Computed-Tomography Body Composition Analysis Complements Pre-Operative Nutrition Screening in Colorectal Cancer Patients on an Enhanced Recovery after Surgery Pathway
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Nutrition Risk Factors by Patient-Generated Subjective Global Assessment Short Form
3.3. CT-Defined Skeletal Muscle Analysis
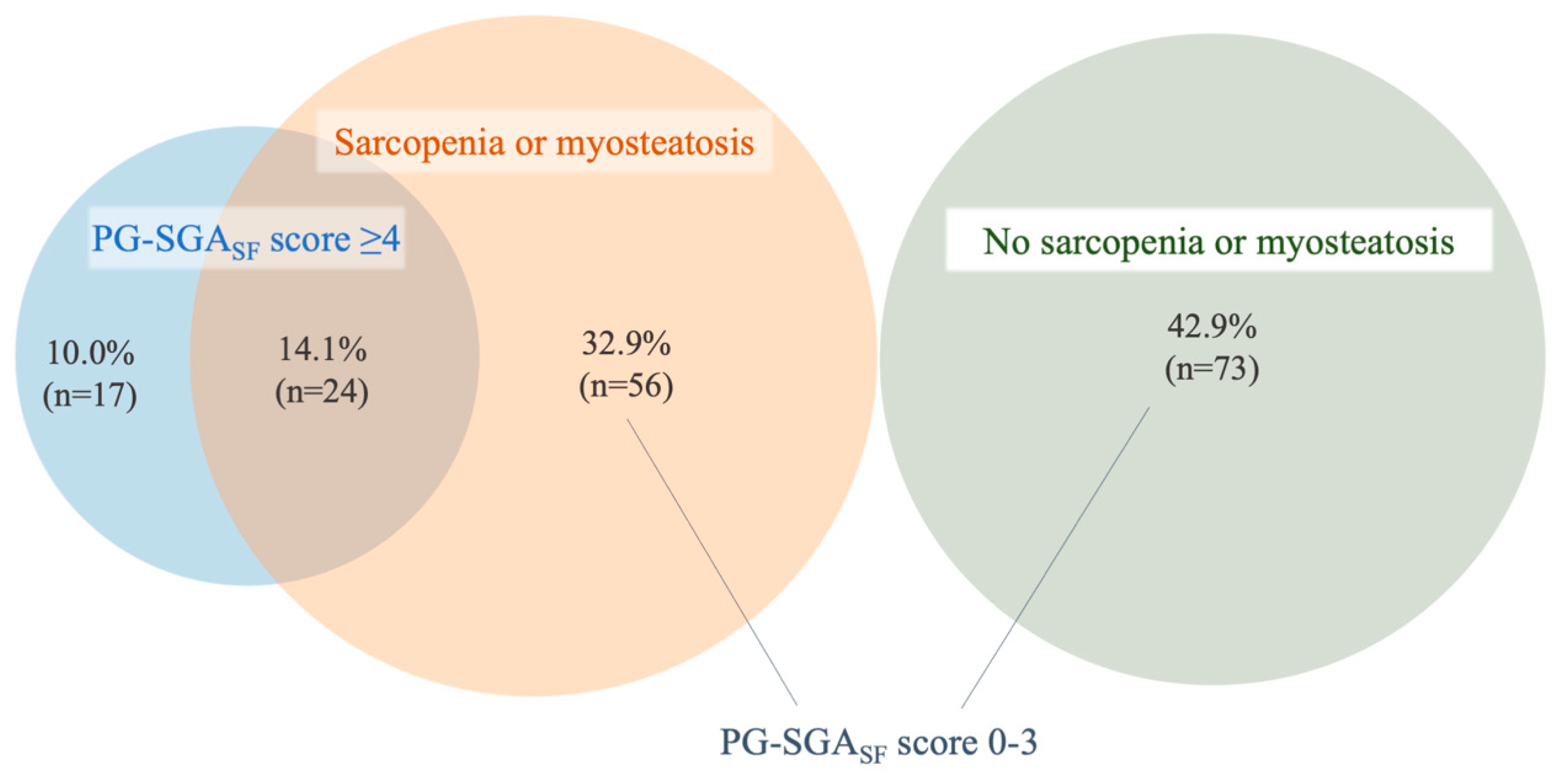
3.4. Co-Existence of Nutrition Risk by PG-SGASF and CT-Defined Sarcopenia and Myosteatosis
4. Discussion
4.1. Early Nutrition Risk Exists
4.2. Sarcopenia and Myosteatosis Are Prevalent in Pre-Operative CRC Patients
4.3. Nutrition Risk and Skeletal Muscle Aberrations Are Distinct Risk Factors
4.4. Enhancing Identification of At-Risk Patients
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2016, 36, 11–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS ®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, K.L.; Elliott, L.; Fuchs-tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T.; Ld, N. Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297–310.e47. [Google Scholar] [CrossRef]
- Martin, L.; Gillis, C.; Atkins, M.; Gillam, M.; Sheppard, C.; Buhler, S.; Hammond, C.B.; Nelson, G.; Gramlich, L. Implementation of an Enhanced Recovery After Surgery Program Can Change Nutrition Care Practice: A Multicenter Experience in Elective Colorectal Surgery. J. Parenter. Enter. Nutr. 2019, 43, 206–219. [Google Scholar] [CrossRef]
- Maurício, S.F.; Xiao, J.; Prado, C.M.; Gonzalez, M.C.; Correia, M.I.T.D. Different nutritional assessment tools as predictors of postoperative complications in patients undergoing colorectal cancer resection. Clin. Nutr. 2018, 37, 1505–1511. [Google Scholar] [CrossRef]
- Schwegler, I.; Von Holzen, A.; Gutzwiller, J.P.; Schlumpf, R.; Mühlebach, S.; Stanga, Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br. J. Surg. 2010, 97, 92–97. [Google Scholar] [CrossRef]
- Huang, T.H.; Hsieh, C.C.; Kuo, L.M.; Chang, C.C.; Chen, C.H.; Chi, C.C.; Liu, C.H. Malnutrition associated with an increased risk of postoperative complications following hepatectomy in patients with hepatocellular carcinoma. HPB 2019, 21, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Garth, A.K.; Newsome, C.M.; Simmance, N.; Crowe, T.C. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J. Hum. Nutr. Diet. 2010, 23, 393–401. [Google Scholar] [CrossRef]
- Vashi, P.G.; Gorsuch, K.; Wan, L.; Hill, D.; Block, C.; Gupta, D. Sarcopenia supersedes subjective global assessment as a predictor of survival in colorectal cancer. PLoS ONE 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Martin, L.; Gioulbasanis, I.; Senesse, P.; Baracos, V.E. Cancer-Associated Malnutrition and CT-Defined Sarcopenia and Myosteatosis Are Endemic in Overweight and Obese Patients. J. Parenter. Enter. Nutr. 2020, 44, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, A.S.; McSorley, S.T.; Dolan, R.D.; Edwards, C.A.; McMillan, D.C. The relation between Malnutrition Universal Screening Tool (MUST), computed tomography–derived body composition, systemic inflammation, and clinical outcomes in patients undergoing surgery for colorectal cancer. Am. J. Clin. Nutr. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, D.K.; Scaffidi, D.; Leung, E.; Stoyanoff, L.; Robinson, J.; Nisenbaum, R.; Brezden-Masley, C.; Darling, P.B. Use of an abridged scored patient-generated subjective global assessment (abPG-SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr. Cancer 2013, 65, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.; Teleni, L.; McKavanagh, D.; Watson, J.; McCarthy, A.L.; Isenring, E. Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) is a valid screening tool in chemotherapy outpatients. Support. Care Cancer 2016. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, R.; Oki, E.; Sasaki, S.; Hirose, K.; Jogo, T.; Edahiro, K.; Korehisa, S.; Taniguchi, D.; Kudo, K.; Kurashige, J.; et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg. Today 2018, 48, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Aro, R.; Mäkäräinen-Uhlbäck, E.; Ämmälä, N.; Rautio, T.; Ohtonen, P.; Saarnio, J.; Meriläinen, S. The impact of sarcopenia and myosteatosis on postoperative outcomes and 5-year survival in curatively operated colorectal cancer patients–A retrospective register study. Eur. J. Surg. Oncol. 2020. [Google Scholar] [CrossRef]
- Martin, L.; Hopkins, J.; Malietzis, G.; Jenkins, J.T.; Sawyer, M.B.; Brisebois, R.; MacLean, A.; Nelson, G.; Gramlich, L.; Baracos, V.E. Assessment of Computed Tomography (CT)-Defined Muscle and Adipose Tissue Features in Relation to Short-Term Outcomes After Elective Surgery for Colorectal Cancer: A Multicenter Approach. Ann. Surg. Oncol. 2018, 25, 2669–2680. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of Systemic Inflammation and Sarcopenia with Survival in Nonmetastatic Colorectal Cancer, Results from the C SCANS Study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Hopkins, J.J.; Reif, R.L.; Bigam, D.L.; Baracos, V.E.; Eurich, D.T.; Sawyer, M.B. The impact of muscle and adipose tissue on long-term survival in patients with stage I to III colorectal cancer. Dis. Colon Rectum 2019, 62, 549–560. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Kazemi-Bajestani, S.M.R.; Mazurak, V.C.; Baracos, V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin. Cell Dev. Biol. 2016, 54, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Jevenn, A.; Hipskind, P. Evaluation of muscle and fat loss as diagnostic criteria for malnutrition. Nutr. Clin. Pract. 2015, 30, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, L.; Frykholm, G.; Lydersen, S.; Ulveland, H.; Baracos, V.; Prado, C.M.M.; Birdsell, L.; Falkmer, U. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. Different assessment criteria for nutritional status provide unequal results. Clin. Nutr. 2013. [Google Scholar] [CrossRef] [PubMed]
- Moctezuma-Velazquez, C.; Ebadi, M.; Bhanji, R.A.; Stirnimann, G.; Tandon, P.; Montano-Loza, A.J. Limited performance of subjective global assessment compared to computed tomography-determined sarcopenia in predicting adverse clinical outcomes in patients with cirrhosis. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Ní Bhuachalla, É.B.; Daly, L.E.; Power, D.G.; Cushen, S.J.; MacEneaney, P.; Ryan, A.M. Computed tomography diagnosed cachexia and sarcopenia in 725 oncology patients: Is nutritional screening capturing hidden malnutrition? J. Cachexia Sarcopenia Muscle 2018, 9, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Punyanitya, M.; Wang, Z.M.; Gallagher, D.; St.-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- Gillis, C.; Nguyen, T.H.; Liberman, A.S.; Carli, F. Nutrition adequacy in enhanced recovery after surgery: A single academic center experience. Nutr. Clin. Pract. 2015, 30, 414–419. [Google Scholar] [CrossRef]
- Gillis, C.; Carli, F. Promoting perioperative metabolic and nutritional care. Anesthesiology 2015, 123, 1455–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, J.J.; Reif, R.; Bigam, D.; Baracos, V.E.; Eurich, D.T.; Sawyer, M.M. Change in Skeletal Muscle Following Resection of Stage I–III Colorectal Cancer is Predictive of Poor Survival: A Cohort Study. World J. Surg. 2019, 43, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Caan, B.J.; Weltzien, E.; Cespedes Feliciano, E.M.; Kroenke, C.H.; Meyerhardt, J.A.; Baracos, V.E.; Kwan, M.L.; Castillo, A.L.; Prado, C.M. Associations of pre-existing co-morbidities with skeletal muscle mass and radiodensity in patients with non-metastatic colorectal cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.; Wells, L.; Nwulu, U.; Currow, D.; Johnson, M.J.; Skipworth, R.J.E. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: A systematic review. Am. J. Clin. Nutr. 2018, 108, 1196–1208. [Google Scholar] [CrossRef]
- Gillis, C.; Li, C.; Lee, L.; Awasthi, R.; Augustin, B.; Gamsa, A.; Liberman, A.S.; Stein, B.; Charlebois, P.; Feldman, L.S.; et al. Prehabilitation versus Rehabilitation. Anesthesiology 2014, 121, 937–947. [Google Scholar] [CrossRef]
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.M.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised Prehabilitation in High-risk Patients Undergoing Elective Major Abdominal Surgery. Ann. Surg. 2018, 267, 50–56. [Google Scholar] [CrossRef]
- Majumdar, D.; Dale, E.; Childs, M.; Clough, S.; Loveridge, R. Initiation of physical activity prehabiliation and rehabilitation for major colorectal surgery. Clin. Nutr. ESPEN 2019. [Google Scholar] [CrossRef]
- Silver, J.K.; Baima, J. Cancer prehabilitation: An opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am. J. Phys. Med. Rehabil. 2013, 92, 715–727. [Google Scholar] [CrossRef]
- Moore, J.; Merchant, Z.; Rowlinson, K.; Mcewan, K.; Evison, M.; Faulkner, G.; Sultan, J.; Mcphee, J.S.; Steele, J. European Journal of Surgical Oncology Implementing a system-wide cancer prehabilitation programme: The journey of Greater Manchester’s ‘Prehab4cancer’. Eur. J. Surg. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]


| Demographics | Male | Female | All (N = 176) |
|---|---|---|---|
| Age (years), mean (±SD) | 63.6 (10.7) | 63.9 (13.3) | 63.8 (12.0) |
| Sex, N (%) | 92 (52.3) | 84 (47.7) | |
| Tumor site, N (%) | |||
| colon | 45 (48.9) | 47 (45.0) | 92 (52.3) |
| rectum | 47 (51.1) | 37 (44.0) | 84 (47.7) |
| Cancer stage, N (%) | |||
| Stage I–II | 51 (55.4) | 46 (54.7) | 97 (55.1) |
| Stage III | 32 (34.8) | 30 (35.7) | 62 (35.2) |
| Stage IV | 5 (5.4) | 5 (6.0) | 10 (5.7) |
| Anthropometrics | |||
| Weight, kg, mean (±SD) | 90.7 (18.2) | 69.9 (17.2) | 80.7 (20.5) |
| Height, cm, mean (±SD) | 176.5 (7.0) | 159.0 (8.6) | 168.3 (11.7) |
| BMI, kg/m2, mean (±SD) | 29.0 (5.0) | 27.8 (7.5) | 28.4 (6.3) |
| BMI category, kg/m2, N (%) | |||
| <20 | 2 (2.2) | 9 (11.1) | 11 (6.5) |
| 20–24.9 | 15 (16.9) | 21 (25.9) | 36 (21.2) |
| 25–29.9 | 41 (46.1) | 31 (38.3) | 72 (42.4) |
| 30–34.9 | 19 (21.3) | 10 (12.3) | 29 (17.1) |
| 35–39.9 | 10 (11.2) | 4 (4.9) | 14 (8.2) |
| ≥40 | 2 (2.2) | 6 (7.4) | 8 (4.7) |
| Body composition by CT analysis | |||
| Mean skeletal muscle index (SMI), cm2/m2 | 53.3 (9.8) | 40.9 (7.7) | 47.4 (10.8) |
| Mean skeletal muscle radiodensity (SMR), HU | 35.5 (9.1) | 35.7 (9.3) | 35.6 (9.2) |
| Subcutaneous adipose tissue index (SATI, cm2/m2), mean | 67.1 (29.0) | 103.6 (58.0) | 84.1 (48.4) |
| Visceral adipose tissue index (VATI, cm2/m2), mean | 79.7 (38.1) | 46.9 (36.9) | 64.3 (40.9) |
| Sarcopenia, myosteatosis or both, N (%) | 45 (49.5) | 38 (46.3) | 83 (48.0) |
| Sarcopenia alone, N (%) | 8 (8.8) | 16 (19.8) * | 24 (14.0) |
| Myosteatosis alone, N (%) | 28 (30.8) | 19 (23.5) | 47 (27.3) |
| Sarcopenia and Myosteatosis, N (%) | 9 (9.9) * | 2 (2.5) | 11 (6.4) |
| No sarcopenia or myosteatosis, N (%) | 46 (50.5) | 44 (53.7) | 90 (52.0) |
| Domain | Overall, N = 176 |
|---|---|
| Box 1: Weight Change | |
| Weight change past month, mean % (±SD) | −0.4 (3.4) |
| Weight change past 6 months, mean % (±SD) | −2.0 (5.5) |
| No change/increased weight in past 2 weeks, N (%) | 123 (69.9) |
| Decreased weight in past 2 weeks, N (%) | 53 (30.1) |
| Box 2: Food Intake | |
| Food intake past month, N (%) | |
| Unchanged/more than usual | 149 (84.7) |
| Less than usual | 27 (15.3) |
| Type of food intake, N (%) | |
| Normal food, normal amount | 143 (81.3) |
| Normal food, less than normal amount | 18 (10.2) |
| Little solid food | 3 (1.7) |
| Only liquids or nutritional supplements | 8 (4.5) |
| Very little of anything | 4 (2.3) |
| Only tube feeding/feeding by vein | 0 (0) |
| Box 3: Nutrition Impact Symptoms, N (%) | |
| No problems eating | 150 (85.2) |
| No appetite | 14 (8.0) |
| Nausea | 7 (4.0) |
| Constipation | 14 (8.0) |
| Diarrhea | 18 (10.2) |
| Vomiting | 3 (1.7) |
| Feel full quickly | 6 (3.4) |
| Foods taste funny or have no taste | 3 (1.7) |
| Smells bother me | 3 (1.7) |
| Mouth sores | 0 (0) |
| Problem swallowing | 3 (1.7) |
| Fatigue | 16 (9.1) |
| Pain | 4 (2.3) |
| Dry mouth | 7 (4.0) |
| Other | 4 (2.3) |
| Box 4: Activity and Function, N (%) | |
| Normal, no limitations | 115 (65.3) |
| Not normal self, fairly normal activities | 44 (25.0) |
| Not feeling up to most things, in bed or chair <half day | 8 (4.5) |
| Not able to do most things or pretty much bedridden | 7 (4.0) |
| PG-SGASF Domain | Score 0–3 | Score ≥ 4 | Overall |
|---|---|---|---|
| Box 1: Weight Change (max. 5; mean ± SD) | 0.27 (0.64) | 1.77 (1.38) | 0.64 (1.10) |
| Box 2: Food Intake (max. 5; mean ± SD) | 0.13 (0.53) | 1.68 (1.74) | 0.52 (1.19) |
| Box 3: Nutrition Impact Symptoms (max. 24; mean ± SD) | 0.08 (0.41) | 4.59 (4.30) | 1.20 (2.92) |
| Box 4: Activity and Function (max. 3; mean ± SD) | 0.22 (0.50) | 1.18 (0.95) | 0.47 (0.77) |
| Total Score, mean ± SD | 0.70 (1.05) | 9.23 (5.77) | 2.86 (4.79) |
| Triage Recommendation | N, % | ||
| 0–1 (no intervention, reassess regularly) | 104 (59.8) | ||
| 2–3 (patient/family education; pharmacological intervention as indicated by symptoms) | 26 (14.9) | ||
| 4–8 (intervention by RD and nurse or physician as indicated by symptoms) | 26 (14.9) | ||
| ≥9 (critical need for symptom management and nutrition intervention) | 18 (10.3) | ||
| Characteristic | PG-SGASF Score 0–3 | PG-SGASF Score ≥ 4 |
|---|---|---|
| Sarcopenia alone, N (%) | 15 (11.6) | 7 (17.1) |
| Myosteatosis alone, N (%) | 30 (23.3) | 17 (41.5) |
| Sarcopenia and myosteatosis, N (%) | 11 (8.5) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klassen, P.; Baracos, V.; Gramlich, L.; Nelson, G.; Mazurak, V.; Martin, L. Computed-Tomography Body Composition Analysis Complements Pre-Operative Nutrition Screening in Colorectal Cancer Patients on an Enhanced Recovery after Surgery Pathway. Nutrients 2020, 12, 3745. https://doi.org/10.3390/nu12123745
Klassen P, Baracos V, Gramlich L, Nelson G, Mazurak V, Martin L. Computed-Tomography Body Composition Analysis Complements Pre-Operative Nutrition Screening in Colorectal Cancer Patients on an Enhanced Recovery after Surgery Pathway. Nutrients. 2020; 12(12):3745. https://doi.org/10.3390/nu12123745
Chicago/Turabian StyleKlassen, Pamela, Vickie Baracos, Leah Gramlich, Gregg Nelson, Vera Mazurak, and Lisa Martin. 2020. "Computed-Tomography Body Composition Analysis Complements Pre-Operative Nutrition Screening in Colorectal Cancer Patients on an Enhanced Recovery after Surgery Pathway" Nutrients 12, no. 12: 3745. https://doi.org/10.3390/nu12123745
APA StyleKlassen, P., Baracos, V., Gramlich, L., Nelson, G., Mazurak, V., & Martin, L. (2020). Computed-Tomography Body Composition Analysis Complements Pre-Operative Nutrition Screening in Colorectal Cancer Patients on an Enhanced Recovery after Surgery Pathway. Nutrients, 12(12), 3745. https://doi.org/10.3390/nu12123745





