Oral Bovine Milk Lactoferrin Administration Suppressed Myopia Development through Matrix Metalloproteinase 2 in a Mouse Model
Abstract
1. Introduction
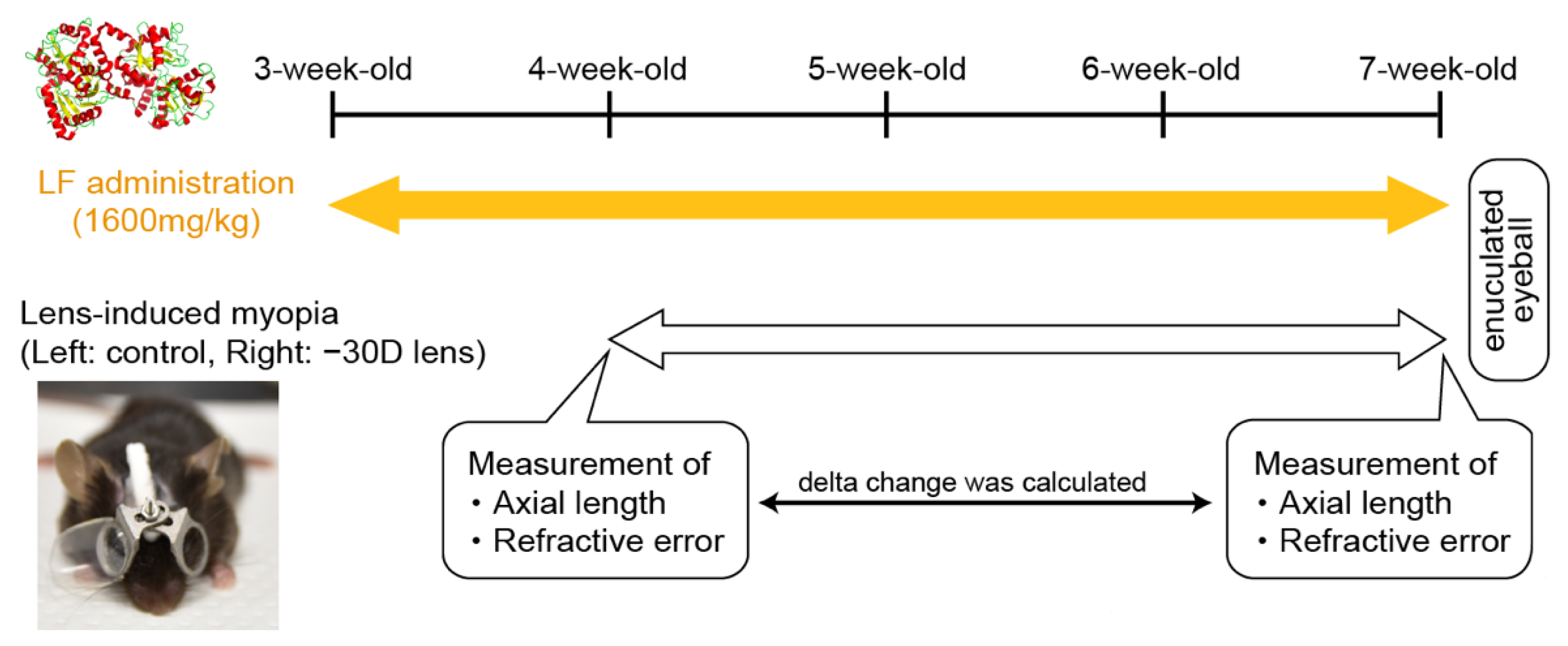
2. Materials and Methods
3. Results
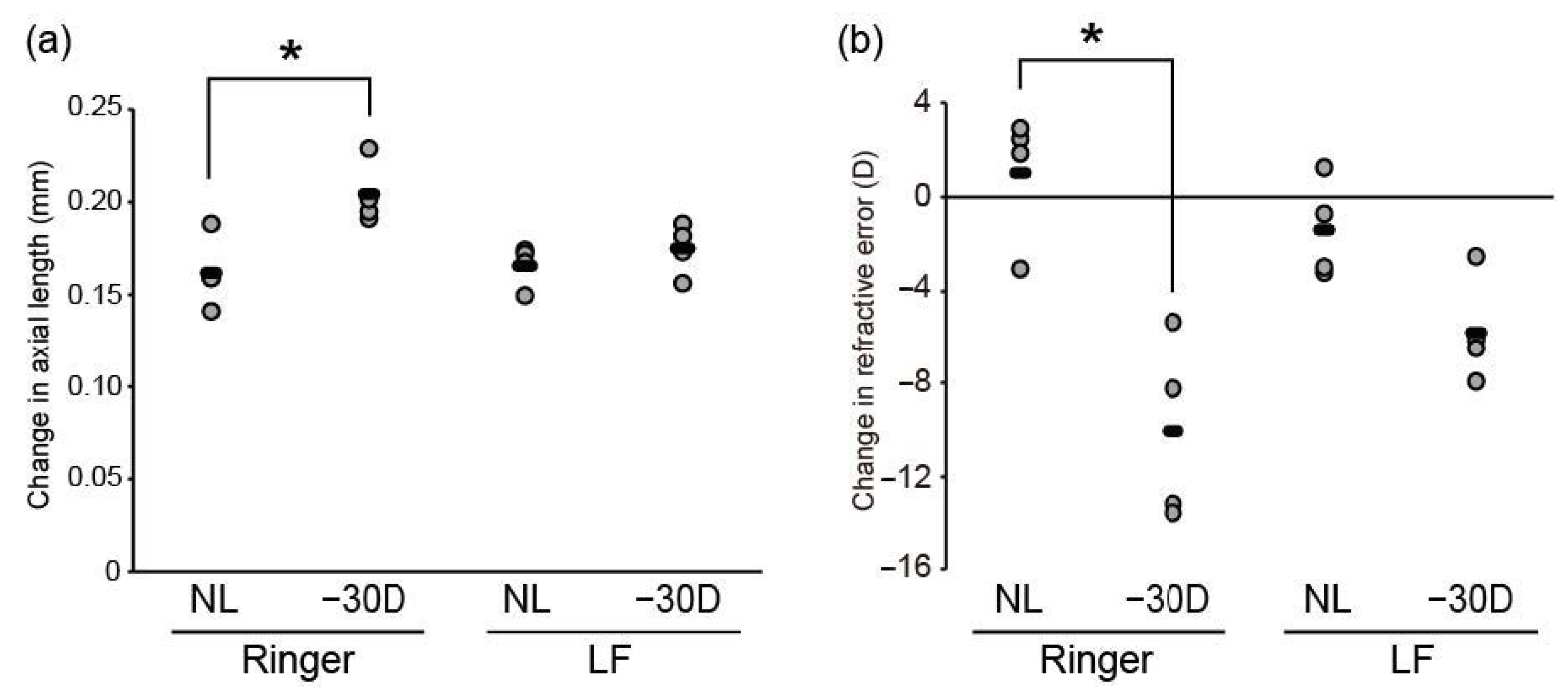
3.1. Oral LF Supplementation Suppressed Minus-Lens-Induced Myopia Development in C57BL/6J Mice
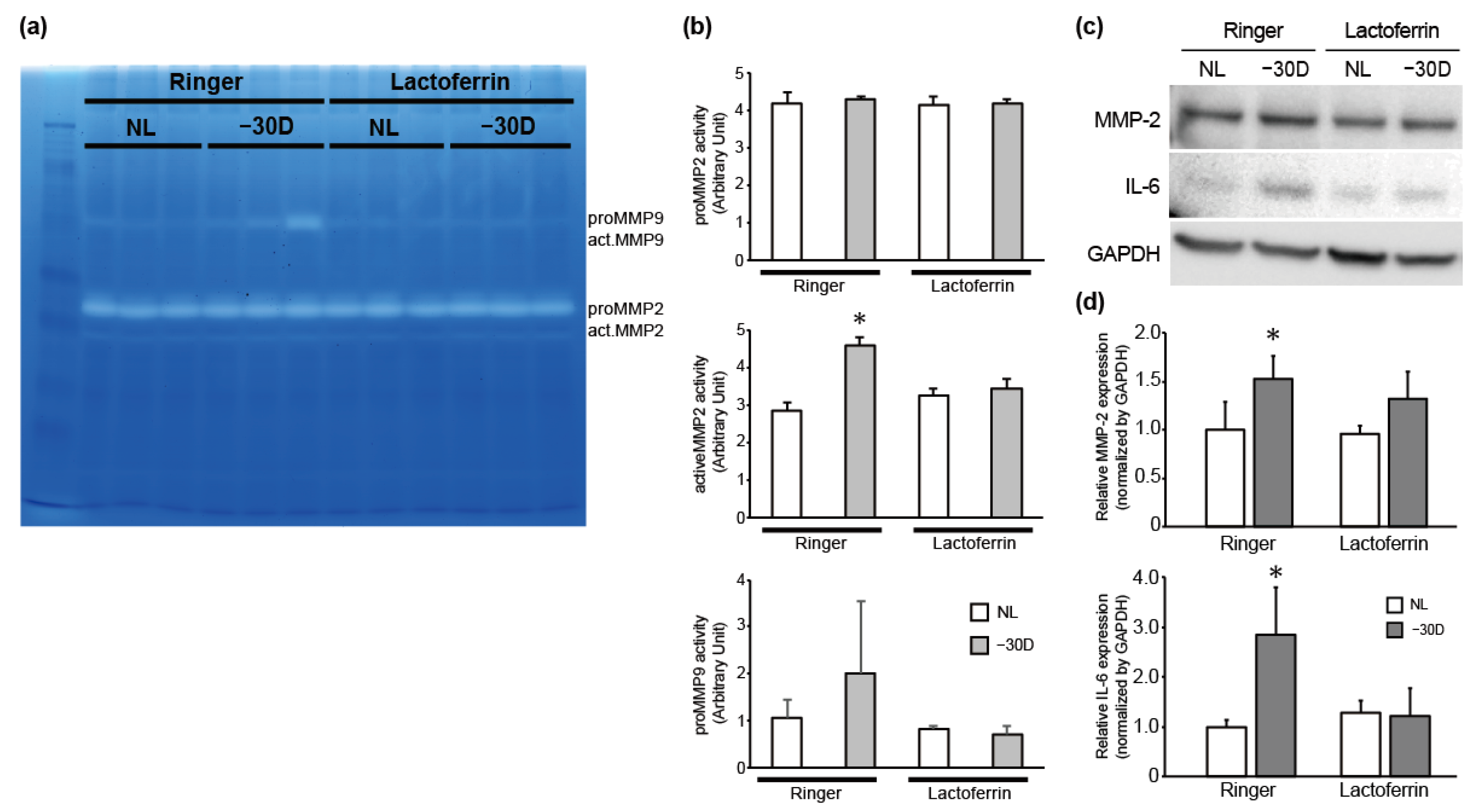
3.2. While LIM Increased Active MMP-2 Activity and IL-6 Expression in the Choroid and Sclera, LF Administration Reversed This Effect
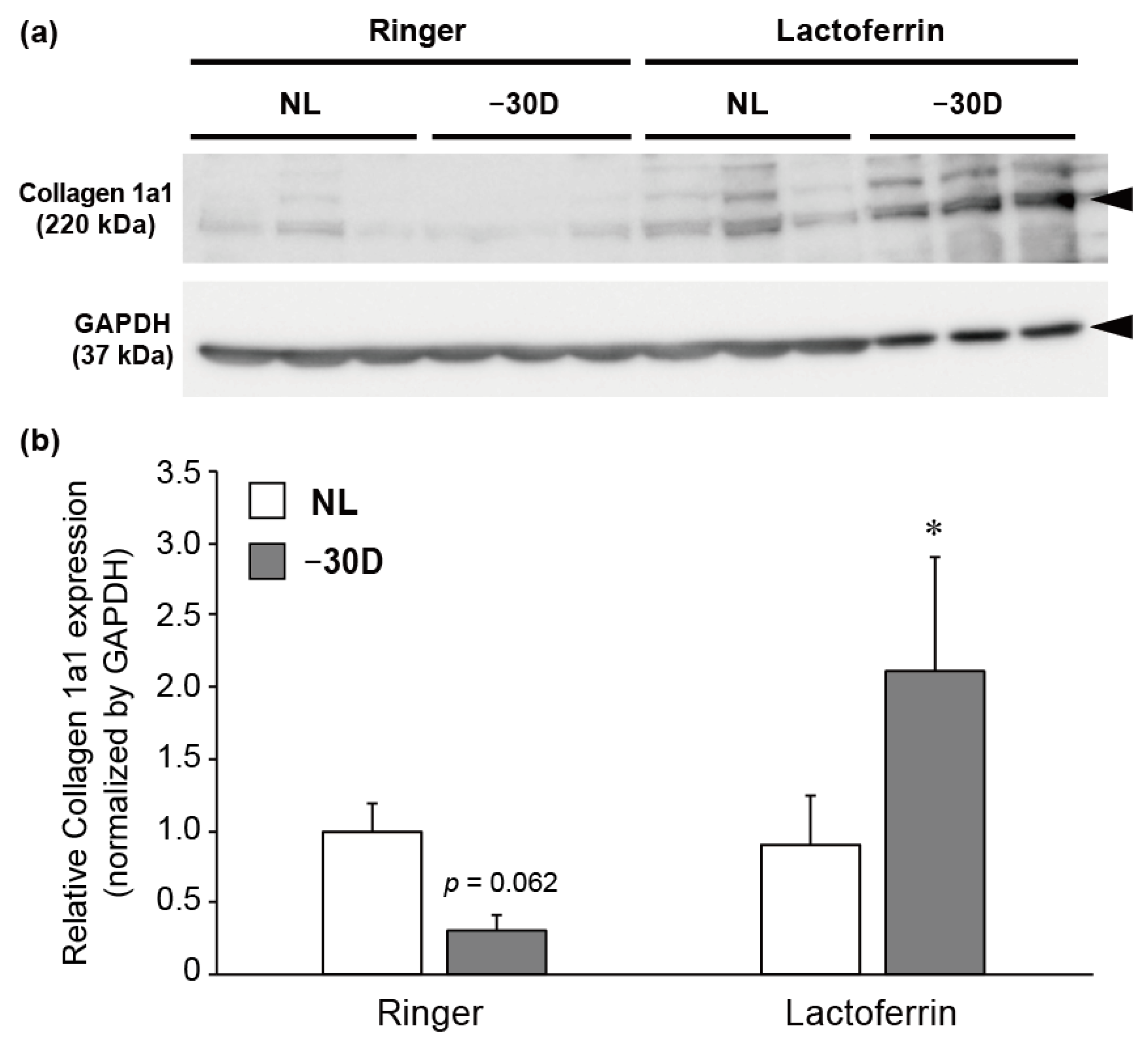
3.3. LIM Decreased the Content of Collagen 1A1 Protein in Choroid and Sclera, and LF Administration Reversed This Effect
4. Discussion
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dolgin, E. The myopia boom. Nature 2015, 519, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Tideman, J.W.; Snabel, M.C.; Tedja, M.S.; van Rijn, G.A.; Wong, K.T.; Kuijpers, R.W.; Vingerling, J.R.; Hofman, A.; Buitendijk, G.H.S.; Keunen, J.E.E.; et al. Association of axial length with risk of uncorrectable visual impairment for Europeans with myopia. JAMA Ophthalmol. 2016, 134, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Herbort, C.P.; Papadia, M.; Neri, P. Myopia and inflammation. J. Ophthalmic Vis. Res. 2011, 6, 270–283. [Google Scholar] [PubMed]
- Harper, A.R.; Summers, J.A. The dynamic sclera: Extracellular matrix remodeling in normal ocular growth and myopia development. Exp. Eye Res. 2015, 133, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Wei, C.C.; Chang, C.Y.; Chen, T.H.; Hsu, Y.A.; Hsieh, Y.C.; Chen, H.-J.; Wan, L. Role of Chronic Inflammation in Myopia Progression: Clinical Evidence and Experimental Validation. EBioMedicine 2016, 10, 269–281. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, S.; Wang, Y.; Pan, S.; Wang, P.; Cheng, L. Inflammatory cytokines in highly myopic eyes. Sci. Rep. 2019, 9, 3517. [Google Scholar] [CrossRef]
- Gentle, A.; Liu, Y.; Martin, J.E.; Conti, G.L.; McBrien, N.A. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J. Biol. Chem. 2003, 278, 16587–16594. [Google Scholar] [CrossRef]
- Schippert, R.; Brand, C.; Schaeffel, F.; Feldkaemper, M.P. Changes in scleral MMP-2, TIMP-2 and TGFbeta-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp. Eye Res. 2006, 82, 710–719. [Google Scholar] [CrossRef]
- Siegwart, J.T., Jr.; Norton, T.T. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2067–2075. [Google Scholar]
- Siegwart, J.T., Jr.; Norton, T.T. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N. Lactoferrin: A multi-tasking protein par excellence. Cell. Mol. Life Sci. 2005, 62, 2529–2530. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Actor, J.K.; Hwang, S.A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Kawakita, T.; Inaba, T.; Okada, N.; Ito, M.; Shimmura, S.; Watanabe, M.; Shinmura, K.; Tsubota, K. Dietary lactoferrin alleviates age-related lacrimal gland dysfunction in mice. PLoS ONE 2012, 7, e33148. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, T.; Tanaka, A.; Yamauchi, K.; Iwatsuki, K. Bovine lactoferrin ingestion protects against inflammation via IL-11 induction in the small intestine of mice with hepatitis. Br. J. Nutr. 2014, 111, 1801–1810. [Google Scholar] [CrossRef]
- Yamauchi, K.; Wakabayashi, H.; Hashimoto, S.; Teraguchi, S.; Hayasawa, H.; Tomita, M. Effects of orally administered bovine lactoferrin on the immune system of healthy volunteers. Adv. Exp. Med. Biol. 1998, 443, 261–265. [Google Scholar]
- Saito, S.; Takayama, Y.; Mizumachi, K.; Suzuki, C. Lactoferrin promotes hyaluronan synthesis in human dermal fibroblasts. Biotechnol. Lett. 2011, 33, 33–39. [Google Scholar] [CrossRef]
- Yan, D.; Chen, D.; Shen, J.; Xiao, G.; van Wijnen, A.J.; Im, H.J. Bovine lactoferricin is anti-inflammatory and anti-catabolic in human articular cartilage and synovium. J. Cell. Physiol. 2013, 228, 447–456. [Google Scholar] [CrossRef]
- Higuchi, A.; Inoue, H.; Kaneko, Y.; Oonishi, E.; Tsubota, K. Selenium-binding lactoferrin is taken into corneal epithelial cells by a receptor and prevents corneal damage in dry eye model animals. Sci. Rep. 2016, 6, 36903. [Google Scholar] [CrossRef]
- Ibuki, M.; Shoda, C.; Miwa, Y.; Ishida, A.; Tsubota, K.; Kurihara, T. Lactoferrin has a therapeutic effect via HIF inhibition in a murine model of choroidal neovascularization. Front. Pharmacol. 2020, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Nakamura, S.; Izuta, Y.; Inoue, S.; Tsubota, K. Dietary supplementation with a combination of lactoferrin, fish oil, and Enterococcus faecium WB2000 for treating dry eye: A Rat Model and Human Clinical Study. Ocul. Surf. 2016, 14, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kurihara, T.; Ikeda, S.-I.; Kunimi, H.; Mori, K.; Torii, H.; Tsubota, K. Inducement and evaluation of a murine model of experimental myopia. J. Vis. Exp. 2019, 2019, e58822. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kurihara, T.; Kunimi, H.; Miyauchi, M.; Ikeda, S.-I.; Mori, K.; Tsubota, K.; Torii, H.; Tsubota, K. A highly efficient murine model of experimental myopia. Sci. Rep. 2018, 8, 2026. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, D.-B.; Li, W.-W.; Zhang, L.-L.; Long, G.-X.; Wang, J.; Mei, Q.; Hu, G. Interleukin-6 promotes the migration and invasion of nasopharyngeal carcinoma cell lines and upregulates the expression of MMP-2 and MMP-9. Int. J. Oncol. 2014, 44, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Kossakowska, A.E.; Edwards, D.R.; Prusinkiewicz, C.; Zhang, M.C.; Guo, D.; Urbanski, S.J.; Grogran, T.; Marquez, L.A.; Janowska-Wieczorek, A. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood 1999, 94, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Shun-Fa, Y.; Zhu, D.; Zhang, L.; Gu, P.; Fan, X.; Zhou, J. MMP-2, MMP-3, TIMP-1, TIMP-2, and TIMP-3 protein levels in human aqueous humor: Relationship with axial length. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3922–3928. [Google Scholar] [CrossRef] [PubMed]
- Rada, J.A.; Perry, C.A.; Slover, M.L.; Achen, V.R. Gelatinase A and TIMP-2 expression in the fibrous sclera of myopic and recovering chick eyes. Investig. Ophthalmol. Vis. Sci. 1999, 40, 3091–3099. [Google Scholar]
- Zhao, F.; Zhou, Q.; Reinach, P.S.; Yang, J.; Ma, L.; Wang, X.; Wen, Y.; Srinivasalu, N.; Qu, J.; Qu, J. Cause and effect relationship between changes in scleral matrix metallopeptidase-2 expression and myopia development in mice. Am. J. Pathol. 2018, 188, 1754–1767. [Google Scholar] [CrossRef]
- Nakayama, K.; Otsuki, K.; Yakuwa, K.; Hasegawa, A.; Sawada, M.; Mitsukawa, K.; Chiba, H.; Nagatsuka, M.; Okai, T. Recombinant human lactoferrin inhibits matrix metalloproteinase (MMP-2, MMP-3, and MMP-9) activity in a rabbit preterm delivery model. J. Obstet. Gynaecol. Res. 2008, 34, 931–934. [Google Scholar] [CrossRef]
- Newsome, A.L.; Johnson, J.P.; Seipelt, R.L.; Thompson, M.W. Apolactoferrin inhibits the catalytic domain of matrix metalloproteinase-2 by zinc chelation. Biochem. Cell Biol. 2007, 85, 563–567. [Google Scholar] [CrossRef] [PubMed]
- McBrien, N.A.; Cornell, L.M.; Gentle, A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2179–2187. [Google Scholar]
- Norton, T.T.; Rada, J.A. Reduced extracellular matrix in mammalian sclera with induced myopia. Vis. Res. 1995, 35, 1271–1281. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, S.-I.; Kurihara, T.; Toda, M.; Jiang, X.; Torii, H.; Tsubota, K. Oral Bovine Milk Lactoferrin Administration Suppressed Myopia Development through Matrix Metalloproteinase 2 in a Mouse Model. Nutrients 2020, 12, 3744. https://doi.org/10.3390/nu12123744
Ikeda S-I, Kurihara T, Toda M, Jiang X, Torii H, Tsubota K. Oral Bovine Milk Lactoferrin Administration Suppressed Myopia Development through Matrix Metalloproteinase 2 in a Mouse Model. Nutrients. 2020; 12(12):3744. https://doi.org/10.3390/nu12123744
Chicago/Turabian StyleIkeda, Shin-Ichi, Toshihide Kurihara, Masataro Toda, Xiaoyan Jiang, Hidemasa Torii, and Kazuo Tsubota. 2020. "Oral Bovine Milk Lactoferrin Administration Suppressed Myopia Development through Matrix Metalloproteinase 2 in a Mouse Model" Nutrients 12, no. 12: 3744. https://doi.org/10.3390/nu12123744
APA StyleIkeda, S.-I., Kurihara, T., Toda, M., Jiang, X., Torii, H., & Tsubota, K. (2020). Oral Bovine Milk Lactoferrin Administration Suppressed Myopia Development through Matrix Metalloproteinase 2 in a Mouse Model. Nutrients, 12(12), 3744. https://doi.org/10.3390/nu12123744






