Functional Connectome Alterations Across the Spectrum of Alzheimer’s Disease
Abstract
1. Introduction
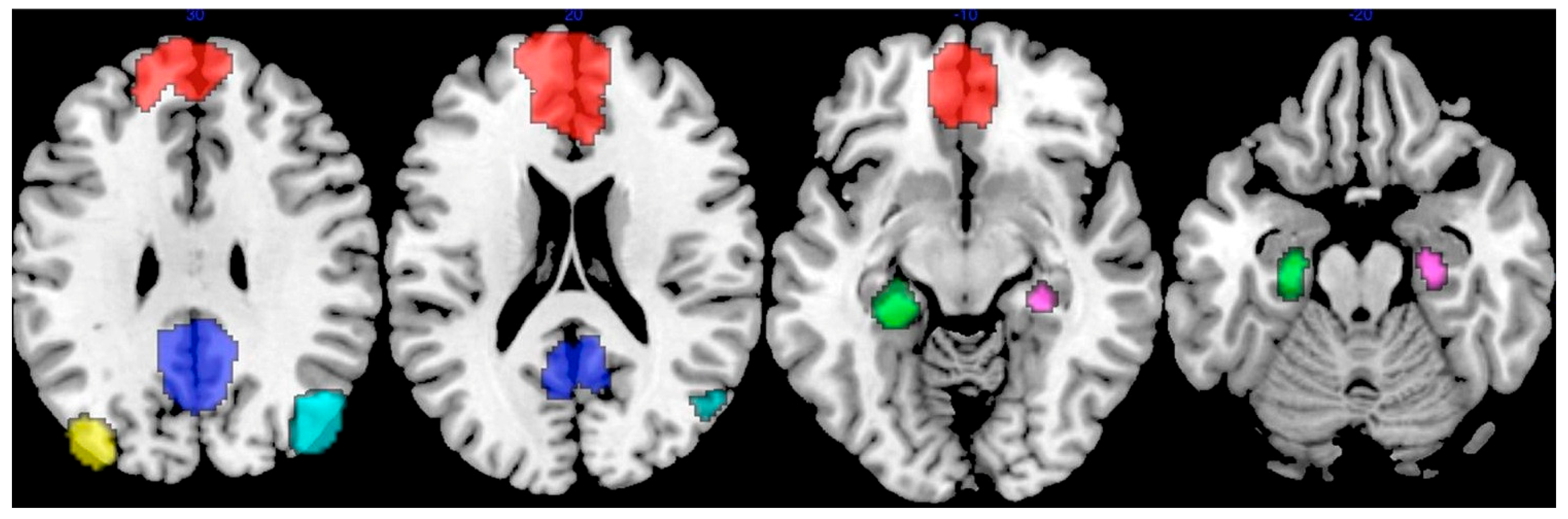
2. Preclinical AD
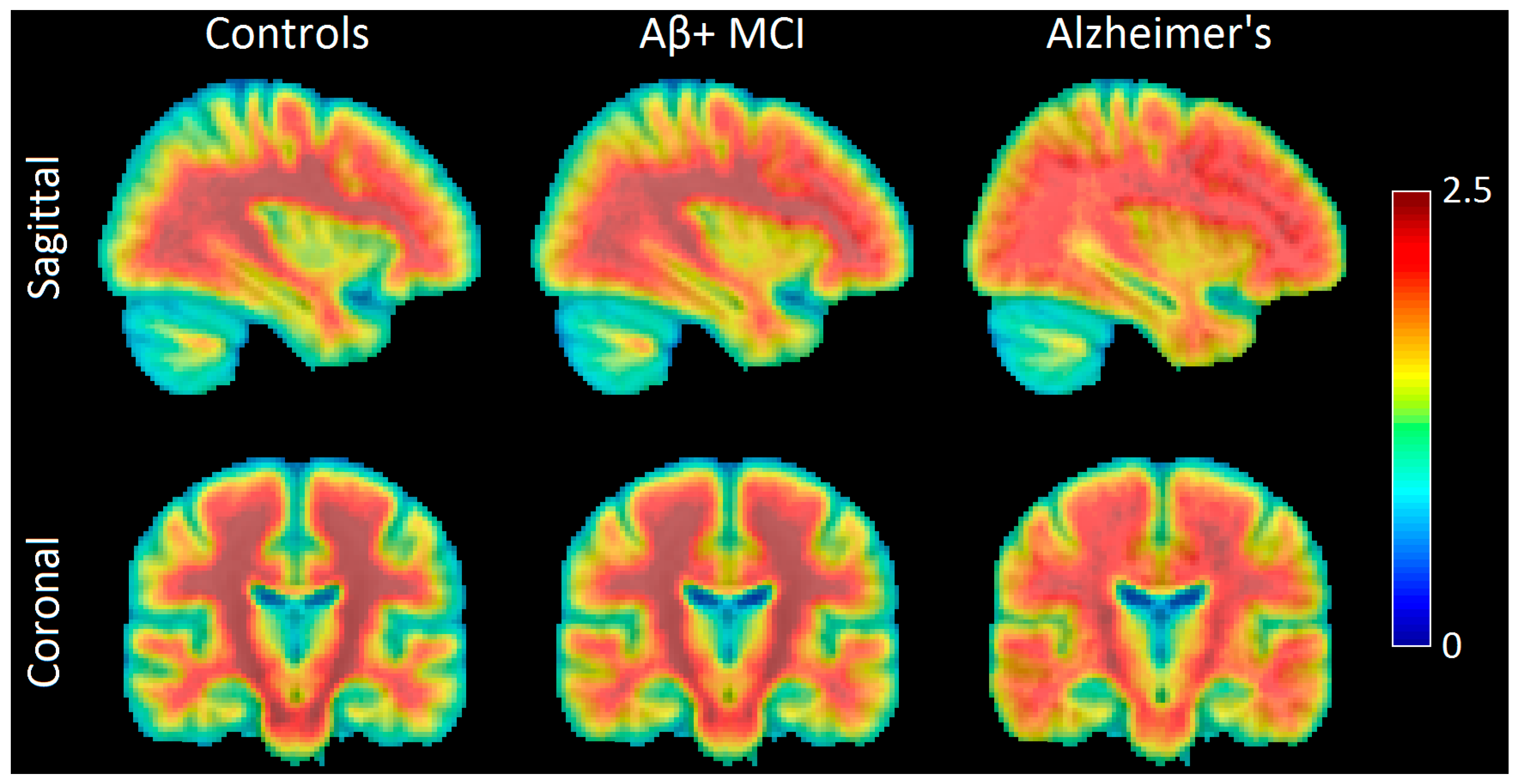
2.1. Aβ-Related Changes
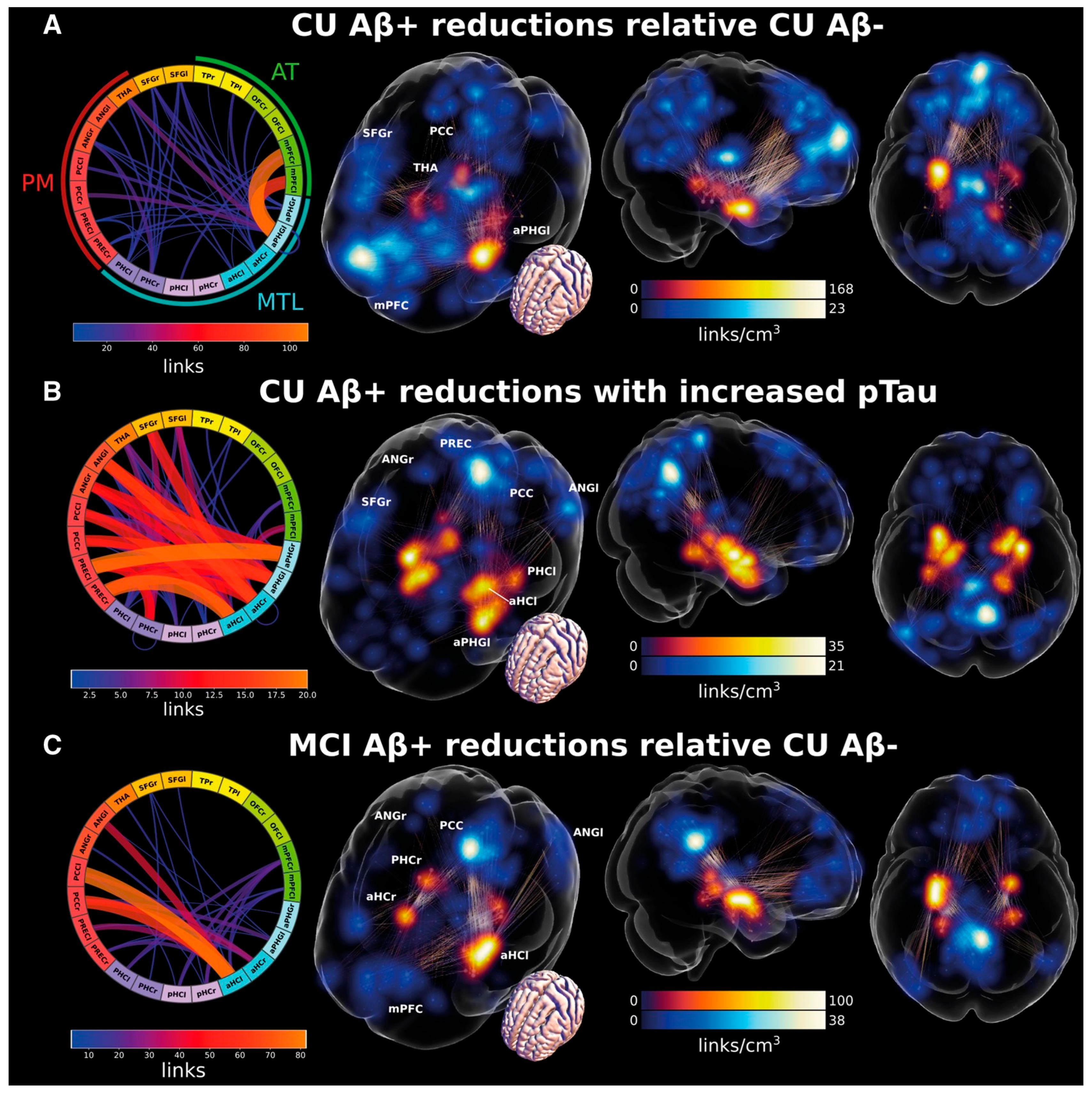
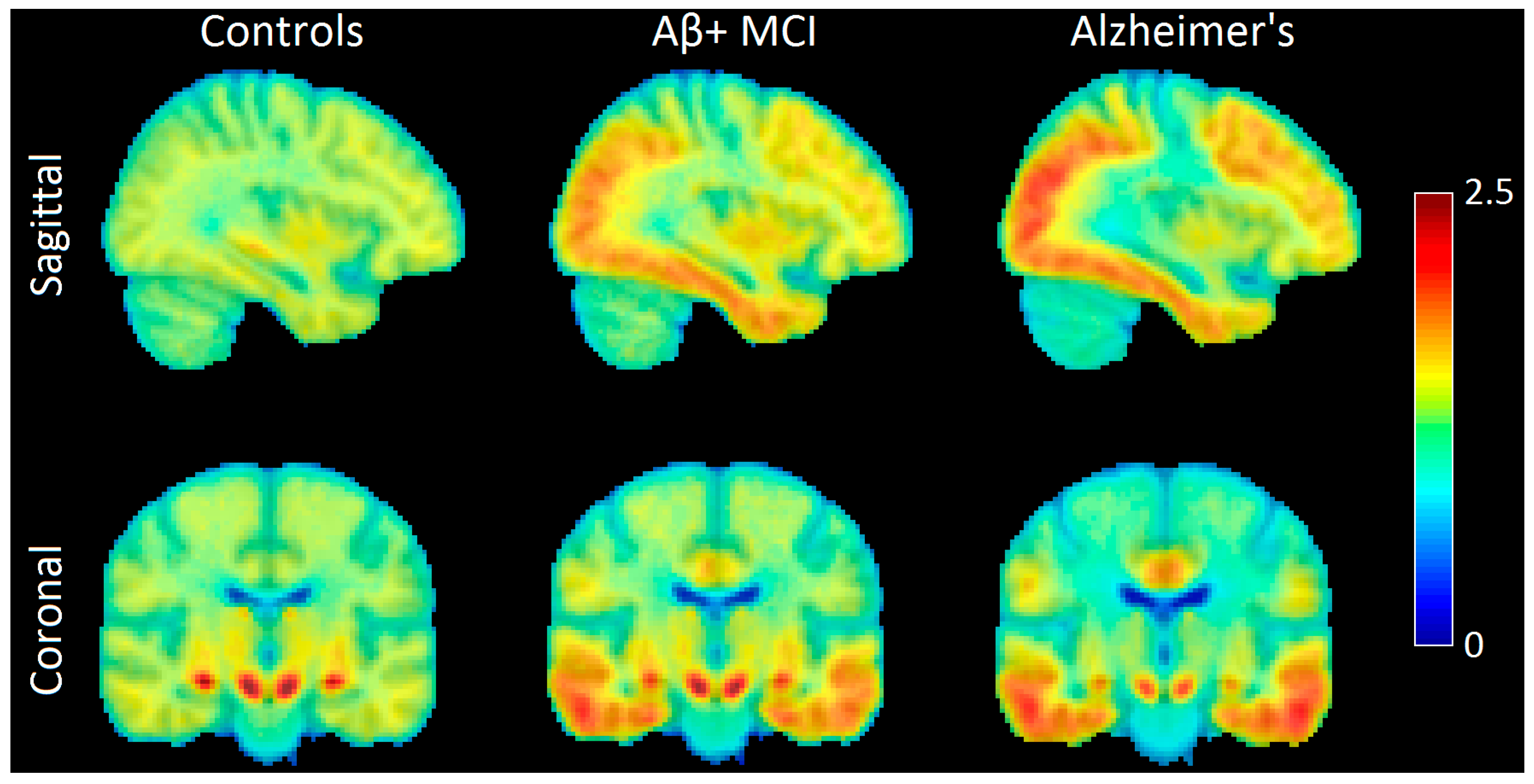
2.2. Tau-Related Changes
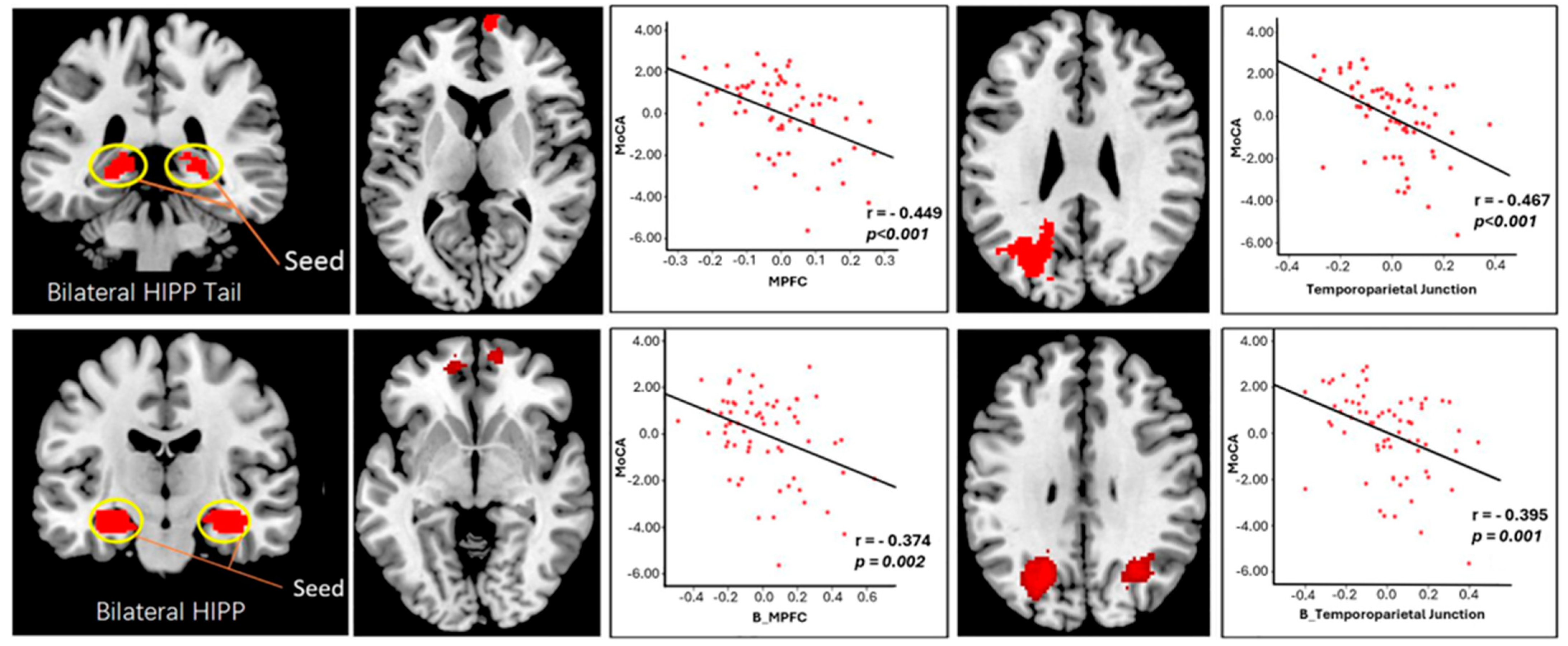
2.3. Connectome Changes in SCD Subjects
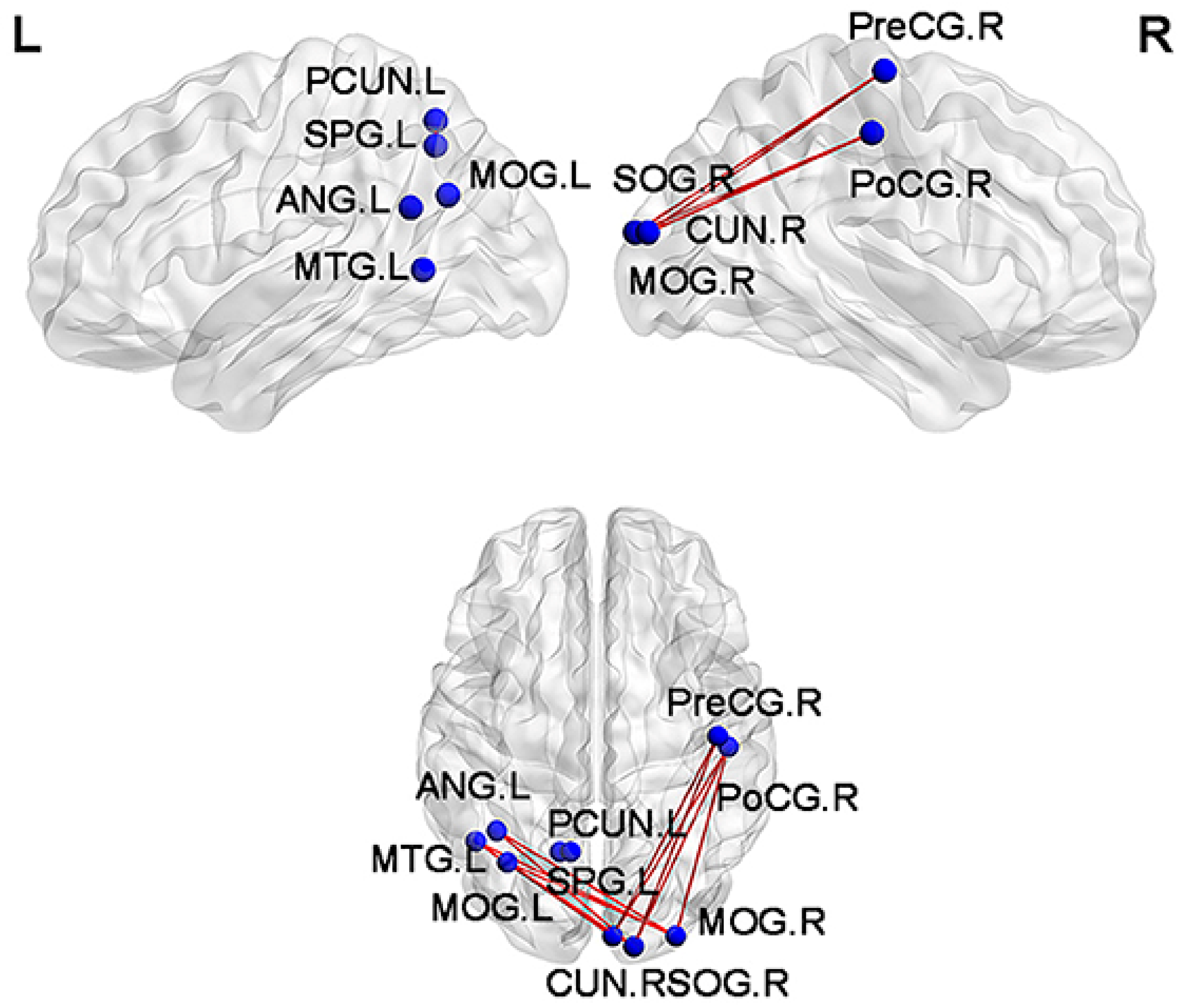
3. Mild Cognitive Impairment
4. Overt Alzheimer’s Disease
5. Brain Fingerprints in AD
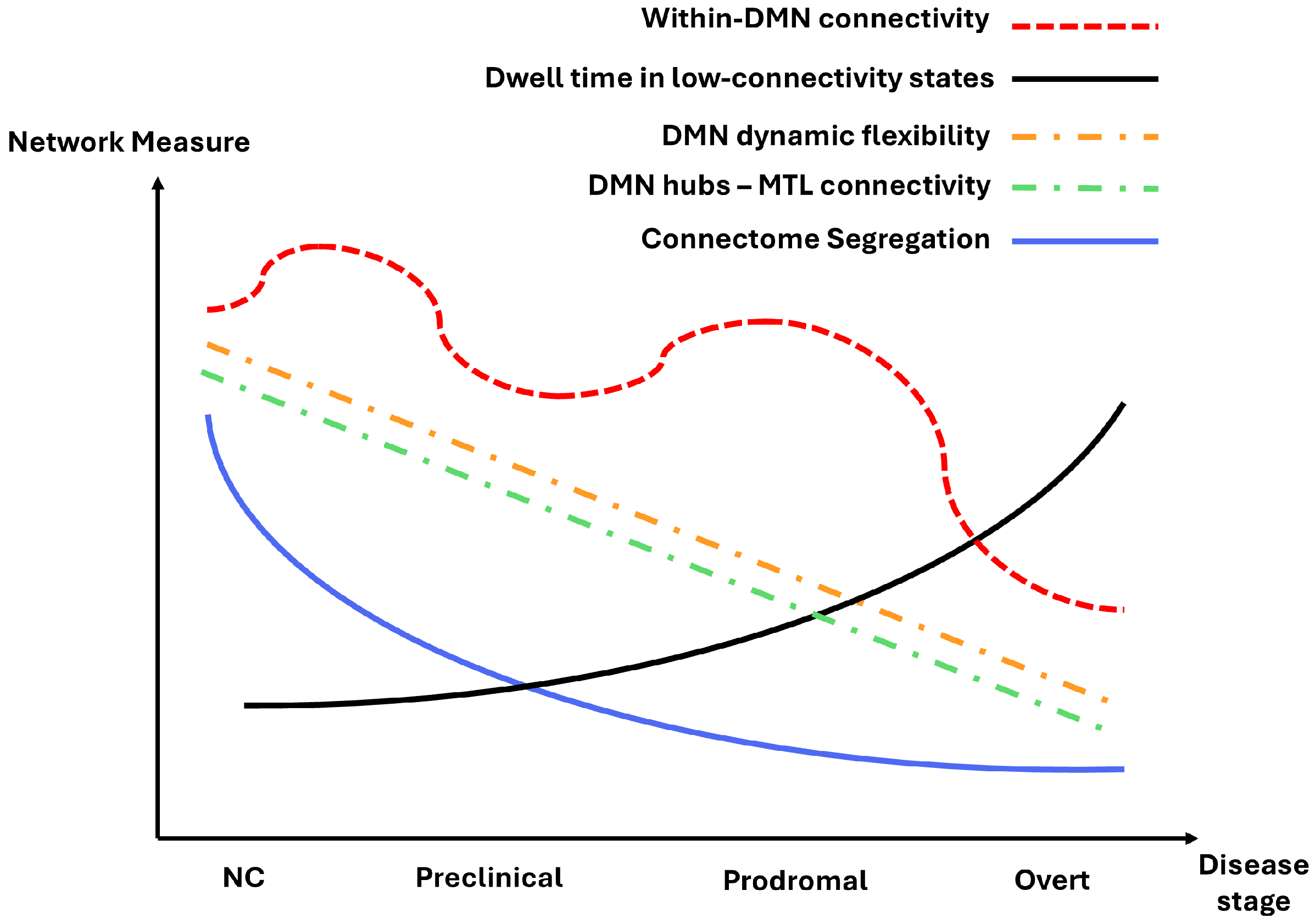
6. Conclusions and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aMCI | amnestic mild cognitive impairment |
| Aβ | amyloid-beta |
| AD | Alzheimer’s disease |
| BNM | basal nucleus of Meynert |
| BOLD | blood oxygen level-dependent |
| CPM | connectome-based predictive modeling |
| CSF | cerebrospinal fluid |
| DAN | dorsal attention network |
| DMN | default mode network |
| EEG | electroencephalography |
| EMCI | Early stage mild cognitive impairment |
| FC | functional connectivity |
| FPN | frontoparietal network |
| ICA | independent component analysis |
| LMCI | late-stage mild cognitive impairment |
| MEG | magnetoencephalography |
| mPFC | medial prefrontal cortex |
| MoCA | Montreal Cognitive Assessment |
| MCI | mild cognitive impairment |
| MMSE | Mini-Mental State Examination |
| MTL | medial temporal lobe |
| NCs | normal controls |
| pMCI | progressive mild cognitive impairment |
| PCC | posterior cingulate cortex |
| PET | positron emission tomography |
| PiB | Pittsburgh Compound B |
| p-tau | phosphorylated tau |
| rTMS | repetitive transcranial magnetic stimulation |
| sMCI | stable mild cognitive impairment |
| SCD | subjective cognitive decline |
| SN | salience network |
| t-tau | total tau |
| TPJ | temporoparietal junction |
References
- Zhang, M.; Chen, H.; Huang, W.; Guo, T.; Ma, G.; Han, Y.; Shu, N. Relationship between topological efficiency of white matter structural connectome and plasma biomarkers across the Alzheimer’s disease continuum. Hum. Brain Mapp. 2024, 45, e26566. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Iacob, L.; Vasilache, C.; Schreiner, O.D. Therapeutic Modalities Targeting Tau Protein in Alzheimer’s Disease. J. Dement. Alzheimer’s Dis. 2025, 2, 32. [Google Scholar] [CrossRef]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.; Langerman, H. Alzheimer’s disease—Why we need early diagnosis. Degener. Neurol. Neuro-Muscular Dis. 2019, 24, 123–130. [Google Scholar] [CrossRef]
- Yu, M.; Sporns, O.; Saykin, A.J. The human connectome in Alzheimer disease—Relationship to biomarkers and genetics. Nat. Rev. Neurol. 2021, 17, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Milanifard, M.; Ramezani, M. Clinical and neurological problems and clinical tests in Alzheimer’s patients specializing in Alzheimer’s disease. Eurasian J. Chem. Med. Pet. Res. 2025, 4, 152–163. [Google Scholar]
- Lin, Y.; Shan, P.-Y.; Jiang, W.-J.; Sheng, C.; Ma, L. Subjective cognitive decline: Preclinical manifestation of Alzheimer’s disease. Neurol. Sci. 2019, 40, 41–49. [Google Scholar] [CrossRef]
- Wilcockson, T.D.W.; Begde, A.; Hogervorst, E. Subjective Cognitive Decline and Antisaccade Latency: Exploring Early Markers of Dementia Risk. J. Dement. Alzheimer’s Dis. 2025, 2, 16. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Perron, J.; Scramstad, C.; Ko, J.H. Brain metabolic imaging-based model identifies cognitive stability in prodromal Alzheimer’s disease. Sci. Rep. 2025, 15, 17187. [Google Scholar] [CrossRef]
- Langley, J.; Bennett, I.J.; Hu, X.P.; Alzheimer’s Disease Neuroimaging Initiative. Examining iron-related off-target binding effects of 18F-AV1451 PET in the cortex of Aβ+ individuals. Eur. J. Neurosci. 2024, 60, 3614–3628. [Google Scholar] [CrossRef] [PubMed]
- Camus, V.; Payoux, P.; Barré, L.; Desgranges, B.; Voisin, T.; Tauber, C.; La Joie, R.; Tafani, M.; Hommet, C.; Chételat, G.; et al. Using PET with 18F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment. Eur. J. Nucl. Med. 2012, 39, 621–631. [Google Scholar] [CrossRef]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergström, M.; Savitcheva, I.; Huang, G.; Estrada, S.; et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Raichle, M.E. Resting state functional connectivity in preclinical Alzheimer’s disease. Biol. Psychiatry 2013, 74, 340–347. [Google Scholar] [CrossRef]
- Hojjati, S.H.; Butler, T.A.; de Leon, M.; Gupta, A.; Nayak, S.; Luchsinger, J.A.; Razlighi, Q.R.; Chiang, G.C. Inter-network functional connectivity increases by beta-amyloid and may facilitate the early stage of tau accumulation. Neurobiol. Aging 2025, 148, 16–26. [Google Scholar] [CrossRef]
- Cacciaglia, R.; Shekari, M.; Salvadó, G.; Milà-Alomà, M.; Falcon, C.; Sánchez-Benavides, G.; Minguillón, C.; Fauria, K.; Grau-Rivera, O.; Molinuevo, J.L.; et al. The CSF p-tau/β-amyloid 42 ratio correlates with brain structure and fibrillary β-amyloid deposition in cognitively unimpaired individuals at the earliest stages of pre-clinical Alzheimer’s disease. Brain Commun. 2025, 7, fcae451. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Del Tredici, K.; Barthélemy, N.R.; Gabitto, M.; van Dyck, C.H.; Lein, E.; Braak, H.; Datta, D. An integrated view of the relationships between amyloid, tau, and inflammatory pathophysiology in Alzheimer’s disease. Alzheimer’s Dement. 2025, 21, e70404. [Google Scholar] [CrossRef] [PubMed]
- Franzmeier, N.; Dewenter, A.; Frontzkowski, L.; Dichgans, M.; Rubinski, A.; Neitzel, J.; Smith, R.; Strandberg, O.; Ossenkoppele, R.; Buerger, K.; et al. Patient-centered connectivity-based prediction of tau pathology spread in Alzheimer’s disease. Sci. Adv. 2020, 6, eabd1327. [Google Scholar] [CrossRef]
- Rubenstein, R.; McQuillan, L.; Wang, K.K.; Robertson, C.S.; Chang, B.; Yang, Z.; Xu, H.; Williamson, J.B.; Wagner, A.K. Temporal Profiles of P-Tau, T-Tau, and P-Tau:Tau Ratios in Cerebrospinal Fluid and Blood from Moderate-Severe Traumatic Brain Injury Patients and Relationship to 6–12 Month Global Outcomes. J. Neurotrauma 2024, 41, 369–392. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Schonhaut, D.R.; Schöll, M.; Lockhart, S.N.; Ayakta, N.; Baker, S.L.; O’neil, J.P.; Janabi, M.; Lazaris, A.; Cantwell, A.; et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 2016, 139, 1551–1567. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Schultz, A.; Betensky, R.A.; Becker, J.A.; Sepulcre, J.; Rentz, D.; Mormino, E.; Chhatwal, J.; Amariglio, R.; Papp, K.; et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 2016, 79, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, L.; Vázquez, R.P.; Hong, Y.; Allinson, K.S.; Williamson, D.; Borchert, R.J.; Sami, S.; Cope, T.; Bevan-Jones, W.; Jones, S.; et al. 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain 2017, 140, 781–791. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.R.; Gbadeyan, O.; Andridge, R.; Schroeder, M.W.; Pugh, E.A.; Scharre, D.W.; Prakash, R.S. p-Tau/Aβ42 ratio associates with cognitive decline in Alzheimer’s disease, mild cognitive impairment, and cognitively unimpaired older adults. Neuropsychology 2025, 39, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Beaumont, H.; Ferguson, D.; Yadegarfar, M.; Stubbs, B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr. Scand. 2014, 130, 439–451. [Google Scholar] [CrossRef]
- Marafioti, G.; Culicetto, L.; Latella, D.; Marra, A.; Quartarone, A.; Buono, V.L. Neural correlates of subjective cognitive decline in Alzheimer’s disease: A systematic review of structural and functional brain changes for early diagnosis and intervention. Front. Aging Neurosci. 2025, 17, 1549134. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, M.; Stiles, W.R.; Choi, H.S. Neuroimaging Modalities in Alzheimer’s Disease: Diagnosis and Clinical Features. Int. J. Mol. Sci. 2022, 23, 6079. [Google Scholar] [CrossRef]
- Moll, L.; Häner, M.; Rössler, R.; Krumm, S. Comparison of Physical Activity Patterns Between Individuals with Early-Stage Alzheimer’s Disease and Cognitively Healthy Adults. J. Dement. Alzheimer’s Dis. 2025, 2, 23. [Google Scholar] [CrossRef]
- Chen, H.; Sheng, X.; Luo, C.; Qin, R.; Ye, Q.; Zhao, H.; Xu, Y.; Bai, F.; Alzheimer’s Disease Neuroimaging Initiative. The compensatory phenomenon of the functional connectome related to pathological biomarkers in individuals with subjective cognitive decline. Transl. Neurodegener. 2020, 9, 21. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Oikonomou, V.P.; Georgiadis, K.; Lazarou, I.; Nikolopoulos, S.; Kompatsiaris, I.; PREDICTOM Consortium. Exploring Functional Brain Networks in Alzheimer’s Disease Using Resting State EEG Signals. J. Dement. Alzheimer’s Dis. 2025, 2, 12. [Google Scholar] [CrossRef]
- Arnold, S.E.; Hyman, B.T.; Betensky, R.A.; Dodge, H.H. Pathways to personalized medicine—Embracing heterogeneity for progress in clinical therapeutics research in Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, 7384–7394. [Google Scholar] [CrossRef]
- Srinivasan, R.; Winter, W.R.; Ding, J.; Nunez, P.L. EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. J. Neurosci. Methods 2007, 166, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Cole, M.W.; Reynolds, J.R.; Power, J.D.; Repovs, G.; Anticevic, A.; Braver, T.S. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 2013, 16, 1348–1355. [Google Scholar] [CrossRef]
- Beckmann, C.F.; DeLuca, M.; Devlin, J.T.; Smith, S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Thomas Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Ramduny, J.; Kelly, C. Connectome-based fingerprinting: Reproducibility, precision, and behavioral prediction. Neuropsychopharmacology 2025, 50, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Finn, E.S.; Scheinost, D.; Rosenberg, M.D.; Chun, M.M.; Papademetris, X.; Constable, R.T. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat. Protoc. 2017, 12, 506–518. [Google Scholar] [CrossRef]
- Ghaffari, A.; Abouzaki, M.; Romero, Y.; Sun, A.; Seitz, A.; Langley, J.; Bennett, I.J.; Hu, X. Connectome-based predictive modelling predicts frailty levels in older adults. bioRxiv 2025. [Google Scholar] [CrossRef]
- Finn, E.S.; Shen, X.; Scheinost, D.; Rosenberg, M.D.; Huang, J.; Chun, M.M.; Papademetris, X.; Constable, R.T. Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015, 18, 1664–1671. [Google Scholar] [CrossRef]
- Foster, M.L.; Ye, J.; Powers, A.R.; Dvornek, N.C.; Scheinost, D. Connectome-based predictive modeling of early and chronic psychosis symptoms. Neuropsychopharmacology 2025, 50, 877–885. [Google Scholar] [CrossRef]
- Treves, I.N.; Kucyi, A.; Park, M.; Kral, T.R.A.; Goldberg, S.B.; Davidson, R.J.; Rosenkranz, M.; Whitfield-Gabrieli, S.; Gabrieli, J.D.E. Connectome-Based Predictive Modeling of Trait Mindfulness. Hum. Brain Mapp. 2025, 46, e70123. [Google Scholar] [CrossRef]
- Adams, J.N.; Chappel-Farley, M.G.; Yaros, J.L.; Taylor, L.; Harris, A.L.; Mikhail, A.; McMillan, L.; Keator, D.B.; Yassa, M.A. Functional network structure supports resilience to memory deficits in cognitively normal older adults with amyloid-β pathology. Sci. Rep. 2023, 13, 13953. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, J.; Xue, Y.; Jiang, J.; Cao, M.; Li, S.; Chen, X.; Alzheimer’s Disease Neuroimaging Initiative. Characterizing structure-function coupling in subjective memory complaints of preclinical Alzheimer’s disease. J. Alzheimer’s Dis. 2025, 107, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J.; van Nifterick, A.M.; de Haan, W.; Gouw, A.A. Network hyperexcitability in early Alzheimer’s disease: Is functional connectivity a potential biomarker? Brain Topogr. 2023, 36, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Franzmeier, N.; Rubinski, A.; Neitzel, J.; Kim, Y.; Damm, A.; Na, D.L.; Kim, H.J.; Lyoo, C.H.; Cho, H.; Finsterwalder, S.; et al. Functional connectivity associated with tau levels in ageing, Alzheimer’s, and small vessel disease. Brain 2019, 142, 1093–1107. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Yao, L.; He, N.; Tang, Y.; Chen, L.; Long, F.; Chen, Y.; Kemp, G.J.; Lui, S.; et al. Shared and differing functional connectivity abnormalities of the default mode network in mild cognitive impairment and Alzheimer’s disease. Cereb. Cortex 2024, 34, bhae094. [Google Scholar] [CrossRef]
- Owens, C.D.; Pinto, C.B.; Mukli, P.; Gulej, R.; Velez, F.S.; Detwiler, S.; Olay, L.; Hoffmeister, J.R.; Szarvas, Z.; Muranyi, M.; et al. Neurovascular coupling, functional connectivity, and cerebrovascular endothelial extracellular vesicles as biomarkers of mild cognitive impairment. Alzheimer’s Dement. 2024, 20, 5590–5606. [Google Scholar] [CrossRef]
- Roemer-Cassiano, S.N.; Wagner, F.; Evangelista, L.; Rauchmann, B.-S.; Dehsarvi, A.; Steward, A.; Dewenter, A.; Biel, D.; Zhu, Z.; Pescoller, J.; et al. Amyloid-associated hyperconnectivity drives tau spread across connected brain regions in Alzheimer’s disease. Sci. Transl. Med. 2025, 17, eadp2564. [Google Scholar] [CrossRef] [PubMed]
- Abuwarda, H.; Trainer, A.; Horien, C.; Shen, X.; Moret, S.; Ju, S.; Constable, R.T.; Fredericks, C. Whole-brain functional connectivity predicts regional tau PET in preclinical Alzheimer’s disease. Brain Commun. 2025, 7, fcaf274. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Zhao, Y.; Chen, X.; Langley, J.; Hu, X. Dynamic fingerprinting of the human functional connectome. bioRxiv 2025. [Google Scholar] [CrossRef]
- Grieder, M.; Wang, D.J.J.; Dierks, T.; Wahlund, L.-O.; Jann, K. Default mode network complexity and cognitive decline in mild Alzheimer’s disease. Front. Neurosci. 2018, 12, 770. [Google Scholar] [CrossRef]
- Millar, P.R.; Ances, B.M.; Gordon, B.A.; Benzinger, T.L.; Fagan, A.M.; Morris, J.C.; Balota, D.A. Evaluating resting-state BOLD variability in relation to biomarkers of preclinical Alzheimer’s disease. Neurobiol. Aging 2020, 96, 233–245. [Google Scholar] [CrossRef]
- Schultz, A.P.; Chhatwal, J.P.; Hedden, T.; Mormino, E.C.; Hanseeuw, B.J.; Sepulcre, J.; Huijbers, W.; LaPoint, M.; Buckley, R.F.; Johnson, K.A.; et al. Phases of hyperconnectivity and hypoconnectivity in the default mode and salience networks track with amyloid and tau in clinically normal individuals. J. Neurosci. 2017, 37, 4323–4331. [Google Scholar] [CrossRef]
- Johnson, K.A.; Sperling, R.A.; Sepulcre, J. Functional connectivity in Alzheimer’s disease: Measurement and meaning. Biol. Psychiatry 2013, 74, 318–319. [Google Scholar] [CrossRef]
- Fischer, L.; Adams, J.N.; Molloy, E.N.; Vockert, N.; Tremblay-Mercier, J.; Remz, J.; Binette, A.P.; Villeneuve, S.; Maass, A.; PREVENT-AD Research Group. Differential effects of aging, Alzheimer’s pathology, and APOE4 on longitudinal functional connectivity and episodic memory in older adults. Alzheimer’s Res. Ther. 2025, 17, 91. [Google Scholar] [CrossRef]
- Ingala, S.; Tomassen, J.; Collij, L.E.; Prent, N.; van ‘t Ent, D.; Kate, M.T.; Konijnenberg, E.; Yaqub, M.; Scheltens, P.; de Geus, E.J.C.; et al. Amyloid-driven disruption of default mode network connectivity in cognitively healthy individuals. Brain Commun. 2021, 3, fcab201. [Google Scholar] [CrossRef]
- Qi, H.; Liu, H.; Hu, H.; He, H.; Zhao, X. Primary disruption of the memory-related subsystems of the default mode network in Alzheimer’s disease: Resting-state functional connectivity MRI study. Front. Aging Neurosci. 2018, 10, 344. [Google Scholar] [CrossRef]
- Demirsoy, I. Tau Pathology in the Medial Temporal Lobe and Neocortex: Implications for Cognitive Unimpaired in Cognitively Unimpaired Older Adults. Balk. Med. J. 2025, 42, 329. [Google Scholar] [CrossRef]
- Kaboodvand, N.; Bäckman, L.; Nyberg, L.; Salami, A. The retrosplenial cortex: A memory gateway between the cortical default mode network and the medial temporal lobe. Hum. Brain Mapp. 2018, 39, 2020–2034. [Google Scholar] [CrossRef] [PubMed]
- Berron, D.; van Westen, D.; Ossenkoppele, R.; Strandberg, O.; Hansson, O. Medial temporal lobe connectivity and its associations with cognition in early Alzheimer’s disease. Brain 2020, 143, 1233–1248. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.P.; Buckley, R.F.; Hampton, O.L.; Scott, M.R.; Properzi, M.J.; Peña-Gómez, C.; Pruzin, J.J.; Yang, H.-S.; Johnson, A.K.; Sperling, A.R.; et al. Longitudinal degradation of the default/salience network axis in symptomatic individuals with elevated amyloid burden. NeuroImage Clin. 2020, 26, 102052. [Google Scholar] [CrossRef] [PubMed]
- Fountain-Zaragoza, S.; Liu, H.; Benitez, A. Functional network alterations associated with cognition in pre-clinical Alzheimer’s Disease. Brain Connect. 2023, 13, 275–286. [Google Scholar] [CrossRef]
- Boyle, R.; Klinger, H.M.; Shirzadi, Z.; Coughlan, G.T.; Seto, M.; Properzi, M.J.; Townsend, D.L.; Yuan, Z.; Scanlon, C.; Jutten, R.J.; et al. Left frontoparietal control network connectivity moderates the effect of amyloid on cognitive decline in preclinical Alzheimer’s disease: The A4 study. J. Prev. Alzheimer’s Dis. 2024, 11, 881–888. [Google Scholar] [CrossRef]
- Zang, F.; Liu, X.; Fan, D.; He, C.; Zhang, Z.; Xie, C.; Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease Metabolomics Consortium. Dynamic functional network connectivity and its association with lipid metabolism in Alzheimer’s disease. CNS Neurosci. Ther. 2024, 30, e70029. [Google Scholar] [CrossRef]
- Canal-Garcia, A.; Veréb, D.; Mijalkov, M.; Westman, E.; Volpe, G.; Pereira, J.B.; Initiative, F.T.A.D.N. Dynamic multilayer functional connectivity detects preclinical and clinical Alzheimer’s disease. Cereb. Cortex 2024, 34, bhad542. [Google Scholar] [CrossRef]
- Lorenzini, L.; Ingala, S.; Collij, E.L.; Wottschel, V.; Haller, S.; Blennow, K.; Frisoni, G.; Chételat, G.; Payoux, P.; Lage-Martinez, P.; et al. Eigenvector centrality dynamics are related to Alzheimer’s disease pathological changes in non-demented individuals. Brain Commun. 2023, 5, fcad088. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Hansson, O. Towards clinical application of tau PET tracers for diagnosing dementia due to Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, 1998–2008. [Google Scholar] [CrossRef]
- Berron, D.; Vogel, J.W.; Insel, P.S.; Pereira, J.B.; Xie, L.; Wisse, L.E.M.; Yushkevich, A.P.; Palmqvist, S.; Mattsson-Carlgren, N.; Stomrud, E.; et al. Early stages of tau pathology and its associations with functional connectivity, atrophy and memory. Brain 2021, 144, 2771–2783. [Google Scholar] [CrossRef]
- Wales, R.M.; Leung, H.-C. The effects of amyloid and tau on functional network connectivity in older populations. Brain Connect. 2021, 11, 599–612. [Google Scholar] [CrossRef]
- Sintini, I.; Graff-Radford, J.; Jones, D.T.; Botha, H.; Martin, P.R.; Machulda, M.M.; Schwarz, C.G.; Senjem, M.L.; Gunter, J.L.; Jack, C.R.; et al. Tau and amyloid relationships with resting-state functional connectivity in atypical Alzheimer’s disease. Cereb. Cortex 2021, 31, 1693–1706. [Google Scholar] [CrossRef]
- Cope, T.E.; Rittman, T.; Borchert, R.J.; Jones, P.S.; Vatansever, D.; Allinson, K.; Passamonti, L.; Vazquez Rodriguez, P.; Bevan-Jones, W.R.; O’Brien, J.T.; et al. Tau burden and the functional connectome in Alzheimer’s disease and progressive supranuclear palsy. Brain 2018, 141, 550–567. [Google Scholar] [CrossRef]
- Wisch, J.K.; Roe, C.M.; Babulal, G.M.; Schindler, S.E.; Fagan, A.M.; Benzinger, T.L.; Morris, J.C.; Ances, B.M. Resting state functional connectivity signature differentiates cognitively normal from individuals who convert to symptomatic Alzheimer’s disease. J. Alzheimer’s Dis. 2020, 74, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Vélez, E.; Diez, I.; Schoemaker, D.; Pardilla-Delgado, E.; Vila-Castelar, C.; Fox-Fuller, J.T.; Baena, A.; Sperling, R.A.; Johnson, K.A.; Lopera, F.; et al. Amyloid-β and tau pathologies relate to distinctive brain dysconnectomics in preclinical autosomal-dominant Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2113641119. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Azargoonjahromi, A.; Nasiri, H.; Yaghoobi, A.; Sadeghi, M.; Chavoshi, S.S.; Baghaeikia, S.; Mahzari, N.; Valipour, A.; Oskouei, R.R.; et al. Altered brain connectivity in mild cognitive impairment is linked to elevated tau and phosphorylated tau, but not to GAP-43 and amyloid-β measurements: A resting-state fMRI study. Mol. Brain 2024, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.M.; Maass, A.; Adams, J.N.; Du, R.; Baker, S.L.; Jagust, W.J. Tau deposition is associated with functional isolation of the hippocampus in aging. Nat. Commun. 2019, 10, 4900. [Google Scholar] [CrossRef]
- Vatansever, D.; Menon, D.; Stamatakis, E. Default mode contributions to automated information processing. Proc. Natl. Acad. Sci. USA 2017, 114, 12821–12826. [Google Scholar] [CrossRef]
- Ottoy, J.; Kang, M.S.; Tan, J.X.M.; Boone, L.; de Wael, R.V.; Park, B.-Y.; Bezgin, G.; Lussier, F.Z.; Pascoal, T.A.; Rahmouni, N.; et al. Tau follows principal axes of functional and structural brain organization in Alzheimer’s disease. Nat. Commun. 2024, 15, 5031. [Google Scholar] [CrossRef]
- Huijbers, W.; Schultz, A.P.; Papp, K.V.; LaPoint, M.R.; Hanseeuw, B.; Chhatwal, J.P.; Hedden, T.; Johnson, K.A.; Sperling, R.A. Tau accumulation in clinically normal older adults is associated with hippocampal hyperactivity. J. Neurosci. 2019, 39, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Sepulcre, J.; Sabuncu, M.R.; Li, Q.; El Fakhri, G.; Sperling, R.; Johnson, K.A. Tau and amyloid β proteins distinctively associate to functional network changes in the aging brain. Alzheimer’s Dement. 2017, 13, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Budak, M.; Fausto, B.A.; Osiecka, Z.; Sheikh, M.; Perna, R.; Ashton, N.; Blennow, K.; Zetterberg, H.; Fitzgerald-Bocarsly, P.; Gluck, M.A. Elevated plasma p-tau231 is associated with reduced generalization and medial temporal lobe dynamic network flexibility among healthy older African Americans. Alzheimer’s Res. Ther. 2024, 16, 253. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.; Mohanty, R.; Westman, E.; Middleton, L. Lower Network Functional Connectivity Is Associated With Higher Regional Tau Burden Among Those At-Risk of Alzheimer’s Disease but Cognitively Unimpaired: Specific Patterns Based on Amyloid Status. Res. Sq. 2025, rs.3.rs-5820051. [Google Scholar] [CrossRef]
- Parker, A.F.; Szoeke, C.; Kwan, H.; Henri-Bhargava, A.; Gawryluk, J.R. Functional connectivity alterations in women with subjective cognitive decline. J. Alzheimer’s Dis. Rep. 2025, 9, 25424823251328340. [Google Scholar] [CrossRef]
- Sperling, R.A.; Dickerson, B.C.; Pihlajamaki, M.; Vannini, P.; LaViolette, P.S.; Vitolo, O.V.; Hedden, T.; Becker, J.A.; Rentz, D.M.; Selkoe, D.J.; et al. Functional alterations in memory networks in early Alzheimer’s disease. Neuromol. Med. 2010, 12, 27–43. [Google Scholar] [CrossRef]
- Tian, H.; Zheng, W.; Wang, J.; Liu, S.; Wang, Z. Altered functional connectivity of insular subregions in subjective cognitive decline. Front. Hum. Neurosci. 2024, 18, 1404759. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Park, D.C. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol. Rev. 2014, 24, 355–370. [Google Scholar] [CrossRef]
- Pievani, M.; Ribaldi, F.; Toussas, K.; Da Costa, S.; Jorge, J.; Reynaud, O.; Chicherio, C.; Blouin, J.; Scheffler, M.; Garibotto, V.; et al. Resting-state functional connectivity abnormalities in subjective cognitive decline: A 7T MRI study. Neurobiol. Aging 2024, 144, 104–113. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.Y.; Kim, W.J. Altered functional connectivity of the default mode and dorsal attention network in subjective cognitive decline. J. Psychiatr. Res. 2023, 159, 165–171. [Google Scholar] [CrossRef]
- Weiler, M.; Fukuda, A.; Massabki, L.H.; Lopes, T.M.; Franco, A.R.; Damasceno, B.P.; Cendes, F.; Balthazar, M.L. Default mode, executive function, and language functional connectivity networks are compromised in mild Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhao, L.; Wei, Y.; Mai, W.; Duan, G.; Su, J.; Nong, X.; Yu, B.; Li, C.; Mo, X.; et al. Structural and functional hippocampal changes in subjective cognitive decline from the community. Front. Aging Neurosci. 2020, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Murari, G.; Vandermorris, S.; Verhoeff, N.P.L.; Herrmann, N.; Chen, J.J.; Mah, L. Functional connectivity between the posterior default mode network and parahippocampal gyrus is disrupted in older adults with subjective cognitive decline and correlates with subjective memory ability. J. Alzheimer’s Dis. 2021, 82, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Viviano, R.P.; Damoiseaux, J.S. Longitudinal change in hippocampal and dorsal anterior insulae functional connectivity in subjective cognitive decline. Alzheimer’s Res. Ther. 2021, 13, 108. [Google Scholar] [CrossRef]
- Binnewijzend, M.A.; Schoonheim, M.M.; Sanz-Arigita, E.; Wink, A.M.; van der Flier, W.M.; Tolboom, N.; Adriaanse, S.M.; Damoiseaux, J.S.; Scheltens, P.; van Berckel, B.N.; et al. Resting-state fMRI changes in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2012, 33, 2018–2028. [Google Scholar] [CrossRef]
- Liang, X.; Xue, C.; Zheng, D.; Yuan, Q.; Qi, W.; Ruan, Y.; Chen, S.; Song, Y.; Wu, H.; Lu, X.; et al. Repetitive transcranial magnetic stimulation regulates effective connectivity patterns of brain networks in the spectrum of preclinical Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1343926. [Google Scholar] [CrossRef]
- Xu, X.; Li, W.; Tao, M.; Xie, Z.; Gao, X.; Yue, L.; Wang, P. Effective and accurate diagnosis of subjective cognitive decline based on functional connection and graph theory view. Front. Neurosci. 2020, 14, 577887. [Google Scholar] [CrossRef]
- Huang, S.; Wang, S.; Che, Z.; Ge, H.; Yan, Z.; Fan, J.; Lu, X.; Liu, L.; Liu, W.; Zhong, Y.; et al. Brain-wide functional connectivity alterations and their cognitive correlates in subjective cognitive decline. Front. Neurosci. 2024, 18, 1438260. [Google Scholar] [CrossRef]
- Xue, C.; Sun, H.; Yue, Y.; Wang, S.; Qi, W.; Hu, G.; Ge, H.; Yuan, Q.; Rao, J.; Tian, L.; et al. Structural and functional disruption of salience network in distinguishing subjective cognitive decline and amnestic mild cognitive impairment. ACS Chem. Neurosci. 2021, 12, 1384–1394. [Google Scholar] [CrossRef]
- Montembeault, M.; Rouleau, I.; Provost, J.-S.; Brambati, S.M. Altered gray matter structural covariance networks in early stages of Alzheimer’s disease. Cereb. Cortex 2016, 26, 2650–2662. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Zhong, X.; Hou, L.; Zhang, M.; Yang, M.; Wu, Z.; Chen, X.; Mai, N.; Zhou, H.; et al. Static and dynamic functional connectivity variability of the anterior-posterior hippocampus with subjective cognitive decline. Alzheimer’s Res. Ther. 2022, 14, 122. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, J.; Zhang, X.; Sun, Y.; Chen, W.; Li, X.; Zhang, W.; Qing, Z.; Zhang, B. Alterations in dynamic functional connectivity in individuals with subjective cognitive decline. Front. Aging Neurosci. 2021, 13, 646017. [Google Scholar] [CrossRef]
- Csukly, G.; Tombor, L.; Hidasi, Z.; Csibri, E.; Fullajtár, M.; Huszár, Z.; Koszovácz, V.; Lányi, O.; Vass, E.; Koleszár, B.; et al. Low Functional network integrity in cognitively unimpaired and MCI subjects with depressive symptoms: Results from a multi-center fMRI study. Transl. Psychiatry 2024, 14, 179. [Google Scholar] [CrossRef]
- Mohammadian, F.; Noroozian, M.; Sadeghi, A.Z.; Malekian, V.; Saffar, A.; Talebi, M.; Hashemi, H.; Salari, H.M.; Samadi, F.; Sodaei, F.; et al. Effective connectivity evaluation of resting-state brain networks in Alzheimer’s disease, amnestic mild cognitive impairment, and normal aging: An exploratory study. Brain Sci. 2023, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Wang, Z.; Sun, P.; Li, K.; Liang, P. Dysfunctional interactions between the default mode network and the dorsal attention network in subtypes of amnestic mild cognitive impairment. Aging 2019, 11, 9147–9166. [Google Scholar] [CrossRef]
- Xu, W.; Rao, J.; Song, Y.; Chen, S.; Xue, C.; Hu, G.; Lin, X.; Chen, J. Altered functional connectivity of the basal nucleus of Meynert in subjective cognitive impairment, early mild cognitive impairment, and late mild cognitive impairment. Front. Aging Neurosci. 2021, 13, 671351. [Google Scholar] [CrossRef]
- Liu, A.K.L.; Chang, R.C.-C.; Pearce, R.K.B.; Gentleman, S.M. Nucleus basalis of Meynert revisited: Anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015, 129, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Urso, D.; Nigro, S.; Tafuri, B.; De Blasi, R.; Pereira, J.B.; Logroscino, G. Nucleus basalis of Meynert degeneration predicts cognitive decline in corticobasal syndrome. Biol. Psychiatry 2024, 95, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Mondragón, J.D.; Maurits, N.M.; De Deyn, P.P.; Alzheimer’s Disease NeuroImaging Initiative. Functional connectivity differences in Alzheimer’s disease and amnestic mild cognitive impairment associated with AT (N) classification and anosognosia. Neurobiol. Aging 2021, 101, 22–39. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Lin, P.-C.; Lin, Y.-C.; Lee, Y.-J.; Wang, C.-Y.; Peng, S.-W.; Wang, P.-N. The clinical course of early and late mild cognitive impairment. Front. Neurol. 2022, 13, 685636. [Google Scholar] [CrossRef]
- Bergamino, M.; McElvogue, M.M.; Stokes, A.M.; Initiative, A.D.N. Distinguishing Early from Late Mild Cognitive Impairment Using Magnetic Resonance Free-Water Diffusion Tensor Imaging. NeuroSci 2025, 6, 8. [Google Scholar] [CrossRef]
- Xue, C.; Qi, W.; Yuan, Q.; Hu, G.; Ge, H.; Rao, J.; Xiao, C.; Chen, J. Disrupted dynamic functional connectivity in distinguishing subjective cognitive decline and amnestic mild cognitive impairment based on the triple-network model. Front. Aging Neurosci. 2021, 13, 711009. [Google Scholar] [CrossRef]
- Penalba-Sánchez, L.; Oliveira-Silva, P.; Sumich, A.L.; Cifre, I. Increased functional connectivity patterns in mild Alzheimer’s disease: A rsfMRI study. Front. Aging Neurosci. 2023, 14, 1037347. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Liao, Z.; Liu, X.; Li, T.; Hu, J.; Le, D.; Pei, Y.; Sun, W.; Lin, J.; Qiu, Y.; et al. Disrupted balance of long and short-range functional connectivity density in Alzheimer’s disease (AD) and mild cognitive impairment (MCI) patients: A resting-state fMRI study. Ann. Transl. Med. 2021, 9, 65. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, C.; Chen, Y.; Zhong, X.; Chen, H.; Sun, R.; Zhang, J.; Zhong, Z.; Huang, M. Altered functional connectivity of basal ganglia in mild cognitive impairment and Alzheimer’s disease. Brain Sci. 2022, 12, 1555. [Google Scholar] [CrossRef] [PubMed]
- Cockrell, J.R.; Folstein, M.F. Mini-mental state examination. In Principles and Practice of Geriatric Psychiatry, 2nd ed.; Abou-Saleh, M.T., Katona, C.L.E., Kumar, A., Eds.; Wiley: Chichester, UK, 2002; pp. 140–141. [Google Scholar]
- Tang, F.; Zhu, D.; Ma, W.; Yao, Q.; Li, Q.; Shi, J. Differences changes in cerebellar functional connectivity between mild cognitive impairment and Alzheimer’s disease: A seed-based approach. Front. Neurol. 2021, 12, 645171. [Google Scholar] [CrossRef]
- Pu, Z.; Huang, H.; Li, M.; Li, H.; Shen, X.; Wu, Q.; Ni, Q.; Lin, Y.; Cui, D. An exploration of distinguishing subjective cognitive decline and mild cognitive impairment based on resting-state prefrontal functional connectivity assessed by functional near-infrared spectroscopy. Front. Aging Neurosci. 2025, 16, 1468246. [Google Scholar] [CrossRef] [PubMed]
- Roy-Côté, F.; Rouleau, I.; Delage, É.; Akzam-Ouellette, M.-A.; Joubert, S. CIMA-Q group Lower semantic verbal fluency at baseline indicates future decline in subjective cognitive decline. Appl. Neuropsychol. Adult 2025, 1–11. [Google Scholar] [CrossRef]
- Benton, A.; Hamsher, S.; Sivan, A. Multilingual Aphasia Examination, 3rd ed.; AJA Associates: Iowa city, IA, USA, 1994. [Google Scholar]
- Malotaux, V.; Dricot, L.; Quenon, L.; Lhommel, R.; Ivanoiu, A.; Hanseeuw, B. Default-Mode Network Connectivity Changes During the Progression Toward Alzheimer’s Dementia: A Longitudinal Functional Magnetic Resonance Imaging Study. Brain Connect. 2023, 13, 287–296. [Google Scholar] [CrossRef]
- Chauveau, L.; Landeau, B.; Dautricourt, S.; Turpin, A.-L.; Delarue, M.; Hébert, O.; de La Sayette, V.; Chételat, G.; de Flores, R. Anterior-temporal network hyperconnectivity is key to Alzheimer’s disease: From ageing to dementia. Brain 2025, 148, 2008–2022. [Google Scholar] [CrossRef]
- Mondragón, J.D.; Marapin, R.; De Deyn, P.P.; Maurits, N. Short-and long-term functional connectivity differences associated with Alzheimer’s disease progression. Dement. Geriatr. Cogn. Disord. Extra 2022, 11, 235–249. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, Y.; Li, B. The Switching Rates of Dynamic Functional Networks Differently Contribute to Cross-Sectional and Longitudinal Cognition in Mild Cognitive Impairment. J. Integr. Neurosci. 2022, 21, 170. [Google Scholar] [CrossRef]
- Brier, M.R.; Thomas, J.B.; Fagan, A.M.; Hassenstab, J.; Holtzman, D.M.; Benzinger, T.L.; Morris, J.C.; Ances, B.M. Functional connectivity and graph theory in preclinical Alzheimer’s disease. Neurobiol. Aging 2014, 35, 757–768. [Google Scholar] [CrossRef]
- Ewers, M.; Luan, Y.; Frontzkowski, L.; Neitzel, J.; Rubinski, A.; Dichgans, M.; Hassenstab, J.; Gordon, B.A.; Chhatwal, J.P.; Levin, J.; et al. Segregation of functional networks is associated with cognitive resilience in Alzheimer’s disease. Brain 2021, 144, 2176–2185. [Google Scholar] [CrossRef]
- Zhang, Z.; Chan, M.Y.; Han, L.; Carreno, C.A.; Winter-Nelson, E.; Wig, G.S.; for the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Dissociable effects of Alzheimer’s disease-related cognitive dysfunction and aging on functional brain network segregation. J. Neurosci. 2023, 43, 7879–7892. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Dou, X.; Wang, W.; Yao, H.; Feng, F.; Wang, P.; Yang, Z.; An, N.; Liu, B.; Zhang, X.; et al. Structural and functional connectivity abnormalities of the default mode network in patients with Alzheimer’s disease and mild cognitive impairment within two independent datasets. Methods 2022, 205, 29–38. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, Y.; Wang, J.; Xu, L.; Yang, K.; Lv, X.; Zhu, Z.; Gong, Q.; Hu, W.; Li, X.; et al. Parietal-hippocampal rTMS improves cognitive function in Alzheimer’s disease and increases dynamic functional connectivity of default mode network. Psychiatry Res. 2022, 315, 114721. [Google Scholar] [CrossRef]
- Seoane, S.; Heuvel, M.v.D.; Acebes, Á.; Janssen, N. The subcortical default mode network and Alzheimer’s disease: A systematic review and meta-analysis. Brain Commun. 2024, 6, fcae128. [Google Scholar] [CrossRef]
- Zhao, Q.; Sang, X.; Metmer, H.; Lu, J.; Alzheimer’s Disease NeuroImaging Initiative. Functional segregation of executive control network and frontoparietal network in Alzheimer’s disease. Cortex 2019, 120, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lu, H.; Metmer, H.; Li, W.X.; Lu, J. Evaluating functional connectivity of executive control network and frontoparietal network in Alzheimer’s disease. Brain Res. 2018, 1678, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, M.L.F.; Pereira, F.R.S.; Lopes, T.M.; da Silva, E.L.; Coan, A.C.; Campos, B.M.; Duncan, N.W.; Stella, F.; Northoff, G.; Damasceno, B.P.; et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum. Brain Mapp. 2014, 35, 1237–1246. [Google Scholar] [CrossRef]
- Gu, Y.; Lin, Y.; Huang, L.; Ma, J.; Zhang, J.; Xiao, Y.; Dai, Z.; Initiative, A.D.N. Abnormal dynamic functional connectivity in Alzheimer’s disease. CNS Neurosci. Ther. 2020, 26, 962–971. [Google Scholar] [CrossRef]
- Sendi, M.S.E.; Zendehrouh, E.; Miller, R.L.; Fu, Z.; Du, Y.; Liu, J.; Mormino, E.C.; Salat, D.H.; Calhoun, V.D. Alzheimer’s disease projection from normal to mild dementia reflected in functional network connectivity: A longitudinal study. Front. Neural Circuits 2021, 14, 593263. [Google Scholar] [CrossRef]
- Pang, X.; Ji, Y.; Hu, C.; Dai, Y.; Hu, P.; Wu, X.; Wang, K. Altered dynamic functional network connectivity patterns in Alzheimer’s disease: Insights into neural dysfunction. Front. Aging Neurosci. 2025, 17, 1617191. [Google Scholar] [CrossRef]
- Zhao, C.; Huang, W.-J.; Feng, F.; Zhou, B.; Yao, H.-X.; Guo, Y.-E.; Wang, P.; Wang, L.-N.; Shu, N.; Zhang, X. Abnormal characterization of dynamic functional connectivity in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 2014–2021. [Google Scholar] [CrossRef]
- Stampacchia, S.; Asadi, S.; Tomczyk, S.; Ribaldi, F.; Scheffler, M.; Lövblad, K.-O.; Pievani, M.; Fall, A.B.; Preti, M.G.; Unschuld, P.G.; et al. Fingerprints of brain disease: Connectome identifiability in Alzheimer’s disease. Commun. Biol. 2024, 7, 1169. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Gao, F.; Xia, X.; Guo, Q.; Zhao, Y.; Huang, S.; Yuan, Z. Individual-specific functional connectivity improves prediction of Alzheimer’s disease’s symptoms in elderly people regardless of APOE ε4 genotype. Commun. Biol. 2023, 6, 581. [Google Scholar] [CrossRef]
- Prakash, R.S.; McKenna, M.R.; Gbadeyan, O.; Shankar, A.R.; Pugh, E.A.; Teng, J.; Andridge, R.; Berry, A.; Scharre, D.W. A whole-brain functional connectivity model of Alzheimer’s disease pathology. Alzheimer’s Dement. 2025, 21, e14349. [Google Scholar] [CrossRef]
- Tripathi, V.; Fox-Fuller, J.; Malotaux, V.; Baena, A.; Felix, N.B.; Alvarez, S.; Aguillon, D.; Lopera, F.; Somers, D.C.; Quiroz, Y.T. Connectome-based predictive modeling of brain pathology and cognition in autosomal dominant Alzheimer’s disease. Alzheimer’s Dement. 2025, 21, e70061. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.T.; Cronin-Golomb, A.; Gatchel, J.R.; Norton, D.J.; Guzmán-Vélez, E.; Jacobs, H.I.L.; Hanseeuw, B.; Pardilla-Delgado, E.; Artola, A.; Baena, A.; et al. Biological and cognitive markers of presenilin1 E280A autosomal dominant Alzheimer’s disease: A comprehensive review of the Colombian kindred. J. Prev. Alzheimer’s Dis. 2019, 6, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ding, S.; Xu, W.; Fredericks, C.; Zhao, Y. GenCPM: A toolbox for generalized connectome-based predictive modeling. Front. Neurosci. 2025, 19, 1627497. [Google Scholar] [CrossRef]
- Natu, V.S.; Lin, J.-J.; Burks, A.; Arora, A.; Rugg, M.D.; Lega, B. Stimulation of the posterior cingulate cortex impairs episodic memory encoding. J. Neurosci. 2019, 39, 7173–7182. [Google Scholar] [CrossRef]
- Ren, Y.; Nguyen, V.T.; Sonkusare, S.; Lv, J.; Pang, T.; Guo, L.; Eickhoff, S.B.; Breakspear, M.; Guo, C.C. Effective connectivity of the anterior hippocampus predicts recollection confidence during natural memory retrieval. Nat. Commun. 2018, 9, 4875. [Google Scholar] [CrossRef]
- Raud, L.; Sneve, M.H.; Vidal-Piñeiro, D.; Sørensen, Ø.; Folvik, L.; Ness, H.T.; Mowinckel, A.M.; Grydeland, H.; Walhovd, K.B.; Fjell, A.M. Hippocampal-cortical functional connectivity during memory encoding and retrieval. NeuroImage 2023, 279, 120309. [Google Scholar] [CrossRef]
- Tao, J.; Liu, J.; Egorova, N.; Chen, X.; Sun, S.; Xue, X.; Huang, J.; Zheng, G.; Wang, Q.; Chen, L.; et al. Increased hippocampus–medial prefrontal cortex resting-state functional connectivity and memory function after Tai Chi Chuan practice in elder adults. Front. Aging Neurosci. 2016, 8, 25. [Google Scholar] [CrossRef]
- Pedersen, M.; Zalesky, A.; Omidvarnia, A.; Jackson, G.D. Multilayer network switching rate predicts brain performance. Proc. Natl. Acad. Sci. USA 2018, 115, 13376–13381. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Xiang, J.; Wang, B.; Li, D.; Cheng, X.; Liu, T.; Cui, X. Frequency-specific analysis of the dynamic reconfiguration of the brain in patients with schizophrenia. Brain Sci. 2022, 12, 727. [Google Scholar] [CrossRef]
- Marek, S.; Dosenbach, N.U. The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialogues Clin. Neurosci. 2018, 20, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.W.; Veitch, D.P.; Aisen, P.S.; Beckett, L.A.; Cairns, N.J.; Green, R.C.; Harvey, D.; Jack, C.R., Jr.; Jagust, W.; Morris, J.C.; et al. The Alzheimer’s Disease Neuroimaging Initiative 3: Continued innovation for clinical trial improvement. Alzheimer’s Dement. 2017, 13, 561–571. [Google Scholar] [CrossRef] [PubMed]
- LaMontagne, P.J.; Benzinger, T.L.; Morris, J.C.; Keefe, S.; Hornbeck, R.; Xiong, C.; Grant, E.; Hassenstab, J.; Moulder, K.; Vlassenko, A.G.; et al. OASIS-3: Longitudinal neuroimaging, clinical, and cognitive dataset for normal aging and Alzheimer disease. medRxiv 2019. medRxiv:2019.12.13.19014902. [Google Scholar]
- Tremblay-Mercier, J.; Madjar, C.; Das, S.; Binette, A.P.; Dyke, S.O.; Étienne, P.; Lafaille-Magnan, M.-E.; Remz, J.; Bellec, P.; Collins, D.L.; et al. Open science datasets from PREVENT-AD, a longitudinal cohort of pre-symptomatic Alzheimer’s disease. NeuroImage Clin. 2021, 31, 102733. [Google Scholar] [CrossRef] [PubMed]
- Galiano, A.; Mengual, E.; De Eulate, R.G.; Galdeano, I.; Vidorreta, M.; Recio, M.; Riverol, M.; Zubieta, J.L.; Fernández-Seara, M.A. Coupling of cerebral blood flow and functional connectivity is decreased in healthy aging. Brain Imaging Behav. 2020, 14, 436–450. [Google Scholar] [CrossRef]
- Passamonti, L.; Tsvetanov, K.; Jones, P.; Bevan-Jones, W.; Arnold, R.; Borchert, R.; Mak, E.; Su, L.; O’Brien, J.; Rowe, J. Neuroinflammation and functional connectivity in Alzheimer’s disease: Interactive influences on cognitive performance. J. Neurosci. 2019, 39, 7218–7226. [Google Scholar] [CrossRef]
- Subramanian, S.; Rajamanickam, K.; Prakash, J.S.; Ramachandran, M.; for Alzheimer’s Disease Neuroimaging Initiative (ADNI). Study on structural atrophy changes and functional connectivity measures in Alzheimer’s disease. J. Med. Imaging 2020, 7, 016002. [Google Scholar] [CrossRef] [PubMed]
- Loaliyan, S.S.; Steeg, G.V. Comparative analysis of generalization and harmonization methods for 3d brain fmri images: A case study on openbhb dataset. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), Seattle, WA, USA, 17–21 June 2024; pp. 4915–4923. [Google Scholar]
- Bazay, F.E.-Z.; Drissi El Maliani, A. Assessing the impact of preprocessing pipelines on fMRI based autism spectrum disorder classification: ABIDE II results. In Proceedings of the International Conference on Engineering Applications of Neural Networks, Corfu, Greece, 27–30 June 2024; pp. 463–477. [Google Scholar]
- Loaliyan, S.S.; Ambite, J.-L.; Thompson, P.M.; Jahanshad, N.; Ver Steeg, G. FedDAPL: Toward Client-Private Generalization in Federated Learning. arXiv 2025, arXiv:2509.23688. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffari, A.; Zhao, Y.; Abouzaki, M.; Romero, Y.; Langley, J.; Hu, X. Functional Connectome Alterations Across the Spectrum of Alzheimer’s Disease. J. Dement. Alzheimer's Dis. 2025, 2, 46. https://doi.org/10.3390/jdad2040046
Ghaffari A, Zhao Y, Abouzaki M, Romero Y, Langley J, Hu X. Functional Connectome Alterations Across the Spectrum of Alzheimer’s Disease. Journal of Dementia and Alzheimer's Disease. 2025; 2(4):46. https://doi.org/10.3390/jdad2040046
Chicago/Turabian StyleGhaffari, Amin, Yufei Zhao, Majd Abouzaki, Yasmine Romero, Jason Langley, and Xiaoping Hu. 2025. "Functional Connectome Alterations Across the Spectrum of Alzheimer’s Disease" Journal of Dementia and Alzheimer's Disease 2, no. 4: 46. https://doi.org/10.3390/jdad2040046
APA StyleGhaffari, A., Zhao, Y., Abouzaki, M., Romero, Y., Langley, J., & Hu, X. (2025). Functional Connectome Alterations Across the Spectrum of Alzheimer’s Disease. Journal of Dementia and Alzheimer's Disease, 2(4), 46. https://doi.org/10.3390/jdad2040046





