Abstract
Two new triterpenes cycloartane-type, named 24-methylencycloartan-12-oxo-3β,22α-diol and trichiliol, were isolated from the leaves of Trichilia casaretti C. DC. together with three known triterpenes—24-methylencycloart-3β,22-diol, 22,25-dihydrocycloart-23(E)-en-3β-ol, and 22(R)-hydroxycycloart-24-en-3-ol. These compounds were characterized on the basis of their spectral data, mainly 1D (1H and 13C) and 2D NMR (1H-1H-COSY, 1H-1H-NOESY, HMQC, HSQC, and HMBC), and mass spectra (EI-MS and HR-ESI-MS), also involving comparison with data from the literature.
1. Introduction
The Meliaceae family has attracted such a great interest among phytochemists because of its relatively complex and diverse chemical structures and its biological activity, mainly against insects [1,2,3,4]. The Trichilia genus (Meliaceae) comprises about 230 species distributed throughout tropical America, which are recognized for their significant economic importance and high commercial value. Phytochemical studies revealed that this genus is a potential source of terpenoids, including triterpenes, limonoids, steroids and other terpenes derivatives [3,4,5,6]. Species of this genus have been also studied for their insecticidal activities and their isolated compounds revealed complex and interesting structures including various limonoids [5,7,8]. The isolation and structural elucidation of two novel sesquiterpenes from the stems of T. casaretti collected in Espírito Santo State, Brazil, were reported by Vieira et al. in 2010 [4].
In the present article, we describe an investigation of methanol extract from leaves of a T. casaretti specimen, which allowed the characterization of five triterpenes, including two novel cycloartane-type triterpenes named 24-methylencycloartane-12-oxo-3β,22α-diol (1) and trichiliol (2). The known triterpenes were identified as 24-methylencycloartane-3β,22-diol (3), cycloartane-22(E)-en-3β,22,25-triol (4), and 22(R)-hydroxycycloart-24-en-3-ol (5). Their structures (Figure 1) were established by spectrometric techniques, mainly 1D and 2D NMR, and HR-ESI-MS, and comparison with literature data.
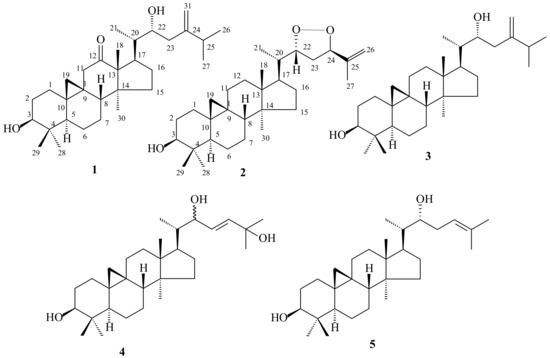
Figure 1.
Triterpenes isolated from Trichilia casaretti.
2. Results and Discussion
Fractionation of the MeOH extract of the leaves from T. casaretti by classical chromatographic methods resulted in the isolation of five cycloartane-type triterpenes 1–5 whose structures are shown in Figure 1. The two novel cycloartane-type triterpenes named 24-methylencycloartan-12-oxo-3β,22α-diol (1) and trichiliol (2), along with three known triterpenes, cycloartane-type 24-methylencycloart-3β,22-diol (3) [9,10], 22,25-dihydrocycloart-23(E)-en-3β-ol (4) [9,11] and 22(R)-hydroxycycloart-24-en-3-ol (5) [12] were characterized on the basis of 1H and 13C-NMR spectral data, especially 2D NMR and mass spectral data, besides comparison with values described in the cited literature.
The new triterpene named 24-methylencycloartan-12-oxo-3β,22α-diol(1) was isolated as yellow oil, = +27.3 (CHCl3, c 0.02). The analysis of the {1H}- and 13C-APT NMR spectra (Table 1) revealed signals corresponding to 31 C-atoms, including seven quaternary C (five sp3 and two sp2, one attributed to methylidene at δC 153.5 (C-24) and one C=O group at δC 216.0 (C-12)), seven CH (all sp3, including two linked to O atom at δC 78.8 (CH-3) and 70.3 (CH-22), nine methylene CH2 (including one sp2 at δC 109.8 (CH2-31)) and seven methyl groups CH3, allowing to deduce the expanded partial formula C6(C=O)CH5(OCH)2(CH2)10(CH3)7 = C31H48O3 or C6(C=O)CH5(HOCH)2(CH2)10(CH3)7 = C31H50O3 based in the presence of two hydroxyl groups.

Table 1.
13C and 1H-NMR data of triterpene 1 and comparison with triterpene 3 (CDCl3), δ in ppm, coupling constants (J in Hz, in parenthesis) *.
The HR-ESI-MS spectrum of 1 showed peak corresponding to the quasi molecular ion ([M + H]+) at m/z 471.3475, that in combination with 1H-NMR (1D and 2D) and comparative analysis of the {1H}- and 13C-APT NMR spectra (Table 1) enabled us to propose the molecular formula C31H50O3 (seven degrees of unsaturation) compatible with a cycloartane-type 24-methylencycloart-3β,22-diol (3) skeleton [9]. The only difference regarding compounds 1 and 3 consists of a carbonyl group at C-12.
The signal at δC 216.0 in the 13C-APT NMR spectrum suggested the presence of a carbonyl group in the triterpene cycloartane 1. The location of the carbonyl group at C-12 was supported in the HMBC spectrum by the correlation (3JHC) between C-12 (δC 216.0) and the hydrogens of the methyl group 3H-18 at δH 0.90. Thus, the new triterpene cycloartane-type was characterized as 24-methylidencycloartan-12-oxo-3β,22-diol (1).
Compound 2 was isolated as a yellow oil, = +48.0 (CHCl3, c 0.001). The analysis of the {1H}- and 13C-NMR spectra (Table 2) revealed signals corresponding to 30 carbon atoms, showing similarity with the triterpene cycloartane 1. Comparison of the 13C-NMR data of compounds 1 and 2 revealed the absence of carbonyl signal in 2, and additional differences can be justified through modifications involving only the side chain (Table 1 and Table 2).

Table 2.
13C- and 1H-NMR data of triterpene 2 (pyridine-d5), δ in ppm, coupling constants (J in Hz, in parenthesis) *.
The 13C-NMR spectrum of 2 with the presence of 30 carbon signals revealed additional carbon signals corresponding to remaining carbon atoms (C)(CH)(OCH)2(CH2)2(CH3)2 = C8H13O2 of the side chain, different of the cycloartane triterpene 1 with signals representing 31 carbon atoms including (C)(CH)2(HOCH)(CH2)2(CH3)3 = C9H17O of the side chain.
The 13C-NMR spectrum involving the side chain of 2 revealed signals representing only two methyl groups when compared with those of 1 with three, one corresponding to CH3-21 at δC 19.5 correlated in the HSQC with the doublet (J = 6.3 Hz) signal at δH 1.22 (3H-21) and one attached to sp2 carbon (CH3-27, δH 2.01\δC 18.5), two sp2 carbon atoms at δC 147.8 (C-25) and 112.5 (CH2-26), featuring a methylidene group =CH2 confirmed by correlations (1JHC) between δC 112.48 (CH2-26) with two broad singlets at δH 5.32 (Ha-26) and 5.10 (Hb-26) observed in the HSQC spectrum.
The location of the methylidene group at the C-25 was confirmed through the 2JHC correlations between C-25 (δC 147.8) and 3H-27 (δH 2.01) and C-26 (δC 112.5) with 3H-27 (δH 2.01). The presence of a isopropenyl group (isopropyl in 1) attached to CH-24 (δC 86.6\δH 5.32, br s) was deduced by HSQC and HMBC correlations between CH-24 and 3H-27 [(δH 2.01, br s\(δC 18.5, 3JHC)]. It was also possible to observe the interaction between CH-22 (δC 69.87.6\(δH 4.55, br d, J = 10.0 Hz) and 3H-21 (δH 1.22, d, J = 6.3 Hz\(δC 12.8, 3JHC). These data allowed to postulate the presence of oxygen atoms linked to carbon 22 (HC-O-22: δH 4.55\69.7) and 24 (HC-O-24 δH 5.32\86.6), in agreement with the 1H-1H-COSY spectrum which showed correlations between the methylene hydrogens 2H-23 (δH 2.10 and 1.90\δC 34.5) and both hydrogen atoms H-22 (δH 4.55) and H-24 at δH 5.32 (chemical shift justified by location in position allylic and carbinolic). Other heteronuclear correlations observed in the HMBC spectrum of 2 were shown in Table 2.
The endoperoxide function in the side chain was also supported by mass spectra of low and high resolution demanding an additional ring to suit the seven degrees of unsaturation. The HRESI-MS spectrum of 2 (Scheme S1, Supplementary Materials) showed peak corresponding to the protonated molecular ion [M + H]+ at m/z 457.3502 which together with the NMR spectrum of 13C-DEPT 135° NMR allowed to propose the molecular formula C30H48O3 compatible with a cycloartane-type skeleton and one heterocyclic involving the peroxide function.
The relative stereochemistry indicated in 2 was deduced by the spatial dipolar interaction observed in the 1H-1H-NOESY spectrum between 3H-30 and H-17 indicating H-17α, interaction of 3H-18 with H-20 and 3H-21with H-12 equatorial suggesting H-20α and CH3-21α, respectively. Other spatial dipolar interaction was shown in Figure 2. These data allowed to identify compound 2 as the new triterpene 22,24-peroxidecycloart-25-en-3β-ol (2), named trichiliol.
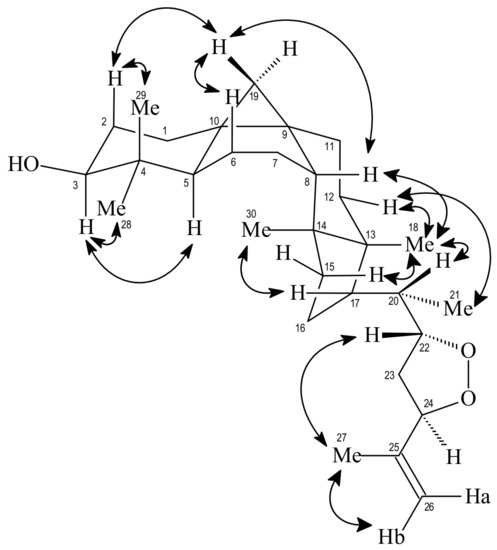
Figure 2.
Selected Nuclear Overhauser Effect (NOE) correlations and relative stereochemistry for compound 2. Arrows denote the main NOE correlations.
3. Material and Methods
3.1. General Methods
Measures of optic rotation were obtained on a Perkin Elmer 343 digital polarimeter. Melting point was obtained on a Microquímica MQRPF. EI-MS (low resolution) mass spectra were obtained on Shimadzu QP5050A mass spectrometer. HRESI-MS (high resolution) mass spectra were obtained by using a ESI-IT-OF-MS SHIMADZU mass spectrometer, using the positive ion mode of analysis. Chromatographic purifications were carried out over silica gel 60 (70–230 mesh). Silica gel 60 F254 was used in thin layer chromatography analysis. 1H and 13C-NMR spectra were measured on a Bruker model DRX-500 and Jeol model Eclipse-400 spectrometers, equipped with inverse probes and field gradient, operating at 500 (1H) and 125 (13C) and 400 (1H) and 100 (13C) MHz. CDCl3 and pyridine-d5 were used as solvents and TMS as internal reference. Chemical shifts are given in the δ scale (ppm) and coupling constants J in Hz. One dimensional (1D) 1H and 13C-NMR spectra were acquired under standard conditions by using a direct detection 5 mm 1H/13C dual probe. Standard pulse sequences were used for two dimensional spectra by using a multinuclear inverse detection 5 mm probe with field gradient.
3.2. Plant Material
The leaves of Trichilia casaretti C. DC. were collected at Vale Cia, Linhares City, Espirito Santo State, Brazil, and identified by Domingos Folly. A voucher specimen (CVRD-449) was deposited at the Vale Cia of Vale herbarium, Vale Cia, Linhares City, Espirito Santo State, Brazil.
3.3. Extract and Isolation
Air dried and powdered leaves (920 g) from Trichilia casaretti C. DC. were extracted with methanol at room temperature, yielding 15.0 g of crude methanol extract. The methanol extract was submitted to liquid-liquid partition (CH2Cl2:H2O, 3:1, v/v). The CH2Cl2 fraction (10.1 g) was submitted to liquid-liquid partition (MeOH:Hexane, 1:1, v/v). The methanol fraction (8.0 g) was chromatographed over a silica gel column with a gradient of ethyl acetate/hexane, affording eight fractions. Fraction 1 (19.3 mg) was similarly rechromatographed, yielding triterpene 5 (8.0 mg). Fraction 5 (518 mg) was analogously rechromatographed, affording eleven fractions. Fraction 5.7 (66.8 mg) was similarly chromatographed, affording triterpene 4 (4.0 mg). Fraction 7 (6.736 mg) was chromatographed affording fifteen fractions. The fraction 7.5 (1.300 mg) was rechromatographed affording fourteen fractions. The triterpene 2 (10 mg) was obtained from the fractions 7.5.3 (45 mg) and 7.5.4 (100 mg). The triterpene 1 (25 mg) was obtained from the fraction 7.6 (500 mg). The fraction 7.9 (163 mg) was chromatographed affording fifteen fractions. The fraction 7.9.2 (50.3 mg) yielded triterpene 3 (11 mg).
Supplementary Materials
Figures S1–S18 and Schemes S1 and S2 are available online.
Acknowledgments
The authors are grateful to Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and to Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES).
Author Contributions
All authors contributed equally to develop this work.
Conflicts of Interest
No potential conflict of interest.
References
- Pennington, T.D.; Styles, B.D. A generic monograph of the Meliaceae. Blumea 1975, 22, 419–540. [Google Scholar]
- Pupo, M.T.; Adorno, M.A.; Vieira, P.C.; Fernandes, J.B.; Silva, M.F.G.F.; Pirani, J.R. Terpenoids and steroids from Trichilia species. J. Braz. Chem. Soc. 2002, 13, 382–388. [Google Scholar] [CrossRef]
- Rodrigues, V.F.; Carmo, H.M.; Braz-Filho, R.; Mathias, L.; Vieira, I.J.C. Two new terpenoids from Trichilia quadrijuga (Meliaceae). Nat. Prod. Comm. 2010, 5, 179–184. [Google Scholar]
- Vieira, I.J.C.; Figueiredo, E.R.; Freitas, V.R.; Mathias, L.; Braz-Filho, R.; Araújo, R.M. A new sesquiterpene from Trichilia casaretti (Meliaceae). Am. J. Anal. Chem. 2010, 1, 70–72. [Google Scholar] [CrossRef]
- Freitas, V.R.; Carmo, H.M.; Oliveira, R.R.; Braz-Filho, R.; Mathias, L.; Vieira, I.J.C. Isolation of terpenoids from Trichilia quadrijuga (Meliaceae) by droplet counter-current chromatography. Chromatographia 2009, 70, 1191–1195. [Google Scholar]
- Ramírez, M.C.; Toscano, R.A.; Arnason, J.; Omar, S.; Cerda-Garcia-Rojas, C.M.; Mata, R. Structure, conformation and absolute configuration of new antifeedant dolabellanes from Trichilia trifolia. Tetrahedron 2000, 56, 5085–5091. [Google Scholar] [CrossRef]
- Xie, Y.S.; Isman, M.B.; Gunning, P.; Mackinnon, S.; Arnason, J.T.; Taylor, D.R.; Sánchez, P.; Hasbun, C.; Towers, G.H.N. Biological activity of extracts of Trichilia species and the limonoid hirtin against lepidopteran larvae. Biochem. Syst. Ecol. 1994, 22, 129–136. [Google Scholar] [CrossRef]
- Cortez, D.A.G.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.G.F.; Ferreira, A.G.; Cass, Q.B.; Pirani, J.R. Meliacin butenolides from Trichilia estipulate. Phytochemistry 1998, 49, 2493–2496. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Roque, N.F. Cycloartane triterpenoids from Guarea macrophylla. Phytochemistry 2002, 60, 329–332. [Google Scholar] [CrossRef]
- Yang, A.; Shang, Q.; Yang, L.; Li, C.; Yuan, H.J. Chemical Constituents of the Flowerbuds of Tussilago farfara. Chem. Nat. Compd. 2017, 53, 584–585. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Khan, S.B.; Ather, A. Tyrosinase inhibitory cycloartane type triterpenoids from the methanol extract of the whole plant of Amberboa ramosa Jafri and their structure–activity relationship. Bioorg. Med. Chem. 2006, 14, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Misra, L.N.; Jakupovic, J.; King, R.M.; Robinson, H. Guaianolides, heliangolides, diterpenes and cycloartenol derivatives from Balsamorhiza sagittata. Phytochemistry 1985, 24, 2029–2036. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).