-
 Exploring DNA Nanostructures as Surface Engineering Techniques for Optimizing Nucleic Acid Biosensor Performance
Exploring DNA Nanostructures as Surface Engineering Techniques for Optimizing Nucleic Acid Biosensor Performance -
 A New Hope for All-Diamond Electrodes? The Integrated Double Diamond Electrode
A New Hope for All-Diamond Electrodes? The Integrated Double Diamond Electrode -
 Electrochemical and Redox Strategies for the Synthesis of Catecholamine- and Dihydroxynaphthalene-Based Materials: A Comparative Review
Electrochemical and Redox Strategies for the Synthesis of Catecholamine- and Dihydroxynaphthalene-Based Materials: A Comparative Review
Journal Description
Electrochem
Electrochem
is an international, peer-reviewed, open access journal on electrochemistry published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, CAPlus / SciFinder, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 20.7 days after submission; acceptance to publication is undertaken in 3.8 days (median values for papers published in this journal in the second half of 2025).
- Journal Rank: CiteScore - Q1 (Materials Chemistry)
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Journal Cluster of Chemical Reactions and Catalysis: Catalysts, Chemistry, Electrochem, Inorganics, Molecules, Organics, Oxygen, Photochem, Reactions, Sustainable Chemistry.
Latest Articles
Yttrium-Enhanced Passive Films in Austenitic Stainless Steel
Electrochem 2026, 7(1), 3; https://doi.org/10.3390/electrochem7010003 - 16 Jan 2026
Abstract
►
Show Figures
It has been demonstrated that a monomolecular surface film with semiconducting characteristics forms on an austenitic, corrosion- and heat-resistant chromium–nickel steel with 0.10 wt.% C, 20 wt.% Cr, 9 wt.% Ni, and 6 wt.% Mn (10Kh20N9G6), microalloyed with yttrium, in aqueous 1 M
[...] Read more.
It has been demonstrated that a monomolecular surface film with semiconducting characteristics forms on an austenitic, corrosion- and heat-resistant chromium–nickel steel with 0.10 wt.% C, 20 wt.% Cr, 9 wt.% Ni, and 6 wt.% Mn (10Kh20N9G6), microalloyed with yttrium, in aqueous 1 M H2SO4. This passive layer exhibits semiconducting behavior, as confirmed by electrochemical impedance and capacitance measurements. For the first time, key electronic parameters, including the flat-band potential, the thickness of the semiconductor layer, and the Fermi energy, have been determined from experimental Mott–Schottky plots obtained for the interphase boundary between the yttrium-microalloyed austenitic Cr–Ni steel (10Kh20N9G6) and aqueous 1 M H2SO4. The results reveal a systematic shift in the flat-band potential toward more negative values with increasing yttrium content in the alloy, indicating a modification of the electronic structure of the passive film. Simultaneously, a decrease in the Fermi energy is observed, suggesting an increase in the work function of the metal surface due to the presence of yttrium. These findings contribute to a deeper understanding of passivation mechanisms in yttrium-containing stainless steels. The formation of a semiconducting passive film is essential for enhancing the electrochemical stability of stainless steels, and the role of rare-earth microalloying elements, such as yttrium, in this process is of both fundamental and practical interest.
Full article
Open AccessArticle
Ageing and Water Detection in Hydroscopic Organic Electrolytes
by
Eva Alonso-Muñoz, Janwa El Maiss, Wejdene Gongi, Divya Balakrishnan, Delphine Faye, Karine Mougin and César Pascual García
Electrochem 2026, 7(1), 2; https://doi.org/10.3390/electrochem7010002 - 16 Jan 2026
Abstract
Electrolyte degradation and trace water contamination critically affect the lifetime and safety of lithium-ion batteries. In organic-based electrolytes such as acetonitrile (MeCN), even small amounts of water can trigger
Electrolyte degradation and trace water contamination critically affect the lifetime and safety of lithium-ion batteries. In organic-based electrolytes such as acetonitrile (MeCN), even small amounts of water can trigger
(This article belongs to the Special Issue Feature Papers in Electrochemistry)
►▼
Show Figures

Figure 1
Open AccessArticle
Synthesis and Structural Characterization of Ni/Mn-Doped Co-RGO Composites for Supercapacitor Electrodes
by
Andriono Manalu, Moraida Hasanah, Winfrontstein Naibaho, Mario Geraldi Simanjuntak and Maren Sius Girsang
Electrochem 2026, 7(1), 1; https://doi.org/10.3390/electrochem7010001 - 24 Dec 2025
Abstract
►▼
Show Figures
In this study, Ni/Mn-doped cobalt–reduced graphene oxide (Co-RGO) composites were successfully synthesized as advanced electrode materials for supercapacitors. The structural and morphological properties of the composites were characterized using FTIR, XRD, SEM, TEM, and UV–Vis spectroscopy. Their electrochemical performance was evaluated through electrochemical
[...] Read more.
In this study, Ni/Mn-doped cobalt–reduced graphene oxide (Co-RGO) composites were successfully synthesized as advanced electrode materials for supercapacitors. The structural and morphological properties of the composites were characterized using FTIR, XRD, SEM, TEM, and UV–Vis spectroscopy. Their electrochemical performance was evaluated through electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), and galvanostatic charge–discharge (GCD). Among the prepared samples, Co-RGO doped with Ni/Mn at a 40:10 ratio exhibited the most outstanding capacitive behavior, achieving a specific capacitance of 7414 F g−1 at a current density of 10 A g−1, along with a high energy density of 565 Wh kg−1 and a power density of 4998 W kg−1. The high capacitance arises from faradaic pseudocapacitive reactions rather than electric double-layer capacitance, eliminating the need for a large surface area. These results confirm that Ni doping significantly enhances pseudocapacitance and conductivity in the Co-RGO matrix, making Ni/Mn (40:10)–Co-RGO a potential material for advanced energy storage systems.
Full article

Graphical abstract
Open AccessArticle
Experimental-Based Optimal Parameter Extraction for PEM Fuel Cell Semi-Empirical Model Using the Cloud Drift Optimization Algorithm
by
Mohamed A. El-Hameed, Mahmoud M. Elkholy, Mahfouz Saeed, Adnan Kabbani, Essa Al-Hajri and Mohammed Jufaili
Electrochem 2025, 6(4), 45; https://doi.org/10.3390/electrochem6040045 - 17 Dec 2025
Abstract
►▼
Show Figures
Accurate modeling of proton exchange membrane fuel cells (PEMFCs) is essential for predicting system performance under diverse operating conditions. This study introduces a refined semi-empirical modeling that combines experimental validation with an enhanced parameter estimation method based on the Cloud Drift Optimization (CDO)
[...] Read more.
Accurate modeling of proton exchange membrane fuel cells (PEMFCs) is essential for predicting system performance under diverse operating conditions. This study introduces a refined semi-empirical modeling that combines experimental validation with an enhanced parameter estimation method based on the Cloud Drift Optimization (CDO) algorithm. The approach focuses on identifying seven key parameters of the nonlinear PEMFC model by minimizing the difference between experimentally measured and simulated cell voltages. To assess its effectiveness, the proposed CDO-based estimator was compared with several established metaheuristic algorithms, including the particle swarm optimizer and the tetragonula carbonaria optimization algorithm. The evaluation was performed using three commercial PEMFC stacks rated at 250 W, 500 W, and the NedStack PS6, as well as experimental data obtained from the Renewable Energy Laboratory at A’Sharqiyah University. Results demonstrate that the CDO algorithm consistently produced the lowest sum of squared errors (SSE) of 1.0337 and exhibited stable convergence across multiple independent runs with a standard deviation of 1.2114 × 10−7. Its reliable performance under both normal and degraded conditions confirms the algorithm’s robustness and adaptability, establishing CDO as an efficient and dependable technique for PEMFC modeling and parameter identification.
Full article
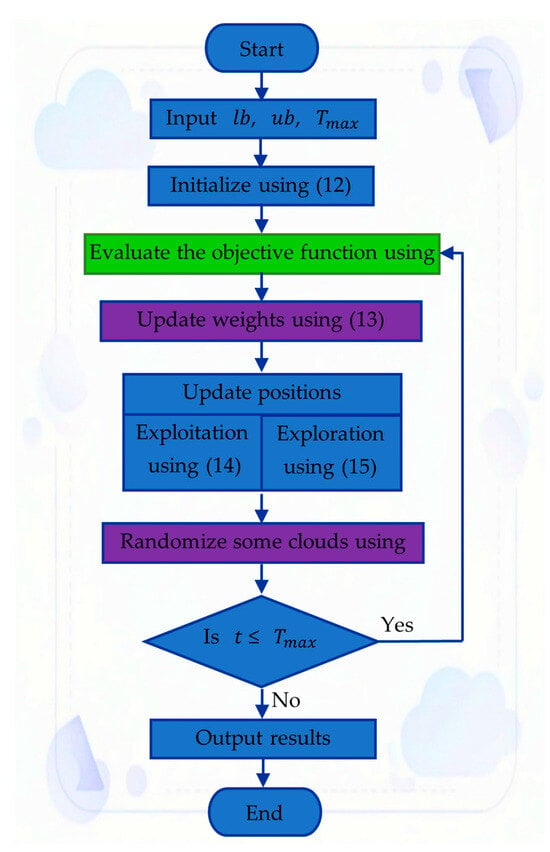
Figure 1
Open AccessReview
A Review of Recent Advances in Multivalent Ion Batteries for Next Generation Energy Storage
by
Raj Shah, Kate Marussich and Vikram Mittal
Electrochem 2025, 6(4), 44; https://doi.org/10.3390/electrochem6040044 - 10 Dec 2025
Cited by 1
Abstract
►▼
Show Figures
As demand for high-performance energy storage grows across grid and mobility sectors, multivalent ion batteries (MVIBs) have emerged as promising alternatives to lithium-based systems due to their potential for higher volumetric energy density and material abundance. This review comprehensively examines recent breakthroughs in
[...] Read more.
As demand for high-performance energy storage grows across grid and mobility sectors, multivalent ion batteries (MVIBs) have emerged as promising alternatives to lithium-based systems due to their potential for higher volumetric energy density and material abundance. This review comprehensively examines recent breakthroughs in magnesium, zinc, aluminum, and calcium-based battery chemistries, with a focus on overcoming barriers related to slow ion transport, limited reversibility, and electrode degradation. Advances in aqueous and non-aqueous electrolyte formulations, including solvation shell engineering, interfacial passivation, and dual-zone ion transport, are discussed for their role in improving compatibility and cycling stability. Particular focus is placed on three high-impact innovations: solvation-optimized Mg-ion systems for improved mobility and retention, interface-engineered Zn-ion batteries enabling dendrite-free operation, and sustainable Al-ion technologies targeting grid-scale deployment with eco-friendly electrolytes and recyclable materials. Cross-cutting insights from operando characterization techniques and AI-guided materials discovery are also evaluated for their role in accelerating MVIB development. By integrating fundamental materials innovation with practical system design, multivalent ion batteries offer a compelling path toward next-generation, safer, and more sustainable energy storage platforms.
Full article

Figure 1
Open AccessArticle
Electrochemical Behavior of Yttrium–Magnesium Intermediate Alloy Preparation Process by Molten Salt Electrolysis
by
Wenchang Shu, Fang Zhang, Jun Peng, Quanjun Zhang, Yubao Liu and Baige Sun
Electrochem 2025, 6(4), 43; https://doi.org/10.3390/electrochem6040043 - 4 Dec 2025
Abstract
►▼
Show Figures
Yttrium–magnesium alloys are commonly employed as processing additives in magnesium alloy materials. Incorporating yttrium into magnesium alloys via Y-Mg intermediate alloys not only minimizes oxidation and burn-off loss but also simplifies operational procedures. Utilizing yttrium–magnesium alloys ensures a stable composition and reliable quality
[...] Read more.
Yttrium–magnesium alloys are commonly employed as processing additives in magnesium alloy materials. Incorporating yttrium into magnesium alloys via Y-Mg intermediate alloys not only minimizes oxidation and burn-off loss but also simplifies operational procedures. Utilizing yttrium–magnesium alloys ensures a stable composition and reliable quality of magnesium alloy products, while contributing to reduced production costs and minimized environmental pollution. In this study, a molten salt co-reduction method was developed for the preparation Y-Mg intermediate alloys. The electrochemical co-reduction behaviors of Y(III) and Mg(II), as well as the transient states of Y-Mg intermediate alloys, were systematically investigated by transient electrochemical techniques. Results indicated that the reduction of Y(III) at the molybdenum (Mo) cathode is a reversible electrochemical process, whereas the reduction of Mg(II) is irreversible and diffusion-controlled. The diffusion coefficient of Y(III) and Mg(II) in the fluoride salt at 1000 °C were determined to be 3.98 × 10−5 cm2/s and 1.16 × 10−3 cm2/s, respectively. Electrochemical calculations revealed that the reduction of Y(III) involves a single-step transfer of three electrons, while Mg(II) involves a single-step transfer of two electrons. The corresponding electrode reactions are Y(III) + 3e−→Y and Mg(II) + 2e−→Mg, respectively. A Y-Mg alloy sample prepared by constant-current molten salt electrolysis primarily consists of the MgY phase with a composition of 88.38 wt% yttrium and 11.62 wt% magnesium.
Full article

Figure 1
Open AccessArticle
Influence of a Plasma Nitriding Treatment on the Corrosion Behavior of API 5L X70 Steel in Simulated Soil Solution
by
O. A. González Noriega, A. Flores Nicolás, J. Uruchurtu Chavarín, A. Torres Islas, E. C. Menchaca Campos and H. Martínez Valencia
Electrochem 2025, 6(4), 42; https://doi.org/10.3390/electrochem6040042 - 27 Nov 2025
Abstract
In this work, plasma nitriding was carried out to improve the corrosion resistance of API 5L X70 steel. The process was conducted at different treatment times, 4, 6, 8, and 10 h, to determine which one provides greater resistance to corrosion. The conditions
[...] Read more.
In this work, plasma nitriding was carried out to improve the corrosion resistance of API 5L X70 steel. The process was conducted at different treatment times, 4, 6, 8, and 10 h, to determine which one provides greater resistance to corrosion. The conditions under which the nitriding was carried out were as follows: a mixture of 20% N2 and 80% H2 at 3 torr pressure, a current of 2.6 × 10−6 A, a voltage of 360 V, and the temperature inside the plasma chamber was 550 °C. The blank and nitrided materials were characterized using dispersive energy spectroscopy and scanning microscopy to study their morphology and chemical composition. In addition, open potential circuit, electrochemical impedance spectroscopy, and potentiodynamic polarization curves in simulated soil solution were performed to evaluate the materials’ corrosion resistance. The treatment achieved at 10 h presented the greatest corrosion resistance, reducing the corrosion current density up to three orders of magnitude. The thickness reached 678.75 µm for this condition.
Full article
(This article belongs to the Topic Advances in Chemistry and Chemical Engineering, 2nd Edition)
►▼
Show Figures

Figure 1
Open AccessArticle
A New Hope for All-Diamond Electrodes? The Interdigitated Double Diamond Electrode
by
Manuel Zulla, Carolin Messerschmidt, Hanadi Ghanem, Johannes Bähr, Lukas Hegemann and Stefan Rosiwal
Electrochem 2025, 6(4), 41; https://doi.org/10.3390/electrochem6040041 - 22 Nov 2025
Abstract
Nowadays, the development of efficient water treatment processes is increasingly driven by the need to provide solutions for contaminants of emerging concern. Electrochemical advanced oxidation processes (EAOPs) based on diamond electrodes can be part of innovative removal concepts. However, expensive substrates, energy-intensive chemical
[...] Read more.
Nowadays, the development of efficient water treatment processes is increasingly driven by the need to provide solutions for contaminants of emerging concern. Electrochemical advanced oxidation processes (EAOPs) based on diamond electrodes can be part of innovative removal concepts. However, expensive substrates, energy-intensive chemical vapor deposition (CVD) of diamond, and market availability complicate matters for diamond electrodes to gain traction in the water treatment sector. In addition, it has to be stated that the mining and complex processing of necessary substrates like Si, Ti, Nb, or Ta need a significant amount of fresh water, which counteracts the need for more sustainability in the field of EAOPs. In this context, a ceramic-based boron-doped diamond (BDD) electrode is presented, which addresses this dilemma. The presented concept of the so-called interdigitated double diamond electrode (iDDE) consumes 14–46% less energy in batch-mode experiments to degrade an organic model molecule compared to standard BDD technology in a poorly conductive electrolyte (κ < 350 µS/cm). Laser-induced micro-structuring of the BDD layer reduces the interelectrode spacing (IES) of the iDDE to below 50 µm. The structuring approach at the micrometer scale enables the treatment of electrically low-conductivity electrolytes more energy efficiently, while reducing the need for a supporting electrolyte or a proton exchange membrane. Degradation experiments and Raman measurements reveal different properties of an iDDE compared to standard BDD technology. The iDDE concept highlights the need to understand the significance of non-uniform current density distributions on the general electrochemical activity of BDD electrodes.
Full article
(This article belongs to the Special Issue Feature Papers in Electrochemistry)
►▼
Show Figures

Graphical abstract
Open AccessReview
Exploring DNA Nanostructures as Surface Engineering Techniques for Optimizing Nucleic Acid Biosensor Performance
by
Kepler Pyle, Naz Savranoğlu, Selin Naz Avdan and Soha Ahmadi
Electrochem 2025, 6(4), 40; https://doi.org/10.3390/electrochem6040040 - 20 Nov 2025
Abstract
►▼
Show Figures
Surface modification of nucleic acid-based electrochemical biosensors has been at the forefront of research since their inception. Effective modification ensures the optimization of the sensitivity, specificity, and stability of modern biosensors. Recent advances in DNA nanotechnology have enabled the development of novel electrochemical
[...] Read more.
Surface modification of nucleic acid-based electrochemical biosensors has been at the forefront of research since their inception. Effective modification ensures the optimization of the sensitivity, specificity, and stability of modern biosensors. Recent advances in DNA nanotechnology have enabled the development of novel electrochemical biosensor interfaces with precise assembly and high biocompatibility. In this review, we explore three strategies for enhancing biosensor performance: the integration of tetrahedral DNA nanostructures (TDNs), self-assembled monolayers (SAMs), and DNA-based hydrogels. TDNs offer well-defined geometry and controlled spatial presentation of capture probes, significantly reducing background noise and improving target accessibility. SAMs provide a robust and tunable platform for anchoring these nanostructures, enabling reproducible and chemically stable interfaces. DNA hydrogels serve as a responsive and flexible scaffold capable of signal amplification and analyte retention. These surface architectures enhance sensitivity and minimize non-specific adsorption (NSA). We discuss recent applications and experimental outcomes, highlighting how each component is driving the next generation of nucleic acid-based biosensors.
Full article

Figure 1
Open AccessArticle
Defect Engineering and Na-Ion Transport in NaMnPO4: A Computational Perspective
by
G. M. P. Dananjana Galappaththi, Poobalasingam Abiman, Poobalasuntharam Iyngaran and Navaratnarajah Kuganathan
Electrochem 2025, 6(4), 39; https://doi.org/10.3390/electrochem6040039 - 10 Nov 2025
Abstract
►▼
Show Figures
Rechargeable sodium-ion batteries (SIBs) have attracted considerable attention owing to the natural abundance and accessibility of sodium. Maricite NaMnPO4, a phosphate-based cathode material with high theoretical capacity, suffers from blocked sodium-ion diffusion channels. In this study, atomistic simulations using pair potentials
[...] Read more.
Rechargeable sodium-ion batteries (SIBs) have attracted considerable attention owing to the natural abundance and accessibility of sodium. Maricite NaMnPO4, a phosphate-based cathode material with high theoretical capacity, suffers from blocked sodium-ion diffusion channels. In this study, atomistic simulations using pair potentials and density functional theory (DFT) are employed to investigate intrinsic defect mechanisms, sodium-ion migration pathways, and the role of dopant incorporation at Na, Mn, and P sites in generating Na vacancies and interstitials. Among the intrinsic defects, the Na–Mn anti-site cluster emerges as the most favorable, exhibiting a very low formation energy of 0.12 eV, while the Na Frenkel pair (1.93 eV) is the next most stable defect, indicating that sodium diffusion is primarily facilitated by vacancy formation. Nevertheless, sodium-ion mobility in NaMnPO4 remains limited, as reflected by the relatively high migration activation energy of 1.28 eV. Among the isovalent substitutions, K is predicted to be the most favorable dopant at the Na site, whereas Ca and Cu are the most favorable at the Mn site. Thallium is identified as a promising dopant at the Mn site for generating Na vacancies that facilitate Na-ion migration, while Ge substitution at the P site is predicted to enhance the sodium content in the material.
Full article

Figure 1
Open AccessReview
Water Management Strategies for Proton Exchange Membrane Fuel Cells: A Comprehensive Review
by
Mahfouz Saeed, Mohamed A. El-Hameed, Essa Al-Hajri and Adnan Kabbani
Electrochem 2025, 6(4), 38; https://doi.org/10.3390/electrochem6040038 - 27 Oct 2025
Abstract
►▼
Show Figures
Proton exchange membrane fuel cells (PEMFCs) are a promising clean energy technology due to their zero gas emissions, low operating temperature, and high efficiency. This review synthesizes research from 2015–2025 on (i) materials-level approaches (advanced/modified PFSA membranes and composite membranes) that improve water
[...] Read more.
Proton exchange membrane fuel cells (PEMFCs) are a promising clean energy technology due to their zero gas emissions, low operating temperature, and high efficiency. This review synthesizes research from 2015–2025 on (i) materials-level approaches (advanced/modified PFSA membranes and composite membranes) that improve water retention and ionic conduction, (ii) engineered gas diffusion layers and hydrophobic/hydrophilic gradients (including Janus and asymmetric GDL architectures) that facilitate directional water transport and have been shown to increase peak power density in some reports (e.g., from ≈1.17 to ≈1.89 W·cm−2 with Janus GDL designs), (iii) flow-field design strategies. This review examines the key aspects of water management in PEMFCs, including their impact on cell performance, the underlying causes of related issues, and the mechanisms of water transport within these cells. Additionally, it discusses the methods and materials used to enhance water management, highlighting recent advancements and potential directions for future research. Topics such as water transport, water flooding, and water control strategies in PEMFCs are also addressed. Both excess water (flooding) and water depletion (dehydration) can negatively influence fuel cell performance and lifespan. Particular attention is given to water dehydration, with a detailed discussion of its effects on the cathode, Anode, gas diffusion layer, catalyst layer, and flow channels.
Full article

Figure 1
Open AccessReview
Metal–Organic Frameworks for Seawater Electrolysis and Hydrogen Production: A Review
by
Ivelina Tsacheva, Mehmet Suha Yazici, Abdul Hanif Mahadi, Aytekin Uzunoglu and Dzhamal Uzun
Electrochem 2025, 6(4), 37; https://doi.org/10.3390/electrochem6040037 - 20 Oct 2025
Abstract
►▼
Show Figures
Electrolysis utilizing renewable electricity is an environmentally friendly, non-polluting, and sustainable method of hydrogen production. Seawater is the most desirable and inexpensive electrolyte for this process to achieve commercial acceptance compared to competing hydrogen production technologies. We reviewed metal–organic frameworks as possible electrocatalysts
[...] Read more.
Electrolysis utilizing renewable electricity is an environmentally friendly, non-polluting, and sustainable method of hydrogen production. Seawater is the most desirable and inexpensive electrolyte for this process to achieve commercial acceptance compared to competing hydrogen production technologies. We reviewed metal–organic frameworks as possible electrocatalysts for hydrogen production by seawater electrolysis. Metal–organic frameworks are interesting for seawater electrolysis due to their large surface area, tunable permeability, and ease of functional processing, which makes them extremely suitable for obtaining modifiable electrode structures. Here we discussed the development of metal–organic framework-based electrocatalysts as multifunctional materials with applications for alkaline, PEM, and direct seawater electrolysis for hydrogen production. Their advantages and disadvantages were examined in search of a pathway to a successful and sustainable technology for developing electrode materials to produce hydrogen from seawater.
Full article

Graphical abstract
Open AccessReview
Electrochemical and Redox Strategies for the Synthesis of Catecholamine- and Dihydroxynaphthalene-Based Materials: A Comparative Review
by
Chloé Laporte and Vincent Ball
Electrochem 2025, 6(4), 36; https://doi.org/10.3390/electrochem6040036 - 18 Oct 2025
Abstract
►▼
Show Figures
Melanins are multifunctional biopolymers with unique properties, ranging from UV and radiation protection to antioxidant activity and metal chelation, making them highly attractive for biomedical applications. Despite extensive research, the mechanisms underlying melanin formation remain only partially understood, and access to these biopolymers
[...] Read more.
Melanins are multifunctional biopolymers with unique properties, ranging from UV and radiation protection to antioxidant activity and metal chelation, making them highly attractive for biomedical applications. Despite extensive research, the mechanisms underlying melanin formation remain only partially understood, and access to these biopolymers therefore relies on suitable molecular precursors. While most studies have focused on catecholamine-derived eumelanins such as 3,4-dihydroxyphenylalanine (L-DOPA) and dihydroxyindole (DHI), nitrogen-free precursors such as 1,8-dihydroxynaphthalene (1,8-DHN) are emerging as promising routes to allomelanins. To date, however, these two precursor classes have largely been investigated separately, limiting a broader understanding of structure–function relationships. This review aims to compare electrochemical and redox-based pathways to catecholamine- and DHN-derived materials, emphasizing both their common principles and distinctive features. By bridging these parallel research streams, we propose a methodological framework for guiding future research on melanin-inspired materials and bioelectrochemical technologies.
Full article

Figure 1
Open AccessArticle
Analytical–Computational Integration of Equivalent Circuit Modeling, Hybrid Optimization, and Statistical Validation for Electrochemical Impedance Spectroscopy
by
Francisco Augusto Nuñez Perez
Electrochem 2025, 6(4), 35; https://doi.org/10.3390/electrochem6040035 - 8 Oct 2025
Abstract
►▼
Show Figures
Background: Electrochemical impedance spectroscopy (EIS) is indispensable for disentangling charge-transfer, capacitive, and diffusive phenomena, yet reproducible parameter estimation and objective model selection remain unsettled. Methods: We derive closed-form impedances and analytical Jacobians for seven equivalent-circuit models (Randles, constant-phase element (CPE), and Warburg impedance
[...] Read more.
Background: Electrochemical impedance spectroscopy (EIS) is indispensable for disentangling charge-transfer, capacitive, and diffusive phenomena, yet reproducible parameter estimation and objective model selection remain unsettled. Methods: We derive closed-form impedances and analytical Jacobians for seven equivalent-circuit models (Randles, constant-phase element (CPE), and Warburg impedance (ZW) variants), enforce physical bounds, and fit synthetic spectra with 2.5% and 5.0% Gaussian noise using hybrid optimization (Differential Evolution (DE) → Levenberg–Marquardt (LM)). Uncertainty is quantified via non-parametric bootstrap; parsimony is assessed with root-mean-square error (RMSE), Akaike Information Criterion (AIC), and Bayesian Information Criterion (BIC); physical consistency is checked by Kramers–Kronig (KK) diagnostics. Results: Solution resistance (

Graphical abstract
Open AccessArticle
Exploring Binder–Ionic Liquid Electrolyte Systems in Silicon Oxycarbide Negative Electrodes for Lithium-Ion Batteries
by
Ivonne E. Monje, Nedher Sanchez-Ramírez, Laurence Savignac, Pedro H. Camargo, Steen B. Schougaard, Daniel Bélanger and Roberto M. Torresi
Electrochem 2025, 6(3), 34; https://doi.org/10.3390/electrochem6030034 - 12 Sep 2025
Abstract
Enhancing the safety of lithium-ion batteries (LIBs) by replacing flammable electrolytes is a key challenge. Ionic liquid (IL)-based electrolytes are considered an interesting alternative due to their thermal and chemical stability, high voltage stability window, and tunable properties. This study investigates the electrochemical
[...] Read more.
Enhancing the safety of lithium-ion batteries (LIBs) by replacing flammable electrolytes is a key challenge. Ionic liquid (IL)-based electrolytes are considered an interesting alternative due to their thermal and chemical stability, high voltage stability window, and tunable properties. This study investigates the electrochemical behavior of two newly synthesized ILs, comparing them to conventional alkyl carbonate-based electrolytes. Nitrogen-doped carbon silicon oxycarbide (NC-SiOC), used as the active material in negative electrodes, was combined with two polymeric binders: poly(acrylic acid) (PAA) and poly(acrylonitrile) (PAN). NC-SiOC/PAN electrodes exhibited a significantly higher initial charge capacity—approximately 25–30% greater than their PAA-based counterparts in the first cycle at 0.1 A g−1 (850–990 mAh g−1 vs. 600–700 mAh g−1), and demonstrated an improved initial Coulombic efficiency (67% vs. 62%). Long-term cycling stability over 1000 cycles at 1.6 A g−1 retained 75–80% of the initial 0.1 A g−1 capacity. This outstanding performance is attributed to the synergistic effects of nitrogen-rich carbonaceous phases within the NC-SiOC material and the cyclized-PAN binder, which facilitate structural stability by accommodating volumetric changes and enhancing solid electrolyte interphase (SEI) stability. Notably, despite the lower ionic transport properties of the IL electrolytes, their incorporation did not compromise performance, supporting their feasibility as safer electrolyte alternatives. These findings offer one of the most promising electrochemical performances reported for SiOC materials to date.
Full article
(This article belongs to the Special Issue Silicon Electrochemistry: Fundamentals and Modern Applications)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Estimation of Lead Acid Battery Degradation—A Model for the Optimization of Battery Energy Storage System Using Machine Learning
by
Arief S. Budiman, Rayya Fajarna, Muhammad Asrol, Fitya Syarifa Mozar, Christian Harito, Bens Pardamean, Derrick Speaks and Endang Djuana
Electrochem 2025, 6(3), 33; https://doi.org/10.3390/electrochem6030033 - 5 Sep 2025
Cited by 1
Abstract
►▼
Show Figures
Energy storage systems are becoming increasingly important as more renewable energy systems are integrated into the electrical (or power utility) grid. Low-cost and reliable energy storage is paramount if renewable energy systems are to be increasingly integrated into the power grid. Lead-acid batteries
[...] Read more.
Energy storage systems are becoming increasingly important as more renewable energy systems are integrated into the electrical (or power utility) grid. Low-cost and reliable energy storage is paramount if renewable energy systems are to be increasingly integrated into the power grid. Lead-acid batteries are widely used as energy storage for stationary renewable energy systems and agriculture due to their low cost, especially compared to lithium-ion batteries (LIB). However, lead-acid battery technology suffers from system degradation and a relatively short lifetime, largely due to its charging/discharging cycles. In the present study, we use Machine Learning methodology to estimate the battery degradation in an energy storage system. It uses two types of datasets: discharge condition and lead acid battery data. In the initial analysis, the Support Vector Regression (SVR) method with the RBF kernel showed poor results, with a low accuracy value of 0.0127 and RMSE 5377. On the other hand, the Long Short-Term Memory (LSTM) method demonstrated better estimation results with an RMSE value of 0.0688, which is relatively close to 0.
Full article

Figure 1
Open AccessArticle
Charge Transfer Rates Controlled by Frequency Dispersion of Double-Layer Capacitances
by
Koichi Jeremiah Aoki and Jingyuan Chen
Electrochem 2025, 6(3), 32; https://doi.org/10.3390/electrochem6030032 - 5 Sep 2025
Cited by 3
Abstract
►▼
Show Figures
Reported rate constants of charge transfer reactions (CTs) have ranged widely, depending on techniques and timescales. This fact can be attributed to the time-dependent double-layer capacitance (DLC), caused by solvent interactions such as hydrogen bonds. The time variation of the DLC necessarily affects
[...] Read more.
Reported rate constants of charge transfer reactions (CTs) have ranged widely, depending on techniques and timescales. This fact can be attributed to the time-dependent double-layer capacitance (DLC), caused by solvent interactions such as hydrogen bonds. The time variation of the DLC necessarily affects the heterogeneous electrode kinetics. The delay by the solvation, being frequency dispersion, is incorporated into the CT kinetics in this report on the basis of the conventional reaction rate equations. It is different from the absolute rate theory. This report insists on a half value of the transfer coefficient owing to the segregation of the electrostatic energy from the chemical one. The rate equation here is akin to the Butler–Volmer one, except for the power law of the time caused by the delay of the DLC. The dipoles orient successively other dipoles in a group associated with the delay, which resembles that in the DLC. The delay suppresses the observed currents in the form of a negative capacitance. The above behavior was examined with a ferrocenyl derivative by ac impedance methods. The delay from diffusion control was attributed to the negative capacitance rather than the CT, even if the conventional DLC effect was corrected.
Full article

Figure 1
Open AccessArticle
Stability of TiO2–Polypyrrole Heterojunctions for Photoelectrochemical Water Oxidation
by
Jhon Puerres, Pablo Ortiz and María T. Cortés
Electrochem 2025, 6(3), 31; https://doi.org/10.3390/electrochem6030031 - 20 Aug 2025
Abstract
►▼
Show Figures
TiO2 composites with polypyrrole have gained attention for various applications; however, some reported results on the suitability of this heterojunction for photoelectrochemical water oxidation do not agree. In this sense, it is relevant to further study this material to clarify the role
[...] Read more.
TiO2 composites with polypyrrole have gained attention for various applications; however, some reported results on the suitability of this heterojunction for photoelectrochemical water oxidation do not agree. In this sense, it is relevant to further study this material to clarify the role of polypyrrole in this system. Here, TiO2 nanorods were grown on fluorine-doped tin oxide (FTO) substrates by a hydrothermal route; then, polypyrrole coatings were electrochemically synthetized on TiO2 nanorods using a galvanostatic signal. The heterojunctions were characterized by different spectroscopic, microscopic, and electrochemical techniques. As a result, it was found that the polypyrrole underwent a rapid degradation process and that this process occurred independently of the amount of polymer deposited on the TiO2, the illumination direction (back and front of the photoanode), and the type of light used (UV-Vis and Vis). In addition, from the measurements of the band positions of TiO2 and the HOMO level of polypyrrole, it was shown that the TiO2–polypyrrole heterojunction is not suitable for achieving the transfer of photogenerated holes to the electrolyte. These findings contribute to understanding the properties and interaction of two components of wide interest in materials science.
Full article

Graphical abstract
Open AccessArticle
Ternary Nickel-Iron-Phosphorus (NiFeP) Electrocatalysts for Alkaline Water Splitting
by
Raminta Šakickaitė, Zita Sukackienė, Virginija Kepenienė, Aldona Balčiūnaitė, Raminta Stagniūnaitė, Gitana Valeckytė and Loreta Tamašauskaitė-Tamašiūnaitė
Electrochem 2025, 6(3), 30; https://doi.org/10.3390/electrochem6030030 - 15 Aug 2025
Abstract
►▼
Show Figures
In this study, ternary NiFeP coatings were fabricated on a copper substrate using a simple, fast, and cost-effective electroless deposition method. The coatings were named Ni85Fe4P12, Ni80Fe8P12, and Ni75Fe
[...] Read more.
In this study, ternary NiFeP coatings were fabricated on a copper substrate using a simple, fast, and cost-effective electroless deposition method. The coatings were named Ni85Fe4P12, Ni80Fe8P12, and Ni75Fe12P12, indicating 4, 8, and 12 at % of Fe, respectively. The surface morphology and composition of the coatings were characterized using scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDX). The activity of the prepared coatings was evaluated using the water-splitting reaction to determine the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in a 1 M KOH electrolyte solution. Electrochemical measurements were carried out in a temperature range from 25 °C to 55 °C. The HER and OER current density values increased by up to 2.58 and 2.13 times, respectively, with temperature increase compared to the result at 25 °C. All three coatings demonstrated activity in both reactions. Ni85Fe4P12 exhibited the highest catalytic efficiency in the HER, with the overpotential of 340 mV at 10 mAcm−2 and a Tafel slope of 61 mVdec−1. In the OER, the efficiency of the NiFeP catalysts correlated with their Fe content. The overpotential was 412 mV for Ni80Fe8P12 and 432 mV for Ni75Fe12P12 at 10 mAcm−2 with Tafel slopes of 96 and 91 mVdec−1, respectively. This study underscores the critical influence of Fe content on the catalytic efficiency of NiFeP coatings, with reduced Fe content enhancing HER and increased Fe content benefits OER.
Full article

Figure 1
Open AccessArticle
Unraveling the Role of Aluminum in Boosting Lithium-Ionic Conductivity of LLZO
by
Md Mozammal Raju, Yi Ding and Qifeng Zhang
Electrochem 2025, 6(3), 29; https://doi.org/10.3390/electrochem6030029 - 4 Aug 2025
Cited by 1
Abstract
►▼
Show Figures
The development of high-performance solid electrolytes is critical to advancing solid-state lithium-ion batteries (SSBs), with lithium lanthanum zirconium oxide (LLZO) emerging as a leading candidate due to its chemical stability and wide electrochemical window. In this study, we systematically investigated the effects of
[...] Read more.
The development of high-performance solid electrolytes is critical to advancing solid-state lithium-ion batteries (SSBs), with lithium lanthanum zirconium oxide (LLZO) emerging as a leading candidate due to its chemical stability and wide electrochemical window. In this study, we systematically investigated the effects of cation dopants, including aluminum (Al3+), tantalum (Ta5+), gallium (Ga3+), and rubidium (Rb+), on the structural, electronic, and ionic transport properties of LLZO using density functional theory (DFT) and ab initio molecular dynamics (AIMD) simulations. It appeared that, among all simulated results, Al-LLZO exhibits the highest ionic conductivity of 1.439 × 10−2 S/cm with reduced activation energy of 0.138 eV, driven by enhanced lithium vacancy concentrations and preserved cubic-phase stability. Ta-LLZO follows, with a conductivity of 7.12 × 10−3 S/cm, while Ga-LLZO and Rb-LLZO provide moderate conductivity of 3.73 × 10−3 S/cm and 3.32 × 10−3 S/cm, respectively. Charge density analysis reveals that Al and Ta dopants facilitate smoother lithium-ion migration by minimizing electrostatic barriers. Furthermore, Al-LLZO demonstrates low electronic conductivity (1.72 × 10−8 S/cm) and favorable binding energy, mitigating dendrite formation risks. Comparative evaluations of radial distribution functions (RDFs) and XRD patterns confirm the structural integrity of doped systems. Overall, Al emerges as the most effective and economically viable dopant, optimizing LLZO for scalable, durable, and high-conductivity solid-state batteries.
Full article

Graphical abstract
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Catalysts, Electrochem, Materials, Molecules, Nanomaterials, Electronic Materials, Reactions
Electrocatalytic Advances for Sustainable Energy
Topic Editors: Yang Liu, Bangwei DengDeadline: 31 December 2026

Special Issues
Special Issue in
Electrochem
Lithium-Ion Battery Second-Life Applications and Recycling
Guest Editors: Qi Zhang, Yunlong Shang, Zhongkai ZhouDeadline: 30 June 2026
Special Issue in
Electrochem
Advanced Electrochemical Materials for Next-Generation High-Performance Batteries
Guest Editor: Tao HuangDeadline: 31 January 2027
Special Issue in
Electrochem
Electrochemically-Mediated Approaches for the Capture, Conversion, and Extraction of Environmentally Relevant Species
Guest Editor: Kyumin JangDeadline: 28 February 2027
Special Issue in
Electrochem
Electrochemical Engineering-Driven Fuel Cell Design: From Fundamentals to Industrial Systems
Guest Editors: Srikanth Ponnada, Anandhan SrinivasanDeadline: 31 March 2027








