Abstract
IV line connectors often become contaminated between infusions, which leads to line infections. A flexible shield was developed to prevent this by means of passive protection. It was tested in a simulated bedside environment and protected from touch contamination as well as airborne transmission of skin bacteria to the connector hub. This flexible shield can compensate for the unavoidable human factor infection control lapses that occur during IV line handling by healthcare workers.
Keywords:
flexible; intravenous line connector; Luer Lock; airborne bacterial contamination; skin contamination; protector; protection; safeguard; alcohol cap; line infection; central line associated blood stream infection; hospital acquired infection; human factors; sterilization; connector hub contamination; touch culture; blood agar; human skin; human 1. Introduction
As many as 23 percent of hospital intravenous line connectors have been found to be contaminated with pathogenic bacteria [1]. Healthcare workers, for human factor reasons, such as fatigue, overwork, distractions or not seeing importance, fail to disinfect 3 to 4 percent of connectors before establishing an infusion [2,3]. Bacterial contamination of a connector tip leads to bacterial biofilm formation with subsequent planktonic shedding into the bloodstream and the clot(s) at the end of a line. The consequences are line infections or as the CDC labels them, central line associated bloodstream infections (CLABSI).
They are reported as hospital-acquired infections (HAI) under the preventable subgroup of central-line associated bloodstream infections (CLABSI) and hospitals can be penalized when they exceed their given rate [4].
The rate of CLABSI in intensive care units has been reported to be 1.7 per 1000 central line days (CLD) and, in medical–surgical units, 2.8 per 1000 CLD [4]. CLABSI incidence increases when healthcare delivery systems approach or exceed their surge capacity, during pandemics, armed conflicts, civilian disasters, respiratory infection seasons [5] and financial distress [6].
These avoidable infections carry considerable mortality, interrupt lifesaving treatments, prolong hospital stays, frustrate antimicrobial stewardship efforts and are costly.
A representative pediatric patient with several IV line connectors at risk of contamination and central line-associated bloodstream infection is shown in Figure 1 below.
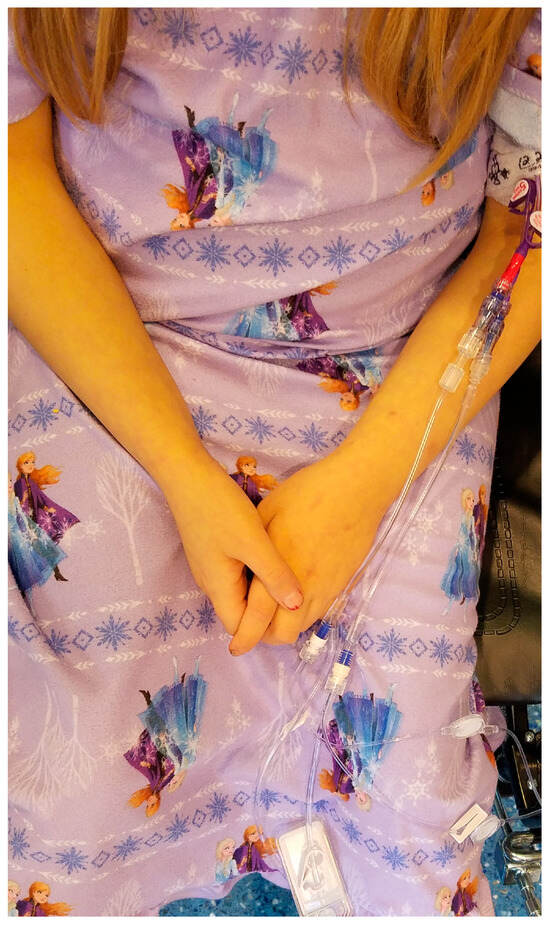
Figure 1.
De-identified icteric pediatric patient with a central line and multiple IV connectors in use.
2. Materials and Methods
A passive, flexible contamination shield was designed to protect IV line connectors when they are uncapped and exposed during the connecting of IV lines.
The flexible shield was tested with nursing staff for its functionalities and for contact and airborne contamination in a human volunteer during a simulated 90 min bedside situation.
The flexible shield design and its evaluated functionalities:
The passive spoon-like protection for when the connector is exposed, disinfection with a 70% alcohol swab, the securely connected (Luer Lock) IV line, and an alcohol cap protector used between infusions are shown in Figure 2 below.

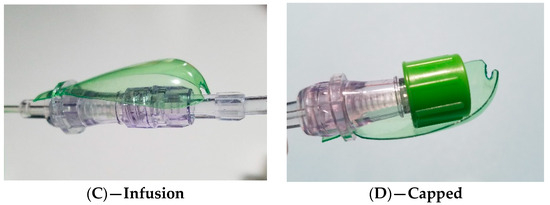
Figure 2.
Passive spoon-like protection for periods when a connector tip is exposed (A), disinfection with a 70% alcohol swab (B), IV line (Luer Lock), securely connected (C), and room for the placement of an alcohol-lined cap between infusions (D).
Simulated 90 min bedside evaluation:
Two shielded and two unshielded needleless valve connectors were disinfected for one minute and placed close to each other on a volunteer’s chest. The paired connectors were subsequently covered by a worn T-shirt and the volunteer was asked to move around for 90 min and occasionally tighten the T-shirt. A total of sixteen connectors, eight shielded and eight unshielded, were prospectively exposed to skin and air movements generated by the T-shirt. This was performed on four different days over four sessions. Imprints of the connector tips were made on blood agar, and the plates were incubated at 36.5 degrees Celsius for 36 h.
The healthy volunteer was informed about the nature and potential risks of the experiment and signed a witnessed written informed consent.
3. Results
The eight unshielded connector tips all yielded various colonies of skin bacteria, whereas the eight imprints from the flexibly shielded connectors remained colony-free; p < 0.001 based on a coin flip probability calculation (posterior probability density function).
4. Discussion
This proposed flexible contamination shield did not interfere with routine IV line care, and it protected the connectors against touch contamination in this 90 min simulated bedside situation. Skin flora and/or colonized skin desquamates were not carried to the protected connector tips by the air currents generated under the T-shirt, thus validating its simple, functional “cobra head” design.
This shield could compensate for the “human factor” infection control lapses that occur during IV line care. CLABSIs are extremely expensive for the patient and any healthcare system.
The current FDA class 2 medical device “needleless IV connector” is a defective medical device from a human factor point of view. The eventual availability of flexible contamination shields could become a legal risk management issue for healthcare providers.
Unfortunately, the FDA does not receive severe adverse reports (caused death or prolongation of hospitalization) to act upon, because any reporter would fear being accused of not following the instructions that were the basis for original market approval.
Manufacturers, understandably, for now, are reluctant to precipitate lawsuits and recalls by making a commodity product safer.
However, there is hope that, ultimately, patient and physicians’ interests will prevail.
Funding
This research received no external funding.
Institutional Review Board Statement
Review was not performed under the perception of no risk discernable to the human subject.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Original data and source documents can be requested from the author mdwalterspiel@gmail.com.
Acknowledgments
The author thanks the volunteer and R.H. Sprinkle, for review.
Conflicts of Interest
The author declares no conflicts of interest except for patent application.
References
- Hankins, R.; Majorant, O.D.; Rupp, M.E.; Cavalieri, R.J.; Fey, P.D.; Lyden, E.; Cawcutt, K.A. Microbial colonization of intravascular catheter connectors in hospitalized patients. Am. J. Infect. Control 2019, 47, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Delahanty, K.M.; Myers, F.E., III. Nursing2009® I.V. Infection control survey report. Nursing 2009, 39, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Delahanty, K.M.; Myers, F.E., III. Nursing2007 Infection control survey report. Nursing 2007, 37, 28–37. [Google Scholar] [CrossRef] [PubMed]
- O’grady, N.P. Prevention of Central Line–Associated Bloodstream Infections. N. Engl. J. Med. 2023, 389, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Weiner-Lastinger, L.M.; Pattabiraman, V.; Konnor, R.Y.; Patel, R.; Wong, E.; Xu, S.Y.; Smith, B.; Edwards, J.R.; Dudeck, M.A. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network. Infect. Control Hosp. Epidemiol. 2022, 43, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Bruch, J.D.; Song, Z. Changes in Hospital Adverse Events and Patient Outcomes Associated with Private Equity Acquisition. JAMA 2023, 330, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).