrides: Synthesis of a mixed alkali metal borohydride. Angew. Chemie Int. Ed. 2008, 47, 2817–2819. [Google Scholar] [CrossRef]
- Nakamori, Y.; Orimo, S.I. Destabilization of Li-based complex hydrides. J. Alloys Compd. 2004, 370, 271–275. [Google Scholar] [CrossRef]
- Paskevicius, M.; Ley, M.B.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Eutectic melting in metal borohydrides. Phys. Chem. Chem. Phys. 2013, 15, 19774–19789. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Kaewsuwan, D. Effects of specific surface area and pore volume of activated carbon nanofibers on nanoconfinement and dehydrogenation of LiBH4. Int. J. Hydrog. Energy 2017, 42, 6189–6201. [Google Scholar] [CrossRef]
- Sofianos, M.V.; Chaudhary, A.; Paskevicius, M.; Sheppard, D.A.; Humphries, T.D.; Dornheim, M.; Buckley, C.E. Hydrogen storage properties of eutectic metal borohydrides melt-infiltrated into porous Al scaffolds. J. Alloys Compd. 2019, 775, 474–480. [Google Scholar] [CrossRef]
- Ley, M.B.; Roedern, E.; Jensen, T.R. Eutectic melting of LiBH4-KBH4. Phys. Chem. Chem. Phys. 2014, 16, 24194–24199. [Google Scholar] [CrossRef]
- Roedern, E.; Hansen, B.R.S.; Ley, M.B.; Jensen, T.R. Effect of Eutectic Melting, Reactive Hydride Composites, and Nanoconfinement on Decomposition and Reversibility of LiBH4-KBH4. J. Phys. Chem. C 2015, 119, 25818–25825. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Adelhelm, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Shao, J.; Xiao, X.; Fan, X.; Huang, X.; Zhai, B.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Enhanced hydrogen storage capacity and reversibility of LiBH4 nanoconfined in the densified zeolite-templated carbon with high mechanical stability. Nano Energy 2015, 15, 244–255. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Chen, X.; Fang, F.; Sun, D.; Li, X.; Guo, Z.; Yu, X. Oxygen-free Layer-by-Layer Assembly of Lithiated Composites on Graphene for Advanced Hydrogen Storage. Adv. Sci. 2017, 4, 1600257. [Google Scholar] [CrossRef]
- Hoang, K.; Van De Walle, C.G. Mechanism for the decomposition of lithium borohydride. Int. J. Hydrog. Energy 2012, 37, 5825–5832. [Google Scholar] [CrossRef]
- Friedrichs, O.; Remhof, A.; Hwang, S.J.; Zuttel, A. Role of Li2B12H12 for the Formation and Decomposition of LiBH4. Chem. Mater. 2010, 22, 3265–3268. [Google Scholar] [CrossRef]
- Hwang, S.; Bowman, R.C.; Reiter, J.J.W.; Rijssenbeek, J.; Soloveichik, G.L.; Zhao, J.; Kabbour, H.; Ahn, C.C. NMR Confirmation for Formation of [B12H12]2-Complexes during Hydrogen Desorption from Metal Borohydrides. J. Phys. Chem. C 2008, 112, 3164–3169. [Google Scholar] [CrossRef]
- Surrey, A.; Bonatto Minella, C.; Fechler, N.; Antonietti, M.; Grafe, H.; Schultz, L. Improved hydrogen storage properties of LiBH4 via nanoconfinement in micro- and mesoporous aerogel-like carbon. Int. J. Hydrog. Energy 2016, 41, 5540–5548. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Lin, H.; Liu, Y.; Zhang, Y.; Li, S. Metal Hydride Nanoparticles with Ultrahigh Structural Stability and Hydrogen Storage Activity Derived from Microencapsulated Nanoconfinement. Adv. Mater. 2017, 29, 1–6. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Ares-Fernández, J.-R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Guo, Z.X.; Aguey-zinsou, K.F. Effects of different carbon materials on MgH2 decomposition. Carbon N. Y. 2008, 46, 126–137. [Google Scholar]
- Adelhelm, P.; Jongh, P.E. De The impact of carbon materials on the hydrogen storage properties of light metal hydrides. J. Mater. Chem. 2011, 21, 2417–2427. [Google Scholar] [CrossRef]
- Xia, G.; Tan, Y.; Chen, X.; Sun, D.; Guo, Z.; Liu, H.; Ouyang, L.; Zhu, M.; Yu, X. Monodisperse Magnesium Hydride Nanoparticles Uniformly Self-Assembled on Graphene. Adv. Mater. 2015, 27, 5981–5988. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of new, nanoporous carbon with hexagonally ordered mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M.; et al. Mechanochemistry of Metal Hydrides: Recent Advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Sholl, D.S. Crystal Structures and Thermodynamic Investigations of LiK(BH4)2, KBH4, and NaBH4 from First-Principles Calculations. J. Phys. Chem. C 2010, 114, 678–686. [Google Scholar] [CrossRef]
- Sartori, S.; Knudsen, K.D.; Zhao-Karger, Z.; Bardaij, E.G.; Fichtner, M.; Hauback, B.C. Small-angle scattering investigations of Mg-borohydride infiltrated in activated carbon. Nanotechnology 2009, 20, 505702. [Google Scholar] [CrossRef] [PubMed]
- Ampoumogli, A.; Charalambopoulou, G.; Javadian, P.; Richter, B.; Jensen, T.R.; Steriotis, T. Hydrogen desorption and cycling properties of composites based on mesoporous carbons and a LiBH4–Ca(BH4)2 eutectic mixture. J. Alloys Compd. 2015, 645, S480–S484. [Google Scholar] [CrossRef]
- Liu, X.; Peaslee, D.; Jost, C.Z.; Baumann, T.F.; Majzoub, E.H. Systematic pore-size effects of nanoconfinement of LiBH4: Elimination of diborane release and tunable behavior for hydrogen storage applications. Chem. Mater. 2011, 23, 1331–1336. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peru, F.; Payandeh, S.; Charalambopoulou, G.; Jensen, T.R.; Steriotis, T. Hydrogen Sorption and Reversibility of the LiBH4-KBH4 Eutectic System Confined in a CMK-3 Type Carbon via Melt Infiltration. C 2020, 6, 19. https://doi.org/10.3390/c6020019
Peru F, Payandeh S, Charalambopoulou G, Jensen TR, Steriotis T. Hydrogen Sorption and Reversibility of the LiBH4-KBH4 Eutectic System Confined in a CMK-3 Type Carbon via Melt Infiltration. C. 2020; 6(2):19. https://doi.org/10.3390/c6020019
Chicago/Turabian StylePeru, Filippo, SeyedHosein Payandeh, Georgia Charalambopoulou, Torben R. Jensen, and Theodore Steriotis. 2020. "Hydrogen Sorption and Reversibility of the LiBH4-KBH4 Eutectic System Confined in a CMK-3 Type Carbon via Melt Infiltration" C 6, no. 2: 19. https://doi.org/10.3390/c6020019
APA StylePeru, F., Payandeh, S., Charalambopoulou, G., Jensen, T. R., & Steriotis, T. (2020). Hydrogen Sorption and Reversibility of the LiBH4-KBH4 Eutectic System Confined in a CMK-3 Type Carbon via Melt Infiltration. C, 6(2), 19. https://doi.org/10.3390/c6020019





