AphaMax®, an Aphanizomenon Flos-Aquae Aqueous Extract, Exerts Intestinal Protective Effects in Experimental Colitis in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of DNBS Colitis and Treatment Protocol
2.3. Evaluation of Colitis
2.3.1. Monitoring of Change in Body Weight, Consistency of Stools and Rectal Bleeding
2.3.2. Macroscopic Scores
2.3.3. Analysis of Microscopic Inflammatory Damage
2.4. Assay of MPO Activity
2.5. ELISA Assay for Pro-Inflammatory Cytokines
2.6. NFκ-B, iNOS and COX-2 mRNA Analysis by Real-Time PCR
2.7. Analysis of Reactive Oxygen Species (ROS) Generation
2.8. Nitric Oxide (NO) Concentration Assay
2.9. Data Analysis and Statistical Tests
3. Results
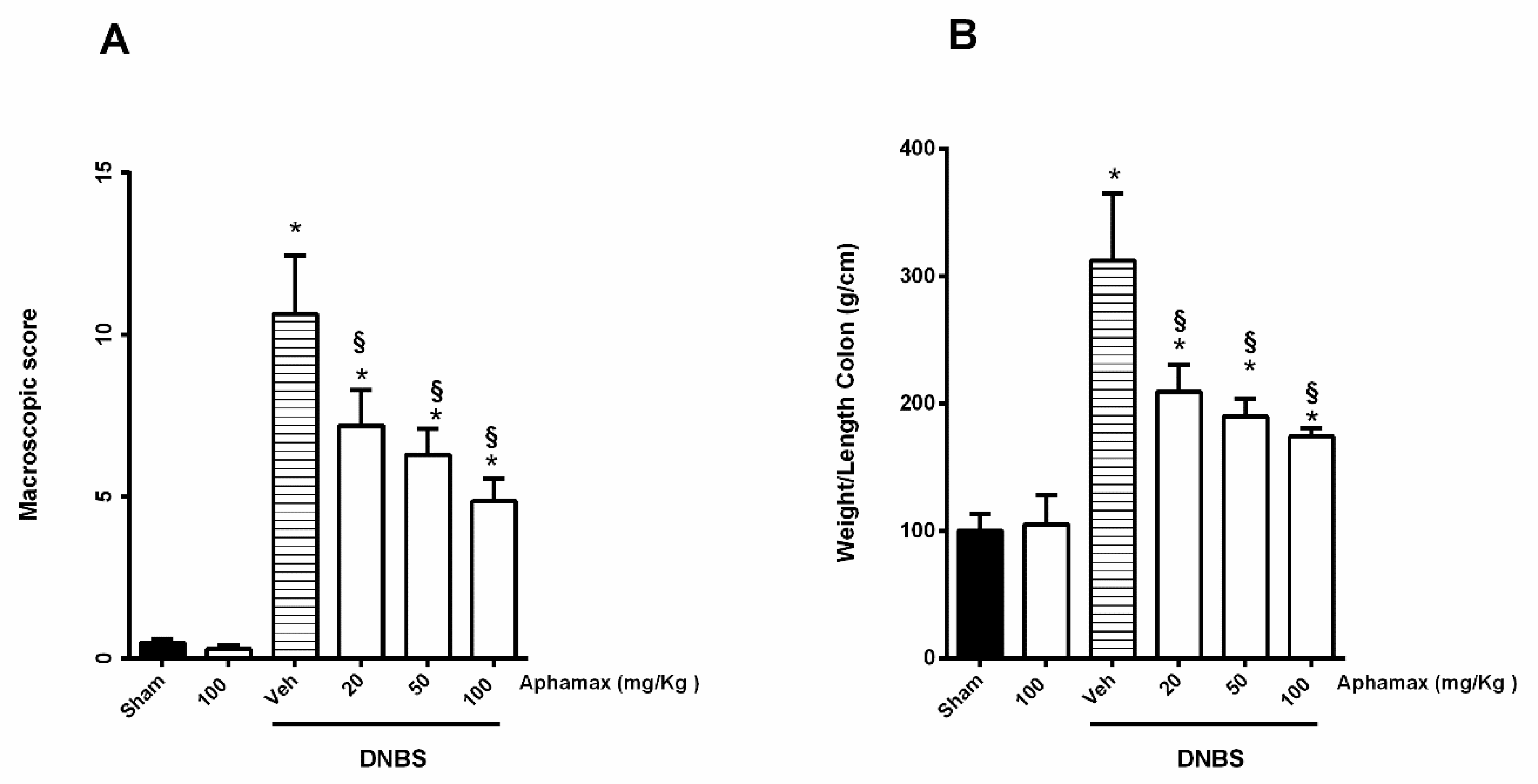
3.1. AphaMax® Effects on Macroscopic Changes in Colon of Colitis Animals
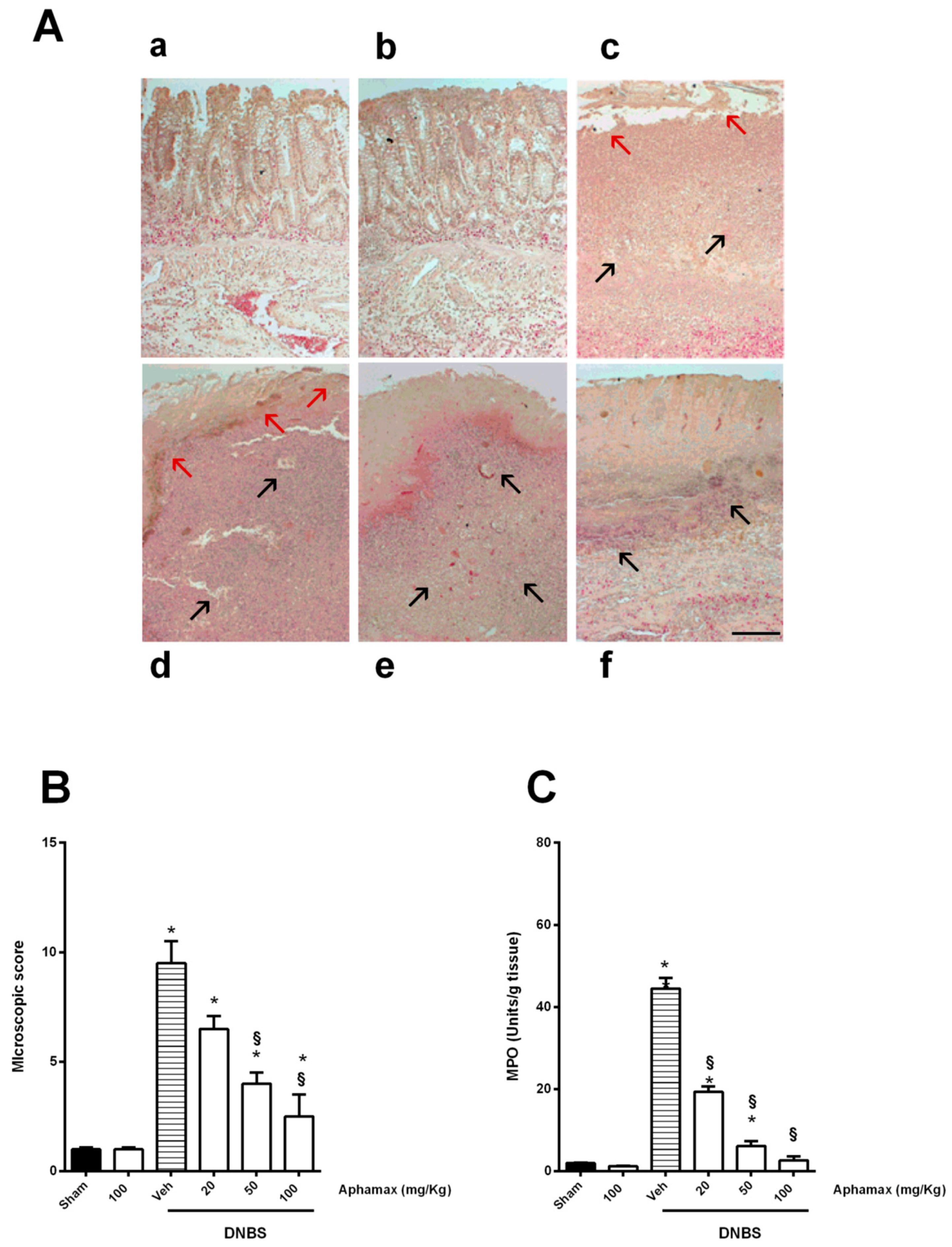
3.2. AphaMax® Effects on Histopathological Changes in Inflamed Colon Tissue
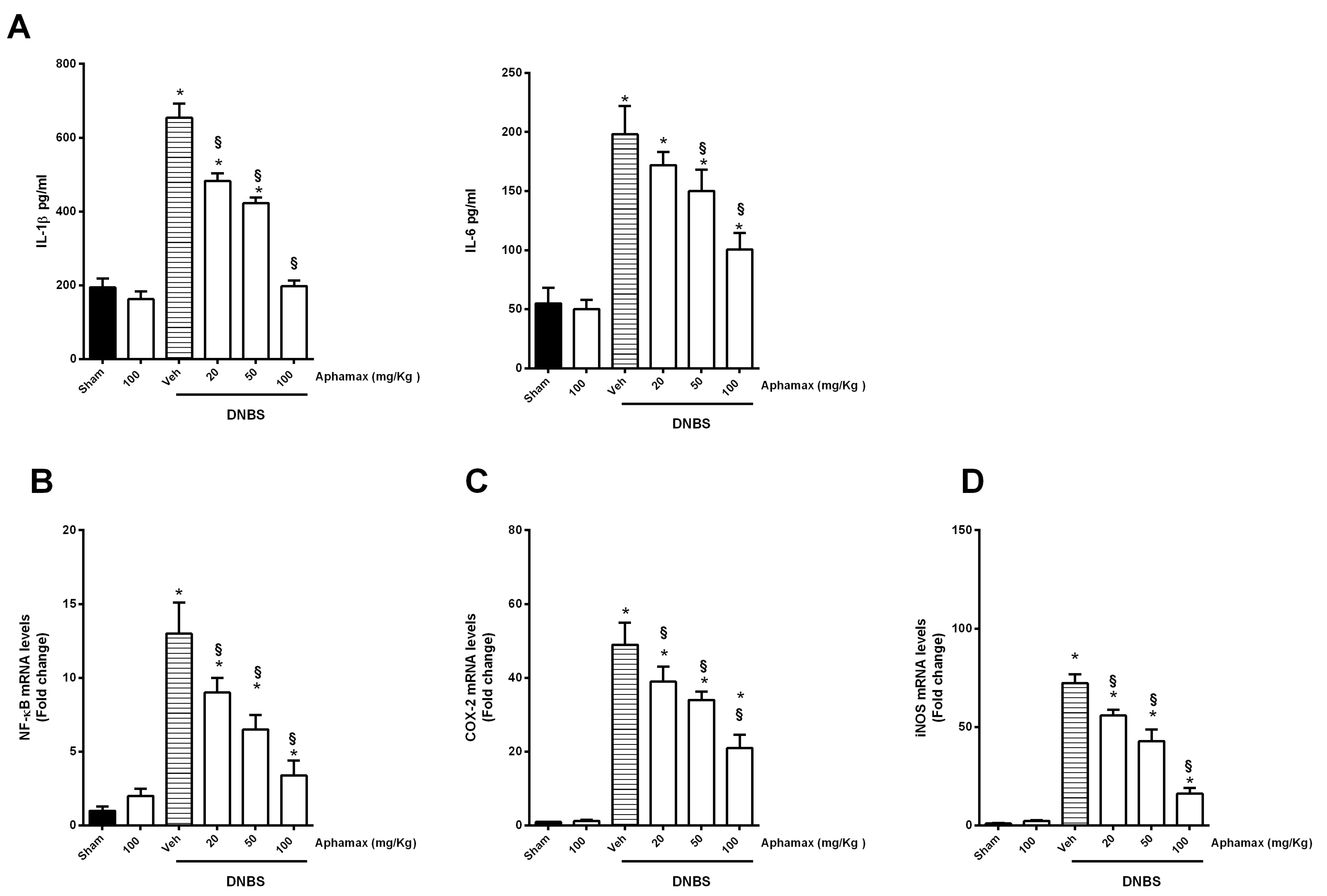
3.3. AphaMax® Effects on MPO and Cytokine Levels
3.4. AphaMax® Effect on NF-κB p65, COX-2 and iNOS mRNA Expression
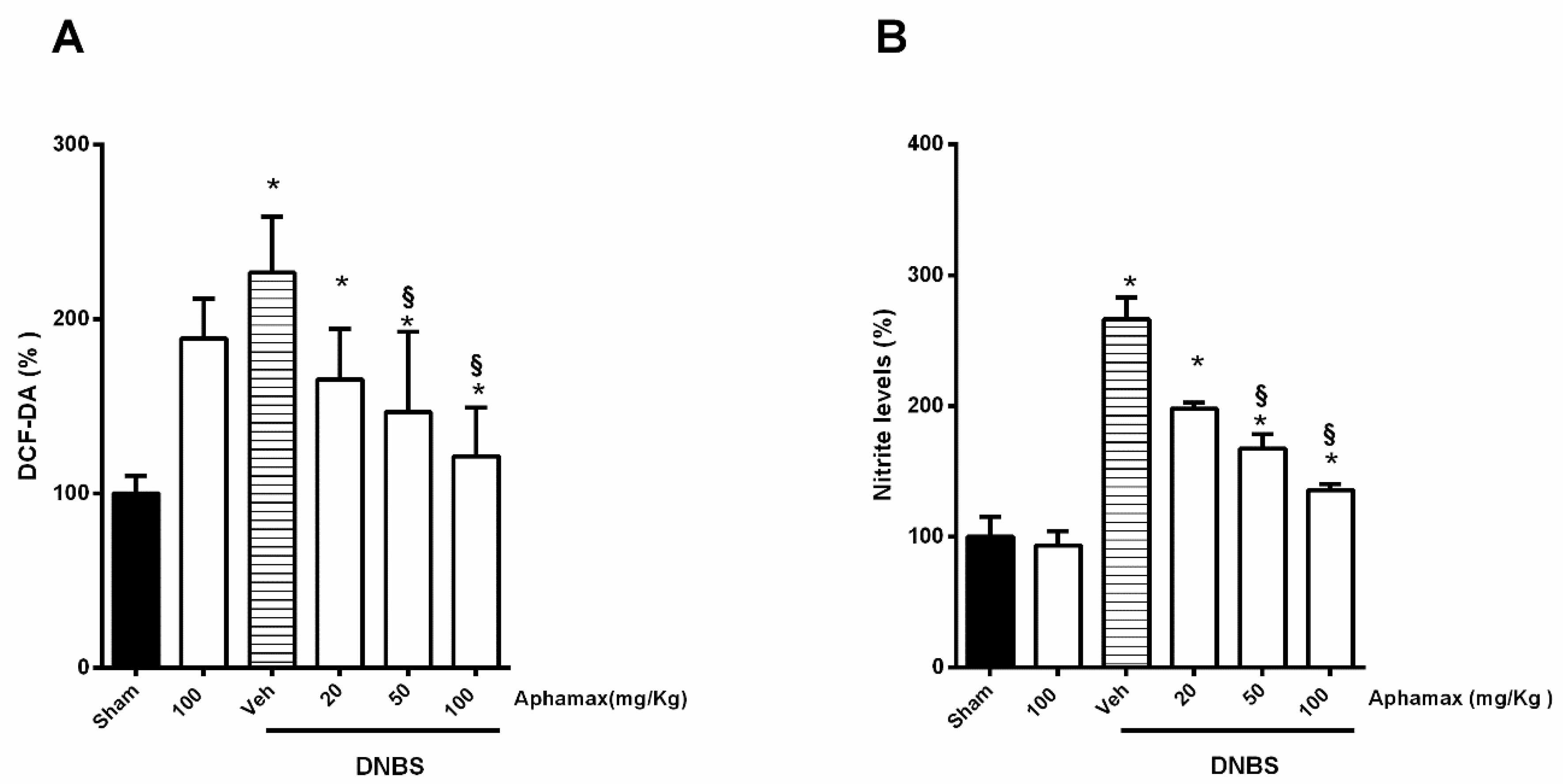
3.5. AphaMax® Effect on Nitrite and ROS Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, A.; Mizoguchi, E.; Takedatsu, H.; Blumberg, R.S.; Bhan, A.K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002, 16, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Pizarro, T.T.; Cominelli, F. Cytokine therapy for Crohn’s disease: Advances in translational research. Annu. Rev. Med. 2007, 58, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Mudter, J.; Amoussina, L.; Schenk, M.; Yu, J.; Brustle, A.; Weigmann, B.; Atreya, R.; Wirtz, S.; Becker, C.; Hoffman, A.; et al. The transcription factor IFN regulatory factor-4 controls experimental colitis in mice via T cell-derived IL-6. J. Clin. Investig. 2008, 118, 2415–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucharzik, T.; Walsh, S.V.; Chen, J.; Parkos, C.A.; Nusrat, A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 2001, 159, 2001–2009. [Google Scholar] [CrossRef] [Green Version]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial tight junctions in intestinal inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef]
- Na, S.Y.; Moon, W. Perspectives on Current and Novel Treatments for Inflammatory Bowel Disease. Gut Liver 2019, 13, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez de, R.C.; Miranda-Zamora, R.; Diaz-Zagoya, J.C.; Juarez-Oropeza, M.A. Preventive effect of Spirulina maxima on the fatty liver induced by a fructose-rich diet in the rat, a preliminary report. Life Sci. 1993, 53, 57–61. [Google Scholar] [CrossRef]
- Ciferri, O. Spirulina, the edible microorganism. Microbiol. Rev. 1983, 47, 551–578. [Google Scholar] [CrossRef]
- Ku, C.S.; Yang, Y.; Park, Y.; Lee, J. Health benefits of blue-green algae: Prevention of cardiovascular disease and nonalcoholic fatty liver disease. J. Med. Food 2013, 16, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, R.; Saidpour, A.; Mottaghi, A. The effects of Spirulina supplementation on metabolic syndrome components, its liver manifestation and related inflammatory markers: A systematic review. Complement. Ther. Med. 2019, 42, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Iyer Uma, M.; Sophia, A.; Mani Uliyar, V. Glycemic and lipemic responses of selected Spirulina supplemented rice-based recipes in normal subjects. Int. J. Diabetes Dev. Ctries 1999, 19, 17–22. [Google Scholar]
- Xue, X.; Lv, Y.; Liu, Q.; Zhang, X.; Zhao, Y.; Zhang, L.; Xu, S. Extracellular polymeric substance from Aphanizomenon flos-aquae induces apoptosis via the mitochondrial pathway in A431 human epidermoid carcinoma cells. Exp. Ther. Med. 2015, 10, 927–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetti, S.; Rinalducci, S.; Benvenuti, F.; Francogli, S.; Pagliarani, S.; Giorgi, L.; Micheloni, M.; D’Amici, G.M.; Zolla, L.; Canestrari, F. Purification and characterization of phycocyanin from the blue-green alga Aphanizomenon flos-aquae. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 833, 12–18. [Google Scholar]
- Benedetti, S.; Benvenuti, F.; Pagliarani, S.; Francogli, S.; Scoglio, S.; Canestrari, F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004, 75, 2353–2362. [Google Scholar] [CrossRef]
- Scoglio, S.; Benedetti, S.; Canino, C.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Canestrari, F.; Genazzani, A.D. Effect of a 2-month treatment with Klamin, a Klamath algae extract, on the general well-being, antioxidant profile and oxidative status of postmenopausal women. Gynecol. Endocrinol. 2009, 25, 235–240. [Google Scholar] [CrossRef]
- Scoglio, S.; Benedetti, Y.; Benvenuti, F.; Battistelli, S.; Canestrari, F.; Benedetti, S. Selective monoamine oxidase B inhibition by an Aphanizomenon flos-aquae extract and by its constitutive active principles phycocyanin and mycosporine-like amino acids. Phytomedicine 2014, 21, 992–997. [Google Scholar] [CrossRef]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Auteri, M.; Amato, A.; Caldara, G.; Nuzzo, D.; Di, C.M.; Serio, R. Angiotensin II type II receptors and colonic dysmotility in 2,4-dinitrofluorobenzenesulfonic acid-induced colitis in rats. Neurogastroenterol. Motil. 2017, 29, e13019. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di, C.M.; Serio, R. Preventive effects of guanosine on intestinal inflammation in 2, 4-dinitrobenzene sulfonic acid (DNBS)-induced colitis in rats. Inflammopharmacology 2019, 27, 349–359. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di, C.M.; Serio, R. PD123319, angiotensin II type II receptor antagonist, inhibits oxidative stress and inflammation in 2, 4-dinitrobenzene sulfonic acid-induced colitis in rat and ameliorates colonic contractility. Inflammopharmacology 2020, 28, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Appleyard, C.B.; Wallace, J.L. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am. J. Physiol. 1995, 269, G119–G125. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.M.; Wang, A.; Hirota, C.L.; McKay, D.M. Neutralizing anti-IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J. Immunol. 2005, 174, 7368–7375. [Google Scholar] [CrossRef] [Green Version]
- Moreels, T.G.; Nieuwendijk, R.J.; De Man, J.G.; De Winter, B.Y.; Herman, A.G.; Van Marck, E.A.; Pelckmans, P.A. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut 2004, 53, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Adachi, M.; Watanabe, M.; Kurata, Y.; Inoue, Y.; Notsu, T.; Yamamoto, K.; Horie, H.; Tanno, S.; Morita, M.; Miake, J.; et al. Beta-Adrenergic Blocker, Carvedilol, Abolishes Ameliorating Actions of Adipose-Derived Stem Cell Sheets on Cardiac Dysfunction and Remodeling After Myocardial Infarction. Circ. J. 2019, 83, 2282–2291. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Candelario-Jalil, E.; de Oliveira, A.C.P.; Olajide, O.A.; Martínez-Sánchez, G.; Fiebich, B.L. Mangiferin inhibits cyclooxygenase-2 expression and prostaglandin E2 production in activated rat microglial cells. Arch. Biochem. Biophys. 2008, 477, 253–258. [Google Scholar] [CrossRef]
- Sönmez, M.F.; Dündar, M. Ameliorative effects of pentoxifylline on NOS induced by diabetes in rat kidney. Ren. Fail. 2016, 38, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Arab, H.H.; Al-Shorbagy, M.Y.; Abdallah, D.M.; Nassar, N.N. Telmisartan Attenuates Colon Inflammation, Oxidative Perturbations and Apoptosis in a Rat Model of Experimental Inflammatory Bowel Disease. PLoS ONE 2014, 9, e97193. [Google Scholar] [CrossRef] [Green Version]
- Roediger, W.E.; Radcliffe, B.C. Role of nitrite and nitrate as a redox couple in the rat colon. Implications for diarrheal conditions. Gastroenterology 1988, 94, 915–922. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kefayat, A.; Ghahremani, F.; Safavi, A.; Hajiaghababa, A.; Moshtaghian, J. C-phycocyanin: A natural product with radiosensitizing property for enhancement of colon cancer radiation therapy efficacy through inhibition of COX-2 expression. Sci. Rep. 2019, 9, 19161. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Farouk, S.M.; Madkour, F.F.; Azab, S.S. Anti-inflammatory and immunomodulatory effects of Spirulina platensis in comparison to Dunaliella salina in acetic acid-induced rat experimental colitis. Immunopharmacol. Immunotoxicol. 2015, 37, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.E.; Andre, C.A. A review of recent clinical trials of the nutritional supplement Chlorella pyrenoidosa in the treatment of fibromyalgia, hypertension, and ulcerative colitis. Altern. Ther. Health Med. 2001, 7, 79–91. [Google Scholar] [PubMed]
- Pleonsil, P.; Soogarun, S.; Suwanwong, Y. Anti-oxidant activity of holo- and apo-c-phycocyanin and their protective effects on human erythrocytes. Int. J. Biol. Macromol. 2013, 60, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Rodriguez, S.; Romay, C.; Gonzalez, A.; Armesto, J.; Remirez, D.; Merino, N. Anti-Inflammatory Activity of Phycocyanin Extract in Acetic Acid-Induced Colitis in Rats. Pharmacol. Res. 1999, 39, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Kong, F.; Vallance, J.E.; Harmel-Laws, E.; Amarachintha, S.; Steinbrecher, K.A.; Rosen, M.J.; Bhattacharyya, S. Activation of TGF-beta activated kinase 1 promotes colon mucosal pathogenesis in inflammatory bowel disease. Physiol. Rep. 2017, 5, e13181. [Google Scholar] [CrossRef]
- Cherng, S.C.; Cheng, S.N.; Tarn, A.; Chou, T.C. Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2016, 81, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Varum, F.; Bravo, R.; Furrer, E.; Bojic, D.; Basit, A.W. Inflammatory bowel disease: Exploring gut pathophysiology for novel therapeutic targets. Transl. Res. 2016, 176, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Hering, N.A.; Schulzke, J.D. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig. Dis. 2009, 27, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Norregaard, R.; Kwon, T.H.; Frokiaer, J. Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res. Clin. Pract. 2015, 34, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Sheibanie, A.F.; Yen, J.H.; Khayrullina, T.; Emig, F.; Zhang, M.; Tuma, R.; Ganea, D. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. J. Immunol. 2007, 178, 8138–8147. [Google Scholar] [CrossRef] [Green Version]
- Eliakim, R.; Karmeli, F.; Chorev, M.; Okon, E.; Rachmilewitz, D. Effect of drugs on colonic eicosanoid accumulation in active ulcerative colitis. Scand. J. Gastroenterol. 1992, 27, 968–972. [Google Scholar] [CrossRef]
- Reddy, M.C.; Subhashini, J.; Mahipal, S.V.; Bhat, V.B.; Srinivas, R.P.; Kiranmai, G.; Madyastha, K.M.; Reddanna, P. C-Phycocyanin, a selective cyclooxygenase-2 inhibitor, induces apoptosis in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2003, 304, 385–392. [Google Scholar] [CrossRef]
- Szalai, Z.; Szasz, A.; Nagy, I.; Puskas, L.G.; Kupai, K.; Kiraly, A.; Berko, A.M.; Posa, A.; Strifler, G.; Barath, Z.; et al. Anti-inflammatory effect of recreational exercise in TNBS-induced colitis in rats: Role of NOS/HO/MPO system. Oxid. Med. Cell. Longev. 2014, 2014, 925981. [Google Scholar] [CrossRef]
- Pallone, F.; Monteleone, G. Mechanisms of tissue damage in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2001, 17, 307–312. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1107. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the NF-kappaB puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef] [Green Version]
- Thomson, A.; Hemphill, D.; Jeejeebhoy, K.N. Oxidative stress and antioxidants in intestinal disease. Dig. Dis. 1998, 16, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lih-Brody, L.; Powell, S.R.; Collier, K.P.; Reddy, G.M.; Cerchia, R.; Kahn, E.; Weissman, G.S.; Katz, S.; Floyd, R.A.; McKinley, M.J.; et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 1996, 41, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The Implications of Oxidative Stress and Antioxidant Therapies in Inflammatory Bowel Disease: Clinical Aspects and Animal Models Saudi. J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Neubauer, K.; Berdowska, I.; Boehm, D.; Zielinski, B.; Petryszyn, P.; Terlecki, G.; Paradowski, L.; Gamian, A. Enhanced formation of advanced oxidation protein products in IBD. Inflamm. Bowel. Dis. 2008, 14, 794–802. [Google Scholar] [CrossRef]
- Jabri, M.A.; Rtibi, K.; Ben-Said, A.; Aouadhi, C.; Hosni, K.; Sakly, M.; Sebai, H. Antidiarrhoeal, antimicrobial and antioxidant effects of myrtle berries (Myrtus communis L.) seeds extract. J. Pharm. Pharmacol. 2016, 68, 264–274. [Google Scholar] [CrossRef]
| Gene Description | Forward Primer | Reverse Primer | Ref. |
|---|---|---|---|
| β-actin | 5′-CTAAGGCCAACCGTGAAAAG-3′ | 5′-GCCTGGATGGCTACGTACA-3′ | [26] |
| COX 2 | 5′-TGCGATGCTCTTCCGAGCTGTGCT-3′ | 5′-TCAGGAAGTTCCTTATTTCCTTTC-3′ | [27] |
| iNOS | 5′-AGAAGGGGACGAACTCAGC-3′ | 5′-TCGAACATCGAACGTCTCAC-3′ | [28] |
| NFκ−B p65 | 5′-GTCATCAGGAAGAGGTTTGGCT-3′ | 5′-TGATAAGCTTAGCCCTTGCAGC-3′ | [29] |
| Groups | % Body Weight Change 2 Days after Colitis Induction | % Body Weight Change 7 Days After Colitis Induction | Stool Consistency 2 Days after Colitis Induction | Stool Consistency 7 Days after Colitis Induction | Rectal Bleeding 2 Days after Colitis Induction | Rectal Bleeding 7 Days after Colitis Induction |
|---|---|---|---|---|---|---|
| Sham | 102.1 ± 0.5 | 109 ± 0.8 | 0 | 0 | 0 | 0 |
| Sham + 100 mg/kg Aphamax® | 103.2 ± 1.2 | 107 ±1.2 | 0 | 0 | 0 | 0 |
| DNBS | 94.2 ± 0.8 * | 95.1 ± 1.5 * | 4.0 ± 0.0 * | 3.8 ± 0.2 * | 4.0 ± 0.0 * | 3.8 ± 0.2 * |
| DNBS+ 20 mg/kg Aphamax® | 95.4 ± 1.3 * | 99.2 ± 0.9 * | 3.2 ± 0.2 * | 2.8 ± 0.2 * | 3.2 ± 0.4 * | 2.0 ± 0.3 * § |
| DNBS+ 50 mg/kg Aphamax® | 96.7 ± 0.9 * | 102.2 ± 0.5 § | 2.8 ± 0.2 * | 2.0 ± 0.4 * § | 2.2 ± 0.4 * § | 1.2 ± 0.2 § |
| DNBS+ 100 mg/kg Aphamax®- | 97.8 ± 1.1 * | 104.4 ± 1.5 § | 2.7 ± 0.4 * § | 1.0 ± 0.3 § | 2.0 ± 0.6 * § | 0.4 ± 0.2 § |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di Carlo, M.; Scoglio, S.; Serio, R. AphaMax®, an Aphanizomenon Flos-Aquae Aqueous Extract, Exerts Intestinal Protective Effects in Experimental Colitis in Rats. Nutrients 2020, 12, 3635. https://doi.org/10.3390/nu12123635
Zizzo MG, Caldara G, Bellanca A, Nuzzo D, Di Carlo M, Scoglio S, Serio R. AphaMax®, an Aphanizomenon Flos-Aquae Aqueous Extract, Exerts Intestinal Protective Effects in Experimental Colitis in Rats. Nutrients. 2020; 12(12):3635. https://doi.org/10.3390/nu12123635
Chicago/Turabian StyleZizzo, Maria Grazia, Gaetano Caldara, Annalisa Bellanca, Domenico Nuzzo, Marta Di Carlo, Stefano Scoglio, and Rosa Serio. 2020. "AphaMax®, an Aphanizomenon Flos-Aquae Aqueous Extract, Exerts Intestinal Protective Effects in Experimental Colitis in Rats" Nutrients 12, no. 12: 3635. https://doi.org/10.3390/nu12123635
APA StyleZizzo, M. G., Caldara, G., Bellanca, A., Nuzzo, D., Di Carlo, M., Scoglio, S., & Serio, R. (2020). AphaMax®, an Aphanizomenon Flos-Aquae Aqueous Extract, Exerts Intestinal Protective Effects in Experimental Colitis in Rats. Nutrients, 12(12), 3635. https://doi.org/10.3390/nu12123635








