Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Undernutrition in Patients with Hip Fracture
3.1. Prevalence of Undernutrition
3.2. Impact of Undernutrition on Clinical Outcomes
3.3. Highlights of Undernutrition in Hip Fracture
4. Sarcopenia in Patients with Hip Fracture
4.1. Definition of Sarcopenia
4.2. Prevalence of Sarcopenia
4.3. Impact of Sarcopenia on Clinical Outcomes
4.4. Highlights of Sarcopenia in Hip Fracture
5. Frailty in Patients with Hip Fracture
5.1. Definition of Frailty
5.2. Prevalence of Frailty
5.3. Impact of Frailty on Clinical Outcomes
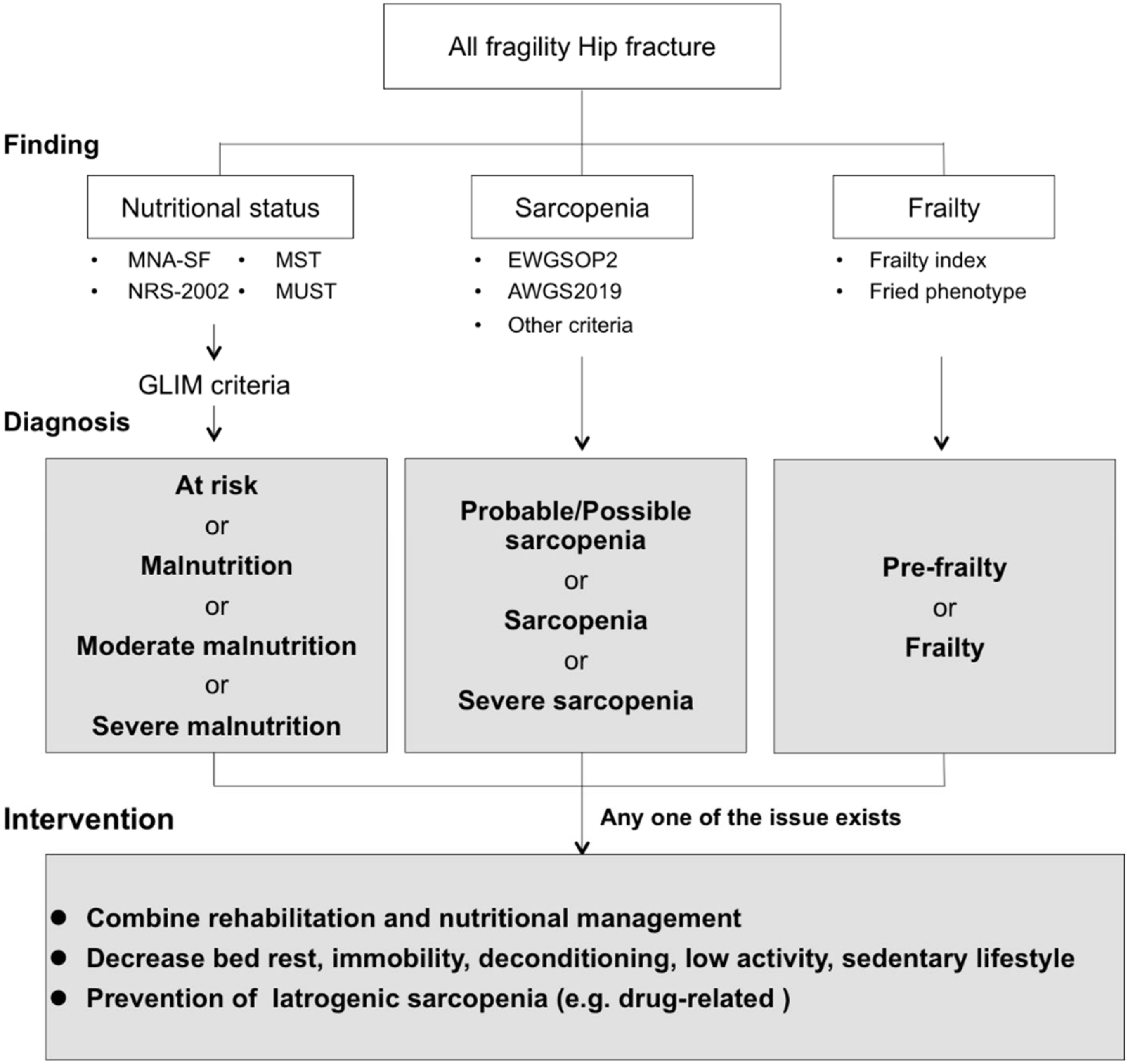
6. Nutritional Intervention for Patients with Hip Fracture
7. Combined Nutritional Intervention with Rehabilitation Exercise
8. Advanced Strategies for Improvement of Clinical Outcomes
9. Comprehensive Intervention Based on Combined Nutritional Intervention with Rehabilitation Exercise for Patients with Hip Fractures
10. Strengths and Limitations
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos. Int. 2004, 15, 897–902. [Google Scholar] [CrossRef]
- Cooper, C.; Campion, G.; Melton, L.J. Hip fractures in the elderly: A world-wide projection. Osteoporos. Int. 1992, 2, 285–289. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005, 16, 6–10. [Google Scholar] [CrossRef]
- Shyu, Y.-I.L.; Chen, M.-C.; Liang, J.; Wu, C.-C.; Su, J.-Y. Predictors of functional recovery for hip fractured elders during 12 months following hospital discharge: A prospective study on a Taiwanese sample. Osteoporos. Int. 2004, 15, 475–482. [Google Scholar] [CrossRef]
- Braithwaite, R.S.; Col, N.F.; Wong, J.B. Estimating hip fracture morbidity, mortality and costs. J. Am. Geriatr. Soc. 2003, 51, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Avenell, A.; Handoll, H. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- National Heart Lung and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 17 November 2020).
- Nishioka, S.; Wakabayashi, H.; Momosaki, R. Nutritional Status Changes and Activities of Daily Living after Hip Fracture in Convalescent Rehabilitation Units: A Retrospective Observational Cohort Study from the Japan Rehabilitation Nutrition Database. J. Acad. Nutr. Diet. 2018, 118, 1270–1276. [Google Scholar] [CrossRef]
- Helminen, H.; Luukkaala, T.; Saarnio, J.; Nuotio, M. Comparison of the Mini-Nutritional Assessment short and long form and serum albumin as prognostic indicators of hip fracture outcomes. Injury 2017, 48, 903–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren-Hakim, T.; Weiss, A.; Hershkovitz, A.; Otzrateni, I.; Grosman, B.; Frishman, S.; Salai, M.; Beloosesky, Y. The relationship between nutritional status of hip fracture operated elderly patients and their functioning, comorbidity and outcome. Clin. Nutr. 2012, 31, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, P.; Ward, L.; Zazzetta, S.; Broggini, V.; Anzuini, A.; Valcarcel, B.; Brathwaite, J.S.; Pasinetti, G.M.; Bellelli, G.; Annoni, G. Association Between Preoperative Malnutrition and Postoperative Delirium after Hip Fracture Surgery in Older Adults. J. Am. Geriatr. Soc. 2017, 65, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Misu, S.; Tanaka, T.; Sakamoto, H.; Iwata, K.; Chuman, Y.; Ono, R. Pre-fracture nutritional status is predictive of functional status at discharge during the acute phase with hip fracture patients: A multicenter prospective cohort study. Clin. Nutr. 2017, 36, 6–11. [Google Scholar] [CrossRef]
- Bajada, S.; Smith, A.; Morgan, D. Pre-operative nutritional serum parameters as predictors of failure after internal fixation in undisplaced intracapsular proximal femur fractures. Injury 2015, 46, 1571–1576. [Google Scholar] [CrossRef]
- Goisser, S.; Schrader, E.; Singler, K.; Bertsch, T.; Gefeller, O.; Biber, R.; Bail, H.J.; Sieber, C.C.; Volkert, D. Malnutrition According to Mini Nutritional Assessment Is Associated with Severe Functional Impairment in Geriatric Patients before and up to 6 Months after Hip Fracture. J. Am. Med. Dir. Assoc. 2015, 1–7. [Google Scholar] [CrossRef]
- Van Wissen, J.; van Stijn, M.F.M.; Doodeman, H.J.; Houdijk, A.P.J. Mini nutritional assessment and mortality after hip fracture surgery in the elderly. J. Nutr. Health Aging 2016, 20, 964–968. [Google Scholar] [CrossRef]
- Zanetti, M.; Gortan Cappellari, G.; Ratti, C.; Ceschia, G.; Murena, L.; De Colle, P.; Barazzoni, R. Poor nutritional status but not cognitive or functional impairment per se independently predict 1 year mortality in elderly patients with hip-fracture. Clin. Nutr. 2019, 38, 1607–1612. [Google Scholar] [CrossRef]
- Milte, R.; Miller, M. Dietetic care of hip fracture patients across Australia: Are we doing enough? Nutr. Diet. 2011, 68, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Yagi, T.; Oshita, Y.; Okano, I.; Kuroda, T.; Ishikawa, K.; Nagai, T.; Inagaki, K. Controlling nutritional status score predicts postoperative complications after hip fracture surgery. BMC Geriatr. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Kotera, A. Geriatric Nutritional Risk Index and Controlling Nutritional Status Score can predict postoperative 180-day mortality in hip fracture surgeries. JA Clin. Rep. 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, L.; Carson, J.L.; Schlussel, Y.; Noveck, H.; Shapses, S.A. Vitamin D deficiency is associated with reduced mobility after hip fracture surgery: A prospective study. Am. J. Clin. Nutr. 2020, 112, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Han, T.S.; Yeong, K.; Lisk, R.; Fluck, D.; Fry, C.H. Prevalence and consequences of malnutrition and malnourishment in older individuals admitted to hospital with a hip fracture. Eur. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vosoughi, A.R.; Emami, M.J.; Pourabbas, B.; Mahdaviazad, H. Factors increasing mortality of the elderly following hip fracture surgery: Role of body mass index, age, and smoking. Musculoskelet. Surg. 2017, 101, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Miyanishi, K.; Jingushi, S.; Torisu, T. Mortality after hip fracture in Japan: The role of nutritional status. J. Orthop. Surg. (Hong Kong) 2010, 18, 265–270. [Google Scholar] [CrossRef]
- Stone, A.V.; Jinnah, A.; Wells, B.J.; Atkinson, H.; Miller, A.N.; Futrell, W.M.; Lenoir, K.; Emory, C.L. Nutritional markers may identify patients with greater risk of re-admission after geriatric hip fractures. Int. Orthop. 2018, 42, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Gumieiro, D.N.; Rafacho, B.P.M.; Gonçalves, A.F.; Tanni, S.E.; Azevedo, P.S.; Sakane, D.T.; Carneiro, C.A.S.; Gaspardo, D.; Zornoff, L.A.M.; Pereira, G.J.C.; et al. Mini Nutritional Assessment predicts gait status and mortality 6 months after hip fracture. Br. J. Nutr. 2012, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Drevet, S.; Bioteau, C.; Mazière, S.; Couturier, P.; Merloz, P.; Tonetti, J.; Gavazzi, G. Prevalence of protein-energy malnutrition in hospital patients over 75 years of age admitted for hip fracture. Orthop. Traumatol. Surg. Res. 2014, 100, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Miu, K.Y.D.; Lam, P.S. Effects of nutritional status on 6-month outcome of hip fractures in elderly patients. Ann. Rehabil. Med. 2017, 41, 1005–1012. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Inoue, T.; Misu, S.; Tanaka, T.; Kakehi, T.; Ono, R. Acute phase nutritional screening tool associated with functional outcomes of hip fracture patients: A longitudinal study to compare MNA-SF, MUST, NRS-2002 and GNRI. Clin. Nutr. 2019, 38, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren-Hakim, T.; Weiss, A.; Hershkovitz, A.; Otzrateni, I.; Anbar, R.; Gross Nevo, R.F.; Schlesinger, A.; Frishman, S.; Salai, M.; Beloosesky, Y. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin. Nutr. 2015, 3–8. [Google Scholar] [CrossRef]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466–485, discussion 485–487. [Google Scholar] [PubMed]
- Cao, L.; Morley, J.E. Sarcopenia Is Recognized as an Independent Condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. J. Am. Med. Dir. Assoc. 2016, 17, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Edwards, M.H.; Dennison, E.M.; Aihie Sayer, A.; Fielding, R.; Cooper, C. Osteoporosis and sarcopenia in older age. Bone 2015, 80, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Nishiguchi, S.; Fukutani, N.; Tanigawa, T.; Yukutake, T.; Kayama, H.; Aoyama, T.; Arai, H. Prevalence of sarcopenia in community-dwelling Japanese older adults. J. Am. Med. Dir. Assoc. 2013, 14, 911–915. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Chen, L.; Woo, J.; Assantachai, P.; Auyeung, T.; Chou, M.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 200–307.e2. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Ortolani, E.; Salini, S.; Martone, A.M.; Santoro, L.; Santoliquido, A.; Sisto, A.; Picca, A.; Marzetti, E. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos. Int. 2017, 28, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- González-Montalvo, J.I.; Alarcón, T.; Gotor, P.; Queipo, R.; Velasco, R.; Hoyos, R.; Pardo, A.; Otero, A. Prevalence of sarcopenia in acute hip fracture patients and its influence on short-term clinical outcome. Geriatr. Gerontol. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, M.; Castiglioni, C.; De Toma, E.; Gardin, L.; Giordano, S.; Di Monaco, R.; Tappero, R. Presarcopenia and sarcopenia in hip-fracture women: Prevalence and association with ability to function in activities of daily living. Aging Clin. Exp. Res. 2015, 27, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.D.; Wu, J.S.; Mhuircheartaigh, J.N.; Hochman, M.G.; Rodriguez, E.K.; Appleton, P.T.; Mcmahon, C.J. Effect of sarcopenia on clinical and surgical outcome in elderly patients with proximal femur fractures. Skelet. Radiol. 2018, 47, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yi, S.R.; Lee, Y.H.; Kwon, J.; Jang, S.I.; Park, S.H. Effect of Sarcopenia on Postoperative Mortality in Osteoporotic Hip Fracture Patients. J. Bone Metab. 2018, 25, 227. [Google Scholar] [CrossRef]
- Yoo, J.I.; Kim, H.; Ha, Y.C.; Kwon, H.B.; Koo, K.H. Osteosarcopenia in patients with hip fracture is related with high mortality. J. Korean Med. Sci. 2018, 33, 1–9. [Google Scholar] [CrossRef]
- Steihaug, O.M.; Gjesdal, C.G.; Bogen, B.; Kristoffersen, M.H.; Lien, G.; Hufthammer, K.O.; Ranhoff, A.H. Does sarcopenia predict change in mobility after hip fracture? A multicenter observational study with one-year follow-up. BMC Geriatr. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Malafarina, V.; Malafarina, C.; Ugarte, A.B.; Martinez, J.A.; Goñi, I.A.; Zulet, M.A. Factors associated with sarcopenia and 7-year mortality in very old patients with hip fracture admitted to rehabilitation units: A pragmatic study. Nutrients 2019, 11, 2243. [Google Scholar] [CrossRef] [Green Version]
- Byun, S.E.; Kim, S.; Kim, K.H.; Ha, Y.C. Psoas cross-sectional area as a predictor of mortality and a diagnostic tool for sarcopenia in hip fracture patients. J. Bone Miner. Metab. 2019, 37, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Wong, P.K.; Tsai, M.J.; Chang, W.C.; Hsieh, T.S.; Leu, T.H.; Jeff Lin, C.F.; Lee, C.H.; Kuo, Y.J.; Lin, C.Y. The high prevalence of sarcopenia and its associated outcomes following hip surgery in Taiwanese geriatric patients with a hip fracture. J. Formos. Med. Assoc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chiles Shaffer, N.; Huang, Y.; Abraham, D.S.; Cheng, Y.J.; Lu, W.; Gruber-Baldini, A.L.; Hochberg, M.C.; Guralnik, J.; Magaziner, J.; Orwig, D. Comparing Longitudinal Sarcopenia Trends by Definitions Across Men and Women After Hip Fracture. J. Am. Geriatr. Soc. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.C.; Jang, J.H.; Seo, H.E.; Suh, K.T.; Moon, N.H. Prevalence and clinical impact of sarcopenia in osteoporotic hip fracture: Single center retrospective cohort study. Acta Orthop. Traumatol. Turc. 2020, 54, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Nagano, A.; Maeda, K.; Shimizu, A.; Nagami, S.; Takigawa, N.; Ueshima, J.; Suenaga, M. Association of sarcopenic dysphagia with underlying sarcopenia following hip fracture surgery in older women. Nutrients 2020, 12, 1365. [Google Scholar] [CrossRef]
- Ha, Y.C.; Won, C.W.; Kim, M.; Chun, K.J.; Yoo, J.-I. SARC-F as a Useful Tool for Screening Sarcopenia in Elderly Patients with Hip Fractures. J. Nutr. Health Aging 2020, 24, 78–82. [Google Scholar] [CrossRef]
- Steihaug, O.M.; Gjesdal, C.G.; Bogen, B.; Kristoffersen, M.H.; Lien, G.; Ranhoff, A.H. Sarcopenia in patients with hip fracture: A multicenter cross-sectional study. PLoS ONE 2017, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ho, A.W.H.; Lee, M.M.L.; Chan, E.W.C.; Ng, H.M.Y.; Lee, C.W.; Ng, W.S.; Wong, S.H. Prevalence of pre-sarcopenia and sarcopenia in Hong Kong Chinese geriatric patients with hip fracture and its correlation with different factors. Hong Kong Med. J. 2015, 22, 8–10. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Lorenzi, M.; Marini, F.; D’Angelo, E.; Martone, A.M.; Celi, M.; Tosato, M.; Bernabei, R.; Landi, F. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older hip fractured patients. Exp. Gerontol. 2014, 60, 79–82. [Google Scholar] [CrossRef]
- Yoo, J.I.; Ha, Y.C.; Choi, H.; Kim, K.H.; Lee, Y.K.; Koo, K.H.; Park, K.S. Malnutrition and chronic inflammation as risk factors for sarcopenia in elderly patients with hip fracture. Asia Pac. J. Clin. Nutr. 2018, 27, 527–532. [Google Scholar] [CrossRef]
- Sánchez-Castellano, C.; Martín-Aragón, S.; Bermejo-Bescós, P.; Vaquero-Pinto, N.; Miret-Corchado, C.; Merello de Miguel, A.; Cruz-Jentoft, A.J. Biomarkers of sarcopenia in very old patients with hip fracture. J. Cachexia Sarcopenia Muscle 2020, 11, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Lorenzi, M.; Tanganelli, F.; Picca, A.; Bossola, M.; Menghi, A.; Bernabei, R.; Landi, F. Association between myocyte quality control signaling and sarcopenia in old hip-fractured patients: Results from the Sarcopenia in HIp FracTure (SHIFT) exploratory study. Exp. Gerontol. 2016, 80, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Lin, H.-S.; Watts, J.N.; Peel, N.M.; Hubbard, R.E. Frailty and post-operative outcomes in older surgical patients: A systematic review. BMC Geriatr. 2016, 16, 157. [Google Scholar] [CrossRef] [Green Version]
- Rolland, Y.; Abellan Van Kan, G.; Benetos, A.; Blain, H.; Bonnefoy, M.; Chassagne, P.; Jeandel, C.; Laroche, M.; Nourhashemi, F.; Orcel, P.; et al. Frailty, osteoporosis and hip fracture: Causes, consequences and therapeutic perspectives. J. Nutr. Health Aging 2008, 12, 335–346. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Med. Sci. 2001, 56, 46–56. [Google Scholar] [CrossRef]
- Rockwood, K.; Mcdowell, I.; Song, X.; Macknight, C.; Bergman, H.; Hogan, D.B.; Hogan, D.; Mcdowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 30, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.V.; Brennan, K.L.; Brennan, M.L.; Jupiter, D.C.; Shar, A.; Davis, M.L. Association of a modified frailty index with mortality after femoral neck fracture in patients aged 60 years and older. Clin. Orthop. Relat. Res. 2014, 472, 1010–1017. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, M.; Beck, S.; Havelock, W.; Eeles, E.; Hubbard, R.E.; Johansen, A. Predicting outcome after hip fracture: Using a frailty index to integrate comprehensive geriatric assessment results. Age Ageing 2014, 43, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Kistler, E.A.; Nicholas, J.A.; Kates, S.L.; Friedman, S.M. Frailty and Short-Term Outcomes in Patients with Hip Fracture. Geriatr. Orthop. Surg. Rehabil. 2015, 6, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleason, L.J.; Benton, E.A.; Alvarez-Nebreda, M.L.; Weaver, M.J.; Harris, M.B.; Javedan, H. FRAIL Questionnaire Screening Tool and Short-Term Outcomes in Geriatric Fracture Patients. J. Am. Med. Dir. Assoc. 2017, 18, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Cho, K.J.; Kim, S.W.; Yoon, S.J.; Kang, M.G.; Kim, K.I.; Lee, Y.K.; Koo, K.H.; Kim, C.H. Prediction of Mortality and Postoperative Complications using the Hip-Multidimensional Frailty Score in Elderly Patients with Hip Fracture. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Winters, A.M.; Hartog, L.C.; Roijen, H.I.F.; Brohet, R.M.; Kamper, A.M. Relationship between clinical outcomes and Dutch frailty score among elderly patients who underwent surgery for hip fracture. Clin. Interv. Aging 2018, 13, 2481–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasu, B.K.; Ramamurthi, K.P.; Rajan, S.; George, M. Geriatric Patients with Hip Fracture: Frailty and Other Risk Factors Affecting the Outcome. Anesth. Essays Res. 2018, 12, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Chen, C.M.; Wang, C.Y.; Ko, P.W.; Chen, C.H.; Hsieh, C.P.; Chiu, H.C. Frailty is Associated with an Increased Risk of Major Adverse Outcomes in Elderly Patients Following Surgical Treatment of Hip Fracture. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, T.; Misu, S.; Tanaka, T.; Kakehi, T.; Kakiuchi, M.; Chuman, Y.; Ono, R. Frailty defined by 19 items as a predictor of short-term functional recovery in patients with hip fracture. Injury 2019, 50, 2272–2276. [Google Scholar] [CrossRef] [PubMed]
- Van De Ree, C.L.P.; Landers, M.J.F.; Kruithof, N.; De Munter, L.; Slaets, J.P.J.; Gosens, T.; Jongh, M.A.C. Effect of frailty on quality of life in elderly patients after hip fracture: A longitudinal study. BMJ Open 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jorissen, R.N.; Lang, C.; Visvanathan, R.; Crotty, M.; Inacio, M.C. The effect of frailty on outcomes of surgically treated hip fractures in older people. Bone 2020, 136, 115327. [Google Scholar] [CrossRef]
- Lu, W.; Dai, L.; Wu, G.; Hu, R. Comparison of two frailty indexes in hip fractures. J. Orthop. Surg. 2020, 28, 1–8. [Google Scholar] [CrossRef]
- Pizzonia, M.; Giannotti, C.; Carmisciano, L.; Signori, A.; Rosa, G.; Santolini, F.; Caffa, I.; Montecucco, F.; Nencioni, A.; Monacelli, F. Frailty assessment, hip fracture, and long-term clinical outcomes in older adults. Eur. J. Clin. Investig. 2020, e13445. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Wee, E.; Dorevitch, M. Impact of place of residence, frailty and other factors on rehabilitation outcomes post hip fracture. Age Ageing 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Narula, S.; Lawless, A.; D’Alessandro, P.; Jones, C.W.; Yates, P.; Seymour, H. Clinical Frailty Scale is a good predictor of mortality after proximal femur fracture. Bone Jt. Open 2020, 1, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Botella-Carretero, J.I.; Iglesias, B.; Balsa, J.A.; Zamarrón, I.; Arrieta, F.; Vázquez, C. Effects of oral nutritional supplements in normally nourished or mildly undernourished geriatric patients after surgery for hip fracture: A randomized clinical trial. JPEN J. Parenter. Enter. Nutr. 2008, 32, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Botella-Carretero, J.I.; Iglesias, B.; Balsa, J.A.; Arrieta, F.; Zamarrón, I.; Vázquez, C. Perioperative oral nutritional supplements in normally or mildly undernourished geriatric patients submitted to surgery for hip fracture: A randomized clinical trial. Clin. Nutr. 2010, 29, 574–579. [Google Scholar] [CrossRef]
- Espaulella, J.; Guyer, H.; Diaz-Escriu, F.; Mellado-Navas, J.A.; Castells, M.; Pladevall, M. Nutritional supplementation of elderly hip fracture patients. A randomized, double-blind, placebo-controlled trial. Age Ageing 2000, 29, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Houwing, R.H.; Rozendaal, M.; Wouters-Wesseling, W.; Beulens, J.W.J.; Buskens, E.; Haalboom, J.R. A randomised, double-blind assessment of the effect of nutritional supplementation on the prevention of pressure ulcers in hip-fracture patients. Clin. Nutr. 2003, 22, 401–405. [Google Scholar] [CrossRef]
- Myint, M.W.W.; Wu, J.; Wong, E.; Chan, S.P.; To, T.S.J.; Chau, M.W.R.; Ting, K.H.; Fung, P.M.; Au, K.S.D. Clinical benefits of oral nutritional supplementation for elderly hip fracture patients: A single blind randomised controlled trial. Age Ageing 2013, 42, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Neumann, M.; Friedmann, J.; Roy, M.A.; Jensen, G.L. Provision of high-protein supplement for patients recovering from hip fracture. Nutrition 2004, 20, 415–419. [Google Scholar] [CrossRef]
- Tidermark, J.; Ponzer, S.; Carlsson, P.; Söderqvist, A.; Brismar, K.; Tengstrand, B.; Cederholm, T. Effects of protein-rich supplementation and nandrolone in lean elderly women with femoral neck fractures. Clin. Nutr. 2004, 23, 587–596. [Google Scholar] [CrossRef]
- Wyers, C.E.; Reijven, P.L.M.; Breedveld-Peters, J.J.L.; Denissen, K.F.M.; Schotanus, M.G.M.; Van Dongen, M.C.J.M.; Eussen, S.J.P.M.; Heyligers, I.C.; Van Den Brandt, P.A.; Willems, P.C.; et al. Efficacy of Nutritional Intervention in Elderly after Hip Fracture: A Multicenter Randomized Controlled Trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1429–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malafarina, V.; Uriz-Otano, F.; Malafarina, C.; Martinez, J.A.; Zulet, M.A. Effectiveness of nutritional supplementation on sarcopenia and recovery in hip fracture patients. A multi-centre randomized trial. Maturitas 2017, 101, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Platz, A.; Orav, E.J.; Stähelin, H.B.; Willett, W.C.; Can, U.; Egli, A.; Mueller, N.J.; Looser, S.; et al. Effect of High-Dosage Cholecalciferol and Extended Physiotherapy on Complications After Hip Fracture. Arch. Intern. Med. 2010, 170, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, A.; Kennedy, C.C.; Giangregorio, L.; Ioannidis, G.; Pritchard, J.; Hanley, D.A.; Farrauto, L.; Debeer, J.; Adachi, J.D. A randomized controlled trial of vitamin D dosing strategies after acute hip fracture: No advantage of loading doses over daily supplementation. BMC Musculoskelet. Disord. 2011, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachrach-Lindström, M.; Unosson, M.; Ek, A.C.; Arnqvist, H.J. Assessment of nutritional status using biochemical and anthropometric variables in a nutritional intervention study of women with hip fracture. Clin. Nutr. 2001, 20, 217–223. [Google Scholar] [CrossRef]
- Chevalley, T.; Hoffmeyer, P.; Bonjour, J.P.; Rizzoli, R. Early serum IGF-I response to oral protein supplements in elderly women with a recent hip fracture. Clin. Nutr. 2010, 29, 78–83. [Google Scholar] [CrossRef]
- Niitsu, M.; Ichinose, D.; Hirooka, T.; Mitsutomi, K.; Morimoto, Y.; Sarukawa, J.; Nishikino, S.; Yamauchi, K.; Yamazaki, K. Effects of combination of whey protein intake and rehabilitation on muscle strength and daily movements in patients with hip fracture in the early postoperative period. Clin. Nutr. 2016, 35, 943–949. [Google Scholar] [CrossRef]
- Ekinci, O.; Yanlk, S.; Terzioǧlu Bebitoǧlu, B.; Yllmaz Akyüz, E.; Dokuyucu, A.; Erdem, Ş. Effect of Calcium β-Hydroxy-β-Methylbutyrate (CaHMB), Vitamin D, and Protein Supplementation on Postoperative Immobilization in Malnourished Older Adult Patients with Hip Fracture. Nutr. Clin. Pract. 2016, 31, 829–835. [Google Scholar] [CrossRef]
- Anbar, R.; Beloosesky, Y.; Cohen, J.; Madar, Z.; Weiss, A.; Theilla, M.; Koren Hakim, T.; Frishman, S.; Singer, P. Tight Calorie Control in geriatric patients following hip fracture decreases complications: A randomized, controlled study. Clin. Nutr. 2014, 33, 23–28. [Google Scholar] [CrossRef]
- Kim, H.K.; Suzuki, T.; Saito, K.; Yoshida, H.; Kobayashi, H.; Kato, H.; Katayama, M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012, 60, 16–23. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, P.T.; Nishino, P.T.; Kuzuhara, P.T.; Tomioka, P.T. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for Treating Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Am. Med. Dir. Assoc. 2017, 18, 553.e1–553.e16. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Dreyer, H.C.; Fry, C.S.; Glynn, E.L.; Rasmussen, B.B. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J. Appl. Physiol. 2009, 106, 1374–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, H. Rehabilitation nutrition in general and family medicine. J. Gen. Fam. Med. 2017, 18, 153–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, A.; Nishioka, S.; Wakabayashi, H. Rehabilitation Nutrition for Iatrogenic Sarcopenia and Sarcopenic Dysphagia. J. Nutr. Health Aging 2019, 23, 256–265. [Google Scholar] [CrossRef]
- Davenport, S.J.; Arnold, M.; Hua, C.; Schenck, A.; Batten, S.; Taylor, N.F. Physical Activity Levels During Acute Inpatient Admission After Hip Fracture are Very Low. Physiother. Res. Int. 2015, 2050, 174–181. [Google Scholar] [CrossRef]
- Martone, A.M.; Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Di Bari, M.; Maggio, M.; Manca, G.M.; Marzetti, E.; et al. The incidence of sarcopenia among hospitalized older patients: Results from the Glisten study. J. Cachexia Sarcopenia Muscle 2017, 8, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Murayama, I.; Asai, T.; Misu, S.; Yamauchi, M.; Miura, A.; Ikemura, T.; Takehisa, T.; Takehisa, Y. Is increased “stay away from bed” time associated with improved clinical rehabilitation outcomes in Japanese rehabilitation hospitals? A prospective observational study and clinical practice. Aging Clin. Exp. Res. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, T.; Misu, S.; Tanaka, T.; Sakamoto, H.; Iwata, K.; Chuman, Y.; Ono, R. Inadequate Postoperative Energy Intake Relative to Total Energy Requirements Diminishes Acute Phase Functional Recovery from Hip Fracture. Arch. Phys. Med. Rehabil. 2019, 100, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Foss, N.B.; Jensen, P.S.; Kehlet, H. Risk factors for insufficient perioperative oral nutrition after hip fracture surgery within a multi-modal rehabilitation programme. Age Ageing 2007, 36, 538–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudge, A.M.; Ross, L.J.; Young, A.M.; Isenring, E.A.; Banks, M.D. Helping understand nutritional gaps in the elderly (HUNGER): A prospective study of patient factors associated with inadequate nutritional intake in older medical inpatients. Clin. Nutr. 2011, 30, 320–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, J.J.; Bauer, J.D.; Capra, S.; Pulle, R.C. Multidisciplinary, multi-modal nutritional care in acute hip fracture inpatients—Results of a pragmatic intervention. Clin. Nutr. 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Sakuma, K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 2014, 5, 269–277. [Google Scholar] [CrossRef]
- Maeda, K.; Akagi, J. Treatment of Sarcopenic Dysphagia with Rehabilitation and Nutritional Support: A Comprehensive Approach. J. Acad. Nutr. Diet. 2016, 116, 573–577. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, Country | Design, Setting | Age (Years) Male/Female, n (%) | Sample Size | Evaluation Tool (Timing of Assessment) | Prevalence of Undernutrition | Outcome | Main Results |
|---|---|---|---|---|---|---|---|
| Miyanishi et al., 2010 [26] Japan | Observational study, acute hospital | Mean 79 24 (18.9)/103 (81.1) | 129 | Serum albumin BMI | Not stated | Four-year mortality | In, multiple logistic regression analysis, serum albumin level (OR 5.854, p < 0.001) and BMI (OR 1.169, p = 0.02) significantly influenced mortality. |
| Koren-Hakim et al., 2012 [13] Israel | Observational study, acute hospital | Mean 83.5 (SD 6.0) 61 (28.4)/154 (71.6) | 215 | MNA-SF (at admission and up to 48 h after admission) | Well-nourished: 44.2% At risk: 44.2% Malnourished: 11.6% | In-hospital complications Mortality (up to 36 months) | Only comorbidity and low functioning can predict long-term mortality (a minimum of 12 up to 36 months). Nutritional status had no effect on outcomes. |
| Gumieiro et al., 2012 [28] Brazil | Prospective observational study, general hospital | Mean 80.2 (SD 7.3) 20 (23.3)/66 (76.7) | 86 | MNA-FF NRS-2002 (within the first 72 h of the patient’s admission) | Not stated | Gait status (patients who could walk or could not walk) and mortality at 6 months after hip fracture | In a multivariate analysis, only the MNA-FF was associated with gait status (OR 0.773, 95% CI 0.663−0.901) and mortality 6 months after hip fracture (HR 0.869, 95% CI 0.757−0.998). |
| Drevet et al., 2014 [29] France | Prospective observational study, university hospital | Mean 86.1 (SD 4.4) 15 (30)/35 (70) | 50 | MNA-FF (no details provided) | At risk for PEM: 58% PEM: 28% | Activities of daily living Hospital stay | PEM was associated with functional dependence (p = 0.002) and 8 days longer mean hospital stay (p = 0.012). |
| Goisser et al., 2015 [17] Germany | Prospective observational study, urban maximum care hospital | Mean 84 (SD 5) (21)/(79) | 97 | MNA-FF (preoperative nutritional status was evaluated retrospectively) | At risk: 38% Malnourished: 17% | Barthel Index after 6 months | Malnourished patients suffered more from remaining losses in ADL ≥25% of initial Barthel Index points (p = 0.033), and regained their prefracture mobility level to a lesser extent (p = 0.020) than well-nourished patients. |
| Bajada et al., 2015 [16] UK | Retrospective observational study, general hospital | Mean 79 years (range: 60–96 years) 19 (18)/89 (82) | 108 | Serum albumin (normal level > 35 g/L) Lymphocyte count (normal 1−4.5 × 109 L) (on admission) | No details provided | Failure of internal fixation | In binary logistic regression analysis, lymphocyte count, and albumin levels were independent predictors of failure of internal fixation. |
| van Wissen et al., 2016 [18] Netherlands | Retrospective cohort study, acute hospital | Mean Malnourished: 85 (SD 5) At risk: 84 (SD 5) Well-nourished: 83 (SD 5) 61 (27.0)/165(73.0) | 226 | MNA-FF (before surgery) | Well-nourished: 4.9% At risk: 26.5% Malnourished: 68.6% | Hospital stay Postoperative complications, Mortality (in-hospital and 1-year) | Preoperative malnutrition is associated with in-hospital (OR 4.4; 95% CI 1.0, 20.4) and 1-year mortality (OR 2.7; 95% CI 1.1, 7.0). Malnutrition was not associated with any other outcome. |
| Miu et al., 2017 [30] China | Observational study, rehabilitation unit | Mean 83.5 (SD 7.5) 74 (33.9)/44 (66.1) | 218 | MNA-SF (within 72 h of admission) | Well-nourished: 21.1% At risk: 52.6% Malnourished: 26.1% | Functional status and place of residence at 6 months Hospital stay Mortality (in-hospital, 6 months) | Functional recovery was slower in the malnourished group. In-patient mortality was higher in malnourished patients than in those at risk of malnourishment and well-nourished individuals. |
| Helminen et al., 2017 [12] Finland | Prospective observational study, acute hospital | No details provided 169 (28.5)/425 (71.5) | 594 | MNA-SF MNA-FF Serum albumin (preoperative period) | MNA-SF Well-nourished: 53% At risk: 40% Malnourished: 7% MNA-FF Well-nourished: 35% At risk: 58% Malnourished: 7% Serum albumin <34 g/L: 46% | Poorer mobility (transfer to more assisted living accommodation) Mortality (1 month, 4 months, and 1 year after fracture) | Risk of malnutrition and malnutrition measured by MNA-FF predicted mobility and living arrangements within 4 months of hip fracture. At 1 year, risk of malnutrition predicted mobility and malnutrition predicted living arrangements when measured by the MNA-FF. Malnutrition, but not risk measured by the MNA-SF, predicted living arrangements at all time points. Neither measure predicted 1-month mobility. |
| Vosoughi et al., 2017 [25] Iran | Cross-sectional study, university hospital | Mean 75.7 (SD 10.6) 318 (43.9)/406 (56.1) | 724 | BMI (at admission) | No details provided | Mortality at 3 months and 1 year | Multivariate logistic regression analysis recognized age (OR 1.08; 95% CI 1.05, 1.11), BMI (OR 0.88; 95% CI 0.82−0.96), and smoking (OR 1.76; 95% CI 1.05−2.96) as major independent risk factors for 1- and 3-year mortality. |
| Mazzola et al., 2017 [14] Italy | Prospective observational study, university hospital | Mean 84.0 (SD 6.6) 106 (25.5)/309 (74.5) | 415 | MNA-SF (within 24 h of admission) | Well-nourished: 36.6% At risk: 44.6% Malnourished: 18.8% | Postoperative delirium | Multivariate regression analysis showed that those at risk of malnutrition (OR 2.42; 95% CI = 1.29–4.53) and those overtly malnourished (OR 2.98; 95% CI = 1.43–6.19) were more likely to develop postoperative delirium. |
| Inoue et al., 2017 [15] Japan | Prospective observational study, three acute hospitals | Mean 82.7 (SD 9.2) 69 (10.1)/165 (80.9) | 204 | MNA-SF (first few days after admission before surgery) | Well-nourished: 27.0% At risk: 48.0% Malnourished: 25.0% | FIM at discharge | In multiple regression analyses, MNA-SF was a significant independent predictor for FIM at discharge (well-nourished vs. malnourished, β = 0.86, p < 0.01). |
| Nishioka et al., 2018 [11] Japan | Retrospective observational cohort study, convalescent rehabilitation units | Mean 85 years (21.8)/(78.2) | 110 | MNA-SF (on admission and at discharge) | Only malnourished patients at admission were included | FIM at discharge Discharged to home | Multivariable analysis revealed a significant association between improvement in nutritional status and higher FIM score at discharge (β = 7.377, p = 0.036). No association with discharge to home. |
| Stone et al., 2018 [27] USA | Retrospective observational cohort study, acute hospital | Not stated 241(39.7)/366(60.3) | 607 | Albumin Prealbumin Total protein Vitamin D | Not stated | Thirty-day readmission | The model incorporated four nutritional makers (albumin, prealbumin, total protein, and vitamin D) with an internally cross-validated C-statistic of 0.811 (95% CI; 0.754, 0.867). |
| Zanetti et al., 2018 [19] Italy | Observational study, acute hospital | Mean 84.7 (SD 7.4) 259 (21.4)/952 (78.6) | 1211 | MNA-FF (within 72 h from admission) | Mean MNA-FF score: 22.3 (SD 5.1) | Three, six and twelve-month mortality | Poor nutritional status was significantly associated with 3, 6, and 12- month mortality after adjustment for potential confounders. |
| Kotera et al. 2019 [22] Japan | Retrospective observational cohort study, acute hospitals | Mean 87 (SD 6) Not stated | 607 | GNRI CONUT | GNRI Normal: 0.8% Light: 3.0% Moderate: 5.7% Severe: 14.4% CONUT Normal: 1.6% Light: 2.7% Moderate: 8.1% Severe: 38.9% | Mortality of 180 days | The GNRI value in the nonsurvivors was significantly lower than that in the survivors. The CONUT value in the nonsurvivors was significantly higher than that in the survivors. |
| Yagi et al., 2020 [21] Japan | Retrospective observational cohort study, community-based hospital | Median 86 years (interquartile range 80–90) (19.9)/(80.1) | 211 | CONUT (admission day) | Malnourished (CONUT score >1): 78.7% | Postoperative complications | Multivariable analysis found that the CONUT score was an independent risk factor for postoperative complications (OR 1.21; 95% CI = 1.01–1.45). |
| Hao et al., 2020 [23] USA | Retrospective observational study (secondary analysis), 47 sites in North America | Mean 82 (SD 7) (27)/(73) | 290 | 25-hydroxyvitamin D GNRI (preoperative) | 25-hydroxyvitamin D (ng/mL) ≥30: 17% 20 to <30: 37% 12 to <20: 34% <12: 12% GNRI No risk: 33 Some risk: 33 Major/moderate risk: 34 | Mortality and mobility at 30 and 60 days after surgery | Compared with patients with <12 ng/mL, those with higher 25(OH)D concentrations had higher rates of walking at 30 days (p = 0.031): 12 to <20 ng/mL (adjusted OR 2.61; 95% CI 1.13, 5.99); 20 to <30 ng/mL (3.48; 1.53, 7.95); ≥30 ng/mL (2.84; 1.12, 7.20). There was also greater mobility at 60 days (p = 0.028) in patients with higher 25(OH)D compared with the reference group (<12 ng/mL). GNRI <92 showed an overall trend to reduce mobility (adjusted p = 0.056) at 30 but not at 60 days. There was no association of vitamin D or GNRI with mortality at either time. |
| Han et al., 2020 [24] UK | Retrospective observational study, National Health Service hospital | Mean 83.8 (SD 8.6) 349(28.2)/890(71.8) | 1239 | MUST | Low risk Medium risk High risk | Mobilization (starting rehabilitation within 1 day after surgery) Pressure ulcers In-patient mortality Change in discharge destination | Compared with the well-nourished group, malnourished individuals showed increased risk for failure to mobilize within 1 day of surgery (OR 1.6; 95% CI 1.0–2.7), pressure ulcers (OR 5.5, 95% CI, 1.8–17.1), in-patient mortality (OR 2.3; 95% CI, 1.1–4.8), and discharge to residential/nursing care (OR 2.8; 95% CI, 1.2–6.6). |
| Author, Year, Country | Design, Setting | Age Male/Female, n (%) | Sample Size | Diagnosis Criteria Measurement Methods of Muscle Strength, Muscle Mass, Physical Function | Prevalence of Sarcopenia | Outcome | Main Results |
|---|---|---|---|---|---|---|---|
| González-Montalvo et al., 2015 [45] Spain | Prospective observational study, university hospital | Mean 85.3 (SD 6.8) 47 (20.3)/382 (79.7) | 479 | EWGSOP Handgrip strength Bioimpedance analysis | 17.1% | Barthel Index at discharge | In the multivariate analysis, sarcopenia was not associated with functional prognosis at discharge (OR 1.68, 95% CI 0.99–2.84). |
| Di Monaco et al., 2015 [46] Italy | Observational study, rehabilitation hospital | Normal: 78.9 (SD 7.7) Presarcopenia: 73.8 (SD 5.5) Sarcopenia: 81.3 (SD 7.5) All female: 138 (100) | 138 | EWGSOP Handgrip strength Dual-energy X-ray absorptiometry | Presarcopenia: 17% Sarcopenia: 58% | Barthel Index (at admission, end of the rehabilitation course) | Sarcopenia was associated with Barthel Index scores at the time of assessment but not at the end of the rehabilitation course after adjusting for multiple adjustments (p < 0.001). |
| Landi et al., 2017 [43] Italy | Observational study, Geriatric Rehabilitation Unit of the hospital | Mean age 81.3 (SD 4.8) 45 (36.4)/82 (64.6) | 127 | FNIH Dual-energy X-ray absorptiometry | Sarcopenia: 48% | Barthel Index (at discharge and 3 months after discharge) | After adjustment for potential confounders, participants with sarcopenia had a significantly increased risk of incomplete functional recovery compared with nonsarcopenic patients (OR 3.07, 95% CI 1.07–8.75). |
| Di Chang et al., 2018 [47] Taiwan | Retrospective observational study, university hospital | Mean age 81.1 (SD 12.2) 24 (26.4)/67 (73.6) | 91 | Computed tomography (total skeletal muscle area at L4) | No details provided | Hospital stay Perioperative mortality Medical complications In-hospital blood transfusion volume Readmission rate at 90 days | Low skeletal muscle index was independently associated with longer length of hospitalization (p = 0.032) but was not associated with any other outcomes. |
| Kim et al., 2018 [48] Korea | Retrospective observational study, National Police Hospital | Mean 78.5 years (range, 65–94 years) 27 (29.7)/64 (70.3) | 91 | Choi et al. reported criteria Computed tomography (L3) | 49.5% | One-year and five-year mortality rates | Kaplan–Meier analysis showed that sarcopenia did not affect the 1-year mortality rate (p = 0.793) but had a significant effect on the 5-year mortality rate (p = 0.028). Both perioperative sarcopenia (p = 0.018) and osteoporosis (p < 0.001) affected the 5-year mortality rate. |
| Yoo et al., 2018 [49] Korea | Retrospective observational study, university hospital | Mean 77.8 (SD 9.7) 78 (24.1)/246 (75.9) | 324 | AWGS Handgrip strength Dual-energy X-ray absorptiometry | 37.7% | One-year mortality | Osteosarcopenia (15.1%) was higher for 1-year mortality than other groups (normal: 7.8%, osteoporosis alone: 5.1%, sarcopenia alone: 10.3%). |
| Steihaug et al., 2018 [50] Norway | Prospective observational study, acute hospital (three hospitals) | Mean 79.4 (SD 8.2) (24)/(76) | 282 | EWGSOP Handgrip strength The formula reported by Heymsfield et al. (using gender, height, arm circumference, and triceps skinfold) New Mobility Score | 38% | Change in New Mobility Score Resident of a nursing home Death | Sarcopenia did not predict change in mobility (p = 0.6), but it was associated with having lower mobility at 1-year (p = 0.003), becoming a resident of a nursing home (OR 3.2, p = 0.048), and the combined endpoint of becoming a resident of a skilled nursing home or death (OR 3.6, p = 0.02). |
| Malafarina et al., 2019 [51] Spain | Prospective observational study, two rehabilitation units | Mean 85.2 (SD 6.3) 49 (26.2)/138 (73.8) | 187 | EWGSOP2 Handgrip strength Bioimpedance analysis 4 meter walking test | Incident sarcopenia during hospitalization: 54 patients Sarcopenia at admission and at discharge (chronic sarcopenia): 41 patients Sarcopenic at admission but reverted sarcopenia during the admission period (reverted sarcopenia): 17 patients | Mortality after 7 years | Cox regression analyses showed that sarcopenia was a risk factor for mortality (HR: 1.67, 95% CI 1.11–2.51) and low handgrip strength (HR: 1.76, 95% CI 1.08–2.88). |
| Byun et al., 2019 [52] Korea | Retrospective study, university hospital | Mean 78.4 (SD 9.7) 121 (24.5)/373 (75.5) | 494 | AWGS Handgrip strength Computed tomography (psoas cross-sectional area at L4–L5 level) | No details provided | One-year mortality | After adjusting for potential confounders, the lowest quintile of psoas cross-sectional area was significantly associated with mortality only in females (HR 1.76, 95% CI 1.05–2.70). |
| Chen et al., 2020 [53] Hong Kong | Prospective observational study, acute hospital | Mean 80.72 (SD 9.66) 36 (25.9)/103 (74.1) | 139 | AWGS Handgrip strength Dual-energy X-ray absorptiometry | 50.36% | EQ5D and Barthel Index at 6 months after the operation | After 6 months, patients with sarcopenia had a poor Barthel Index and a lower EQ5D than patients without sarcopenia before injury. |
| Chiles Shaffer et al., 2020 [54] USA | Prospective observational study, the seventh cohort of the Baltimore Hip Studies | Male: 81.0 (SD 7.5) Female: 80.2 (SD 7.6) 82 (51.3)/78 (48.7) | 160 | EWGSOP IWGS FNIH Handgrip strength Dual-energy X-ray absorptiometry Gait speed | No details provided | Sarcopenia prevalence over 12 months after hip fracture | Sarcopenia prevalence was stable over time in men by all definitions, whereas the prevalence in women by FNIH was lowest at 2 months, significantly increased at 6 months (p = 0.03) and remained higher at 12 months. Sarcopenia prevalence differed significantly by sex and varied by time point and definition; however, when different, men had a higher prevalence than women did (p < 0.05). |
| Shin et al., 2020 [55] Korea | Retrospective cohort study, university Hospital | Mean age 74.1 (range, 25–96) 35 (25.9)/100 (74.1) | 135 | AWGS Dual-energy X-ray absorptiometry | 45.9% | Harris Hip Score Parker’s mobility score at the last follow-up Discharge disposition | In multiple regression analysis, no significant association was found between sarcopenia and the Harris Hip Score of mobility at the last follow-up, nonunion, or time to union. |
| Nagano et al., 2020 [56] Japan | Retrospective observational study, acute hospital | Mean 85.9 (SD 6.5) All female patients, 89 (100) | 89 | AWGS 2019 Handgrip strength Bioimpedance analysis | 76.4% | Incidence of dysphagia on day 7 and discharge | All patients who developed dysphagia had underlying sarcopenia. |
| Ha et al., 2020 [57] Korea | Cross-sectional study, acute hospital | Not sarcopenia: 76.02 (SD 6.87) Sarcopenia: 82.62 (SD 7.72) 22 (19.1)/93 (80.9) | 115 | SARC-F, EWGSSOP2, AWGS, IWGS Handgrip strength Dual-energy X-ray absorptiometry | SARC-F: 63.5% EWGS2: 43 (37.4%) AWGS: 35 (30.4%) IWGS: 60 (52.2%) | Comparison of the results with criteria | Accuracy of SARC-F was that the sensitivity, specificity, positive predictive value, negative predictive value, and positive predictive value with the EWGSOP2 criteria as the reference standard were 95.35%, 56.94%, 56.94%, 95.35%, and 71.3%, respectively. |
| Author, Year, Country | Design, Setting | Age Male/Female, n (%) | Sample Size | Diagnosis Criteria Details of Criteria | Prevalence of Frailty | Outcome | Main Results |
|---|---|---|---|---|---|---|---|
| Patel et al., 2014 [70] USA | Retrospective observational study, acute hospital | Mean 81.05 (SD 8.45) No gender details provided | 697 | Modified frailty index 19 items Comorbidities, cognitive function, and walking ability | No details provided | One-year and two-year mortality rates after femoral neck fracture | Patients with a modified frailty index had an OR of 4.97 for 1-year mortality and an OR of 4.01 for 2-year mortality as compared with patients with an index less than 4. |
| Krishnan et al., 2014 [71] UK | Prospective study, university-affiliated community hospital | Mean 81 (range, 47–101) 47 (26.5)/131 (735) | 178 | Frailty index Fifty-one deficits Motivation, self-rated health, cognitive assessments, clock face drawing, comorbidities, continence, mobility, and functional independence Low-frailty group (FI ≤ 0.25), intermediate (FI > 0.25–0.4), high-FI group (FI > 0.4) | Low-frailty group (FI ≤ 0.25): 56 (31.5%) Intermediate (FI >0.25–0.4): 58 (32.5%) High (FI >0.4): 64 (36%) | Hospital stay Discharge disposition | The mean length of hospital stay for the intermediate group was 36.3 days in the high-FI group compared with 67.8 days in the high-FI group (p < 0.01). 30-day mortality was 3.4% for the intermediate group compared with 17.2% for the high-FI group (p < 0.001). |
| Kistler et al., 2015 [72] USA | Prospective observational study, university-affiliated community hospital | Mean 86 (SD 4) 6 (17)/29 (83) | 35 | Fried frailty index (modified for a post fracture population) Shrinking, exhaustion, slowness, weakness, and physical activity Participants with a total score of 3 or higher were considered frail | 51% | Overall hospital complication rate Hospital stay Complications | Frail patients (67%) versus nonfrail patients (29%) had a complication (p = 0.028). Mean length of stay was longer in patients with frailty (7.3 (SD) 5.9 vs. 4.1 (SD) 1.2 days, p = 0.038). |
| Gleason et al., 2017 [73] USA | Retrospective observational study, acute hospital | Mean 82.3 (SD 7.4) 44 (25.1)/131 (74.9) | 175 | The FRAIL scale Five-question assessment Fatigue, resistance, aerobic capacity, illnesses, and loss of weight Classified the patients into three categories: robust (score = 0), prefrail (score = 1–2), and frail (score = 3–5) | Robust (n = 29): 16.6% Prefrail (n = 73): 41.7% Frail (n = 73): 41.7% | Postoperative complications Unplanned intensive care unit admission Hospital stay Discharge disposition 30-day readmission and mortality | There was a statistically significant association between frailty and both length of stay (4.2, 5.0, and 7.1 days, p = 002, in robust, prefrail, and frail groups) and the development of any complication (3.4%, 26%, and 39.7%, p = 0.03) after surgery. There were also significant differences in discharge disposition (31% of robust vs. 4.1% frailty, p = 0.008) and follow-up completion (97% of robust vs. 69% of frail). |
| Choi et al., 2017 [74] Korea | Retrospective study, university hospital | Mean 80.4 (IQR 75.3–85.3) 139 (28.8)/343 (71.3) | 481 | Hip-Multidimensional Frailty Score Sex, Charlson Comorbidity Index, Albumin, Koval grade, risk of falling, MNA, and mid-arm circumference High risk: >8 and low risk: ≤8 | High risk: 24.3% | One-year all-cause mortality Postoperative complication Hospital stay Institutionalization | High-risk patients showed a higher risk of six-month mortality (HR: 3.545, 95% CI: 1.466–8.572) than low-risk patients after adjustment. Hip-Multidimensional Frailty Score could predict six-month mortality, postoperative complications, and prolonged hospital stay after surgery. Hip-Multidimensional Frailty Score more precisely predicted six-month mortality than age or existing tools (p values of comparison of ROC curve: 0.002, 0.004, and 0.044 for the ASA classification, age, and NHFS, respectively). |
| Winters et al., 2108 [75] Netherlands | Retrospective observational cohort study, general hospital | Mean 83.0 (SD 6.6) 71 (25)/215 (75) | 280 | Groningen Frailty Indicator questionnaire Consisted of 15 questions Physical, cognitive, social, and psychological impairments Score on a scale of 0–15 Score of 4 or higher suggests frailty VeiligheidsManagementSysteem Three items (cognitive impairment or confusion during earlier admissions, falls in the last 6 months, and physical impairments) Falling and another question to determine the frailty | Groningen Frailty Indicator questionnaire: 60% VeiligheidsManagementSysteem: 58% | Mortality 3-years and 30 days after surgery | VMS showed a statistically significant difference in overall survival as compared to nonfrail patients (57 vs 80%, respectively, p < 0.001) with an HR of 3.5 (95% CI 2.1–5.7; p < 0.001)). Classification according to GFI yielded a lower but still significant HR 2.3 (95% CI 1.2–4.1; p = 0.008). |
| Vasu et al., 2018 [76] India | Retrospective observational study, acute hospital | Not stated 34 (56.7)/26 (43.3) | 60 | Modified frailty index Nineteen items Comorbidities, cognitive function, and walking ability | Mean modified frailty index score: 3 | 90 days mortality | Modified frailty index and 90-day mortality showed a significantly direct correlation, with <0.001. |
| Chen et al., 2019 [77] Taiwan | Prospective observational cohort study | ≤75: 34.3% 76–85: 41.2% ≥86: 25.5% 79 (32.2)/166 (67.8) | 245 | Chinese-Canadian Study of Health and Aging Clinical Frailty Scale Ranged from 1 (very fit) to 7 (severely frail). | Robust: 31.4%. Prefrail: 46.1% Frail: 22.4% | 1, 3, and 6-month postoperative emergency department visits Readmissions Mortality | More cumulative events occurred for frail than for robust patients for each adverse outcome. Frailty had a long-term effect on each adverse outcome. |
| Inoue et al., 2019 [78] Japan | Retrospective observational study, two acute hospitals | Mean 83.7 (SD 7.4) 52 (19.3)/217 (80.7) | 274 | Modified frailty index Nineteen items Comorbidities, cognitive function, and walking ability | Mean modified frailty score: 3.2 ±1.9 points (minimum to a maximum range of 0 to 9) | Efficiency on the motor-Functional Independence Measure Postoperative complication Discharge disposition | Higher modified frailty index was significantly associated with increased likelihood of lower functional recovery (OR, 1.60; 95% CI, 1.32–1.93), occurrence of postoperative complication (OR, 1.32; 95% CI, 1.13–1.54) and not returning home (OR, 1.77; 95% CI, 1.38–2.26). |
| Van De Ree et al., 2019 [79] Netherlands | Prospective observational study, 10 participating Dutch hospitals | Mean 80.27 (SD 8.62) 206 (29.6)/490 (70.4) | 696 | Groningen Frailty Indicator questionnaire Consisted of 15 questions Physical, cognitive, social, and psychological impairments Score on a scale of 0–15 Score of 4 or higher suggests frailty | 53.3% | EuroQol-5 Dimensions ICEpop CAPability measure for Older people | Frailty was negatively associated with EuroQol-5 Dimensions (β −0.333; 95% CI −0.366 to −0.299), self-rated health (β −21.9; 95% CI −24.2 to −19.6), and capability and well-being (β −0.296; 95% CI −0.322 to −0.270) 1 year after hip fracture. |
| Jorissen et al., 2020 [80] Australia | Retrospective cohort study, historical national cohort of the Registry of Senior Australians | Mean 85.8 (SD 6.3) 1164 (24.4)/3607 (75.6) | 4771 | Frailty index Forty-four deficits Eight activity limitations, 24 health conditions, and three signs and symptoms 0–0.18 (quartile 1), 0.19–0.23 (quartile 2), 0.24–0.27 (quartile 3), and 0.28–0.41 (quartile 4) | Quartile 1: 1307 (27.4%) Quartile 2: 1158 (24.3%) Quartile 3: 1123 (23.5%) Quartile 4: 1183 (24.8%) | 2 year survival ADL limitations Permanent residential aged care for patients living in the community | The two-year survival of patients following hip fracture was 43.7% (95% CI 40.9–46.7%) in those in the highest quartile of frailty, compared with 54.4% (95% CI 51.8–57.2%) for those in the lowest quartile (HR = 1.25, 95% CI 1.11–1.41). No associations were found between pre-fracture frailty and post fracture ADL limitations. No association of frailty with transition to permanent residential aged care for patients living in the community was observed (HR = 0.98, 95% CI 0.81–1.18). |
| Lu et al., 2020 [81] China | Longitudinal and observational study, university hospital | Mean 77.5 (SD 8.5) 43 (33)/87 (67) | 130 | The modified Krishnan FI Physical health, mental health, cognitive function, self-care ability, life satisfaction, and social function The Canadian study of health and aging frailty index Cognition, existing diseases, self-care deficits, and abnormal physical signs | The modified Krishnan FI Low: 39% Medium: 50% High: 12% The Canadian study of health and aging frailty index Low: 63% Medium: 36% High: 0.8% | Death Rate of readmission to the hospital Fall within 3 months Hip function Daily activities at 3 months after surgery | The modified Krishnan FI correlated with the Japanese Orthopedic Association hip score (pain, activity, walking ability, and ability for daily living; R = 0.249, p = 0.005), whereas the Canadian study of health and aging frailty index was not correlated (R = 0.125, p = 0.170). Both the modified Krishnan FI (R = 0.415, p < 0.001) and the Canadian study of health and aging frailty index (R = 0.332, p < 0.001) were significantly correlated with the functional recovery scale score. |
| Pizzonia et al., 2020 [82] Italy | Prospective observational study, acute hospital | Mean 86.5 (SD 5.65) 80 (22)/284 (78) | 364 | Modified frailty index 19 items Comorbidities, cognitive function, and walking ability | Robust: 2.2% Prefrail: 14.9% Frail: 82.9% | Mortality (median follow-up of 2.4 years) | Modified frailty index was predictive of long-term mortality. |
| Low et al., 2020 [83] Australia | Prospective cohort study, rehabilitation and two geriatric evaluation and management wards | Median 86 years (interquartile range 81–90) 254 (30.1)/590 (69.9) | 844 | Clinical Frailty Scale 9 points scale | 69.9% | FIM efficiency Mobility Discharge disposition | Clinical Frailty Scale was the strongest independent predictor of poorer FIM efficiency, inability to recover pre-fracture mobility, and return to community dwelling. |
| Narula et al., 2020 [84] Australia | Retrospective observational study, acute hospital | Nonfrail: 73.8 (8.8) Vulnerable: 80.3 (9.0) Mildly frail: 84.3 (8.3) Moderately frail: 84.7 (6.9) Severely frail: 86.6 (7.3) 135 (26.5)/374 (73.5) | 509 | Clinical Frailty Scale 9 points scale | Non frail: 15.7% Vulnerable: 17.9% Mildly frail: 23.0% Moderately frail: 13.8% Severely frail: 29.7% | 30 day and 1-year mortality | The Clinical Frailty Scale demonstrated superior discriminative ability in predicting mortality (area under the curve 0.699; 95% CI 0.651 to 0.747) when compared with the ASA and chronological age groups. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, T.; Maeda, K.; Nagano, A.; Shimizu, A.; Ueshima, J.; Murotani, K.; Sato, K.; Tsubaki, A. Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes. Nutrients 2020, 12, 3743. https://doi.org/10.3390/nu12123743
Inoue T, Maeda K, Nagano A, Shimizu A, Ueshima J, Murotani K, Sato K, Tsubaki A. Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes. Nutrients. 2020; 12(12):3743. https://doi.org/10.3390/nu12123743
Chicago/Turabian StyleInoue, Tatsuro, Keisuke Maeda, Ayano Nagano, Akio Shimizu, Junko Ueshima, Kenta Murotani, Keisuke Sato, and Atsuhiro Tsubaki. 2020. "Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes" Nutrients 12, no. 12: 3743. https://doi.org/10.3390/nu12123743
APA StyleInoue, T., Maeda, K., Nagano, A., Shimizu, A., Ueshima, J., Murotani, K., Sato, K., & Tsubaki, A. (2020). Undernutrition, Sarcopenia, and Frailty in Fragility Hip Fracture: Advanced Strategies for Improving Clinical Outcomes. Nutrients, 12(12), 3743. https://doi.org/10.3390/nu12123743






