Risk Factors for Relapses in Multiple Sclerosis Beyond Disease-Modifying Therapy: An Umbrella Review of Systematic Reviews and Meta-Analyses
Abstract
1. Introduction
2. Methods
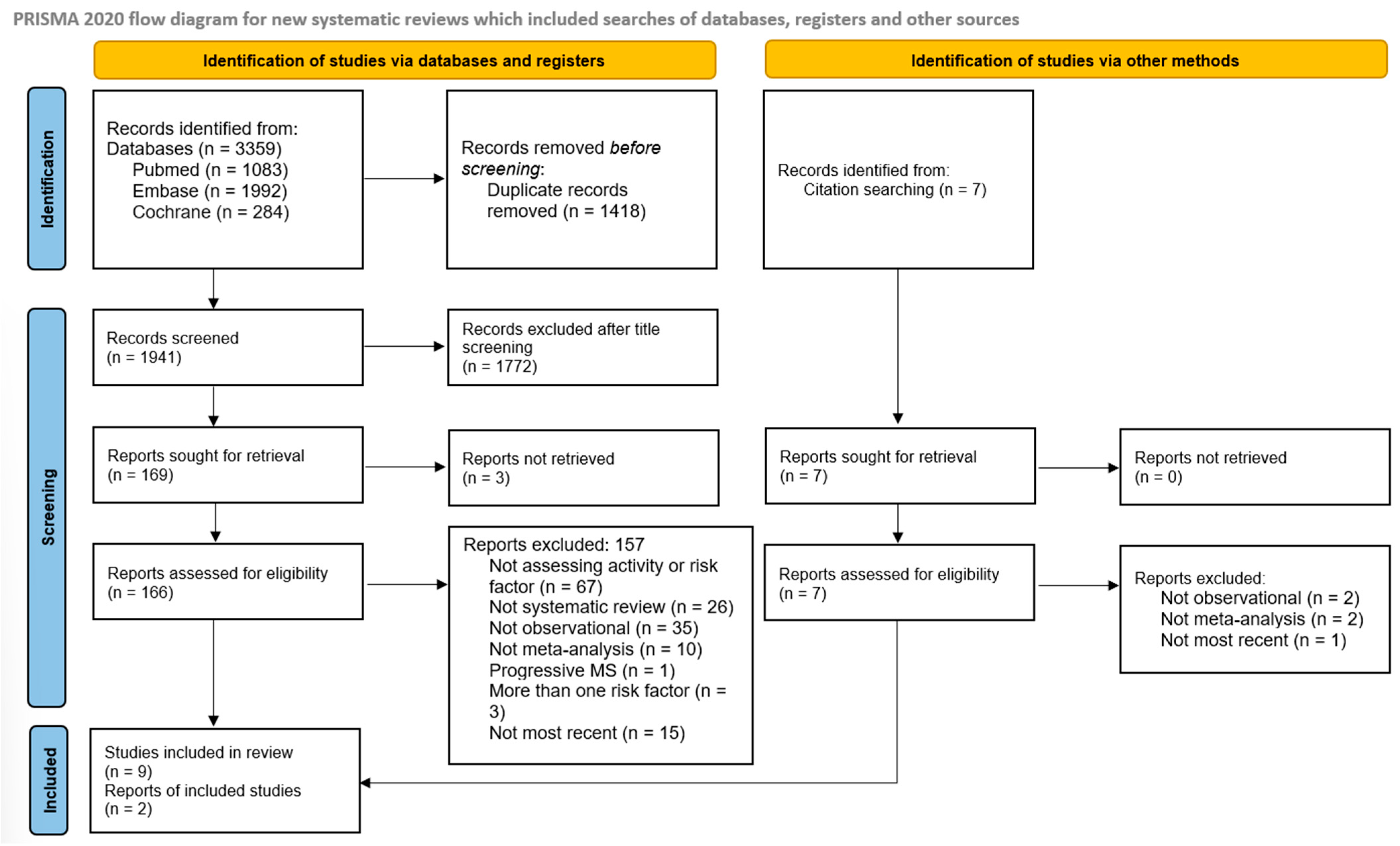
3. Results
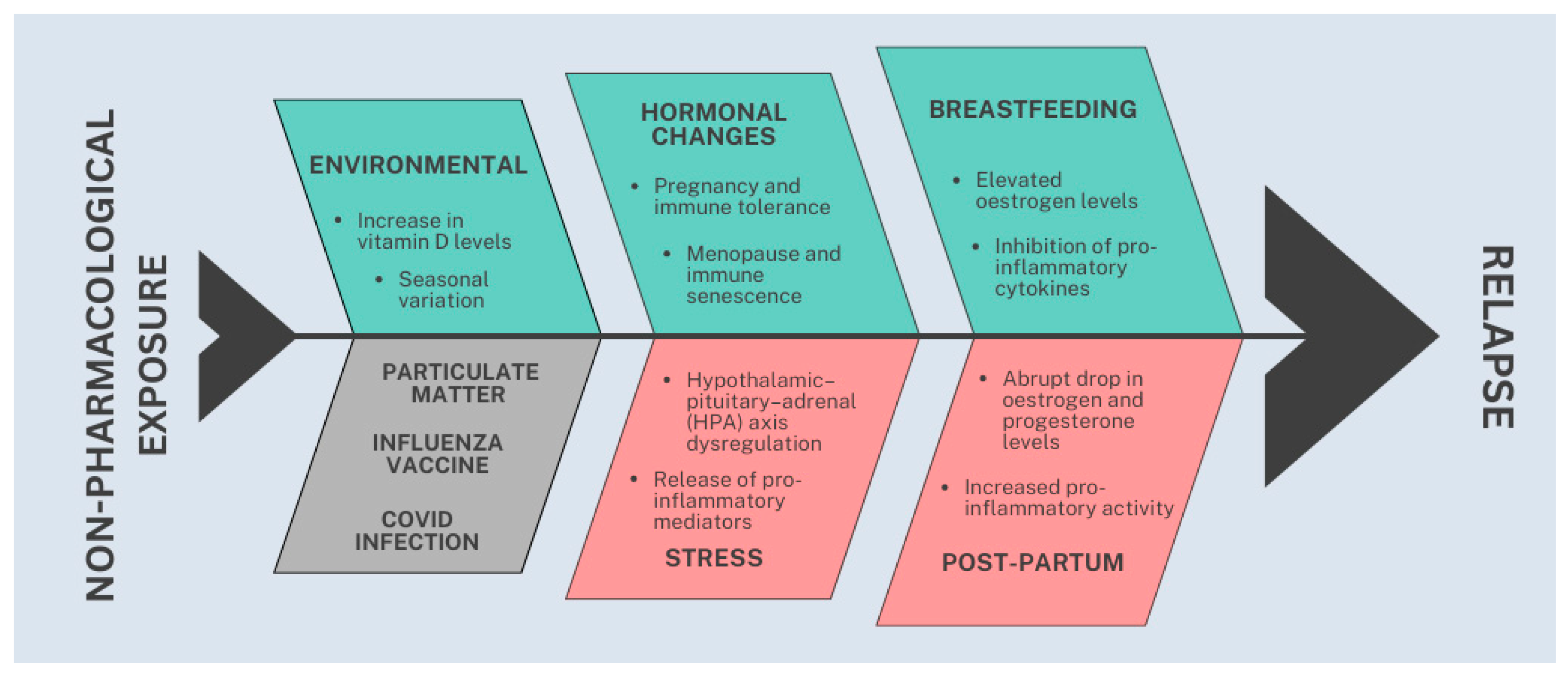
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frohman, E.M.; Racke, M.K.; Raine, C.S. Multiple sclerosis—The plaque and its pathogenesis. N. Engl. J. Med. 2006, 354, 942–955. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Lublin, F.D.; Häring, D.A.; Ganjgahi, H.; Ocampo, A.; Hatami, F.; Čuklina, J.; Aarden, P.; Dahlke, F.; Arnold, D.L.; Wiendl, H.; et al. How patients with multiple sclerosis acquire disability. Brain 2022, 145, 3147–3161. [Google Scholar] [CrossRef]
- Gavoille, A.; Rollot, F.; Casey, R.; Kerbrat, A.; Le Page, E.; Bigaut, K.; Mathey, G.; Michel, L.; Ciron, J.; Ruet, A.; et al. Acute Clinical Events Identified as Relapses with Stable Magnetic Resonance Imaging in Multiple Sclerosis. JAMA Neurol. 2024, 81, 814–823. [Google Scholar] [CrossRef]
- Rosalem, R.A.; Spricigo, M.G.P.; De Oliveira, M.B.; Adoni, T.; Apóstolos-Pereira, S.L.; Callegaro, D.; Silva, G.D. Delayed access and adherence are real-world challenges that compromises effectiveness of natalizumab in multiple sclerosis. Mult. Scler. Relat. Disord. 2025, 103, 106627. [Google Scholar] [CrossRef] [PubMed]
- Simon-Nogueira, A.B.; De Holanda, A.C.; Rosalem, R.A.; Santillan, T.I.V.; de Oliveira, M.B.; Apóstolos-Pereira, S.L.; Adoni, T.; Callegaro, D.; Silva, G.D. Fingolimod in multiple sclerosis: Insights into drug survival and discontinuation factors. Mult. Scler. Relat. Disord. 2025, 101, 106554. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.D.; Pipek, L.Z.; Oliveira, M.B.D.; Apóstolos-Pereira, S.L.; Adoni, T.; Lino, A.M.M.; Callegaro, D.; Castro, L.H.M. Could rituximab revolutionize multiple sclerosis treatment in Brazil? The missed opportunity for fewer relapses and lower costs. Lancet Reg. Health-Am. 2025, 49, 101171. [Google Scholar] [CrossRef]
- Hobart, J.; Bowen, A.; Pepper, G.; Crofts, H.; Eberhard, L.; Berger, T.; Boyko, A.; Boz, C.; Butzkueven, H.; Celius, E.G.; et al. International consensus on quality standards for brain health-focused care in multiple sclerosis. Mult. Scler. Houndmills Basingstoke Engl. 2019, 25, 1809–1818. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, Z.; Han, F.; Liang, S.; Gao, Y.; Wu, D. Factors associated with relapses in relapsing-remitting multiple sclerosis. Medicine 2020, 99, e20885. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Krysko, K.M.; Rutatangwa, A.; Graves, J.; Lazar, A.; Waubant, E. Association Between Breastfeeding and Postpartum Multiple Sclerosis Relapses: A Systematic Review and Meta-Analysis. JAMA Neurol. 2020, 77, 327–338. [Google Scholar] [CrossRef]
- Seyedmirzaei, H.; Salabat, D.; KamaliZonouzi, S.; Teixeira, A.L.; Rezaei, N. Risk of MS relapse and deterioration after COVID-19: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2024, 83, 105472. [Google Scholar] [CrossRef]
- Modrego, P.J.; Urrea, M.A.; de Cerio, L.D. The effects of pregnancy on relapse rates, disability and peripartum outcomes in women with multiple sclerosis: A systematic review and meta-analysis. J. Comp. Eff. Res. 2021, 10, 175–186. [Google Scholar] [CrossRef]
- Shahraki, Z.; Rastkar, M.; Rastkar, E.; Mohammadifar, M.; Mohamadi, A.; Ghajarzadeh, M. Impact of menopause on relapse rate and disability level in patients with multiple sclerosis (MS): A systematic review and meta-analysis. BMC Neurol. 2023, 23, 316. [Google Scholar] [CrossRef]
- Lotfi, F.; Mansourian, M.; Mirmoayyeb, O.; Najdaghi, S.; Shaygannejad, V.; Esmaeil, N. Association of Exposure to Particulate Matters and Multiple Sclerosis: A Systematic Review and Meta-Analysis. Neuroimmunomodulation 2022, 29, 21–27. [Google Scholar] [CrossRef]
- Schubert, C.; Steinberg, L.; Peper, J.; Ramien, C.; Hellwig, K.; Köpke, S.; Solari, A.; Giordano, A.; Gold, S.M.; Friede, T.; et al. Postpartum relapse risk in multiple sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2023, 94, 718–725. [Google Scholar] [CrossRef]
- Nabizadeh, F.; Valizadeh, P.; Yazdani Tabrizi, M.; Moayyed, K.; Ghomashi, N.; Mirmosayyeb, O. Seasonal and monthly variation in multiple sclerosis relapses: A systematic review and meta-analysis. Acta Neurol. Belg. 2022, 122, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- von Drathen, S.; Gold, S.M.; Peper, J.; Rahn, A.C.; Ramien, C.; Magyari, M.; Hansen, H.-C.; Friede, T.; Heesen, C. Stress and Multiple Sclerosis—Systematic review and meta-analysis of the association with disease onset, relapse risk and disability progression. Brain Behav. Immun. 2024, 120, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Mohr, D.C.; Hart, S.L.; Julian, L.; Cox, D.; Pelletier, D. Association between stressful life events and exacerbation in multiple sclerosis: A meta-analysis. BMJ 2004, 328, 731. [Google Scholar] [CrossRef]
- Farez, M.F.; Correale, J. Immunizations and risk of multiple sclerosis: Systematic review and meta-analysis. J. Neurol. 2011, 258, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lapiscina, E.H.; Mahatanan, R.; Lee, C.H.; Charoenpong, P.; Hong, J.P. Associations of serum 25(OH) vitamin D levels with clinical and radiological outcomes in multiple sclerosis, a systematic review and meta-analysis. J. Neurol. Sci. 2020, 411, 116668. [Google Scholar] [CrossRef]
- Hellwig, K.; Rockhoff, M.; Herbstritt, S.; Borisow, N.; Haghikia, A.; Elias-Hamp, B.; Menck, S.; Gold, R.; Langer-Gould, A. Exclusive Breastfeeding and the Effect on Postpartum Multiple Sclerosis Relapses. JAMA Neurol. 2015, 72, 1132–1138. [Google Scholar] [CrossRef]
- Howie, P.W.; Mcneilly, A.S. Breast-feeding and postpartum ovulation. IPPF Med. Bull. 1982, 16, 1–3. [Google Scholar] [PubMed]
- Baroncini, D.; Annovazzi, P.O.; De Rossi, N.; Mallucci, G.; Clerici, V.T.; Tonietti, S.; Mantero, V.; Ferrò, M.T.; Messina, M.J.; Barcella, V.; et al. Impact of natural menopause on multiple sclerosis: A multicentre study. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Maglione, A.; Rolla, S.; Mercanti, S.F.D.; Cutrupi, S.; Clerico, M. The Adaptive Immune System in Multiple Sclerosis: An Estrogen-Mediated Point of View. Cells 2019, 8, 1280. [Google Scholar] [CrossRef]
- Confavreux, C.; Hutchinson, M.; Hours, M.M.; Cortinovis-Tourniaire, P.; Moreau, T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med. 1998, 339, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Bansal, A.; Culberson, J.; Peiris, A.N. The Role of Sex Hormones in Multiple Sclerosis. Eur. Neurol. 2018, 80, 93–99. [Google Scholar] [CrossRef]
- Koetzier, S.C.; Neuteboom, R.F.; Wierenga-Wolf, A.F.; Melief, M.-J.; de Mol, C.L.; van Rijswijk, A.; Dik, W.A.; Broux, B.; van der Wal, R.; Berg, S.A.A.v.D.; et al. Effector T Helper Cells Are Selectively Controlled During Pregnancy and Related to a Postpartum Relapse in Multiple Sclerosis. Front. Immunol. 2021, 12, 642038. [Google Scholar] [CrossRef]
- Vukusic, S.; Hutchinson, M.; Hours, M.; Moreau, T.; Cortinovis-Tourniaire, P.; Adeleine, P.; Confavreux, C. Pregnancy and multiple sclerosis (the PRIMS study): Clinical predictors of post-partum relapse. Brain J. Neurol. 2004, 127 Pt 6, 1353–1360. [Google Scholar] [CrossRef]
- Graves, J.S.; Krysko, K.M.; Hua, L.H.; Absinta, M.; Franklin, R.J.M.; Segal, B.M. Ageing and multiple sclerosis. Lancet Neurol. 2023, 22, 66–77. [Google Scholar] [CrossRef]
- Agorastos, A.; Chrousos, G.P. The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Mol. Psychiatry 2022, 27, 502–513. [Google Scholar] [CrossRef]
- Karagkouni, A.; Alevizos, M.; Theoharides, T.C. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun. Rev. 2013, 12, 947–953. [Google Scholar] [CrossRef]
- Zapanti, E.; Dermentzoglou, A.; Kazakou, P.; Kilindireas, K.; Mastorakos, G. The role of the stress adaptive response in multiple sclerosis. Front. Neuroendocrinol. 2025, 78, 101204. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; de Pedro-Cuesta, J.; Söderström, M.; Stawiarz, L.; Link, H. Seasonal patterns in optic neuritis and multiple sclerosis: A meta-analysis. J. Neurol. Sci. 2000, 181, 56–64. [Google Scholar] [CrossRef]
- Martynova, E.; Khaibullin, T.; Salafutdinov, I.; Markelova, M.; Laikov, A.; Lopukhov, L.; Liu, R.; Sahay, K.; Goyal, M.; Baranwal, M.; et al. Seasonal Changes in Serum Metabolites in Multiple Sclerosis Relapse. Int. J. Mol. Sci. 2023, 24, 3542. [Google Scholar] [CrossRef]
- Carlberg, C.; Mycko, M.P. Linking Mechanisms of Vitamin D Signaling with Multiple Sclerosis. Cells 2023, 12, 2391. [Google Scholar] [CrossRef]
- Mahler, J.V.; Solti, M.; Apóstolos-Pereira, S.L.; Adoni, T.; Silva, G.D.; Callegaro, D. Vitamin D3 as an add-on treatment for multiple sclerosis: A systematic review and meta-analysis of randomized controlled trials. Mult. Scler. Relat. Disord. 2024, 82, 105433. [Google Scholar] [CrossRef]
- Thouvenot, E.; Laplaud, D.; Lebrun-Frenay, C.; Derache, N.; Le Page, E.; Maillart, E.; Froment-Tilikete, C.; Castelnovo, G.; Casez, O.; Coustans, M.; et al. High-Dose Vitamin D in Clinically Isolated Syndrome Typical of Multiple Sclerosis: The D-Lay MS Randomized Clinical Trial. JAMA 2025, 333, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Esmaeil Mousavi, S.; Heydarpour, P.; Reis, J.; Amiri, M.; Sahraian, M.A. Multiple sclerosis and air pollution exposure: Mechanisms toward brain autoimmunity. Med. Hypotheses 2017, 100, 23–30. [Google Scholar] [CrossRef]
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 42390–42404. [Google Scholar] [CrossRef] [PubMed]
- Cortese, A.; Lova, L.; Comoli, P.; Volpe, E.; Villa, S.; Mallucci, G.; La Salvia, S.; Romani, A.; Franciotta, D.; Bollati, V.; et al. Air pollution as a contributor to the inflammatory activity of multiple sclerosis. J. Neuroinflamm. 2020, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Steelman, A.J. Infection as an Environmental Trigger of Multiple Sclerosis Disease Exacerbation. Front. Immunol. 2015, 6, 520. [Google Scholar] [CrossRef]
- Miele, G.; Cepparulo, S.; Abbadessa, G.; Lavorgna, L.; Sparaco, M.; Simeon, V.; Guizzaro, L.; Bonavita, S. Clinically Manifest Infections Do Not Increase the Relapse Risk in People with Multiple Sclerosis Treated with Disease-Modifying Therapies: A Prospective Study. J. Clin. Med. 2023, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, L.; Papeix, C.; Hamon, Y.; Buchard, A.; Moride, Y.; Benichou, J.; Duchemin, T.; Abenhaim, L. Vaccines and the Risk of Hospitalization for Multiple Sclerosis Flare-Ups. JAMA Neurol. 2023, 80, 1098–1104. [Google Scholar] [CrossRef]
| Risk Factor | Number of Primary Studies | Total Number of Cases/Controls | Random-Effects Summary Effect Size | p Random | Largest Study a | I2 |
|---|---|---|---|---|---|---|
| Protective factors | ||||||
| Breastfeeding [11] | 15 | 1053/751 | OR = 0.63 (0.45–0.88) | 0.006 | 0.65 (0.41–1.02) | 48.2% |
| Pregnancy [12] | 15 | 2426 b | SMD = −0.5 (0.67–0.38) | <0.001 | NA | 89.7% |
| Menopause [13] | 4 | 343 c | SMD = −0.52 (−0.88−0.15) | NA | 0.46 (0.23; 0.69) | 73.6% |
| Season variation (fall) [14] | 24 | 29,106 | RR = 0.97 (0.95–0.98) | NA | 0.97 (0.95; 0.98) | 0.02% |
| Increase by 25 nmol/L in vitamin D levels [15] | 8 | 3130 | RR = 0.9 (0.83–0.99) | NA | 0.94 (0.86; 1.02) | 51.5% |
| Risk factors | ||||||
| Stress (war) [16] | 2 | 372 | HR = 3.0 (1.56–5.81) | NA | 2.74 (1.76; 4.25) | NA |
| Stress [17] | 14 | 1082 | d = 0.53 (0.4–0.65) | <0.0001 | NA | 21.8% |
| Postpartum 0–3 months [18] | 11 | 2679/2739 | RR = 1.87 (1.4–2.5) | <0.0001 | 1.71 (1.29; 2.27) | 82% |
| Postpartum 0–12 months [18] | 8 | 2455/2498 | RR = 1.29 (1.08–1.54) | 0.004 | 1.31 (1.06; 1.61) | 73% |
| Factors with no association | ||||||
| COVID [19] | 10 | 2783/2 d | RR = 0.97 (0.67–1.41) | 0.87 | 1.12 (0.65–1.95) | 30% |
| Influenza vaccine [20] | 5 | 156/157 | RR = 1.24 (0.89–1.72) | 0.2 | NA | 0% |
| Serum vitamin D > 50 nmol/L [15] | 2 | 296 patients with MS e | RR = 0.47 (0.19–1.17) | 0.105 | NA | NA |
| Particulate matter [21] | 3 | NA | RR = 1.24 (0.95–1.62) | NA | NA | NA f |
| Risk Factor | Sample Size (Number of Cases) | Significance Threshold Reached (Under the Random-Effects Model) | 95% Prediction Interval Rule | Estimate of Heterogeneity a | Certainty of Evidence |
|---|---|---|---|---|---|
| Breastfeeding | >1000 | <0.05 | Excluding the null value | Not large | High |
| COVID | >1000 | >0.05 | Including the null value | Not large | Low |
| Pregnancy | >1000 | <0.05 | Excluding the null value | Very large | Moderate |
| Menopause | <500 | NA | Excluding the null value | Large | Low |
| Particulate matter | <500 | NA | Including the null value | Very large | Low |
| Postpartum | >1000 | <0.05 | Excluding the null value | Large | Moderate |
| Season variation | >1000 | NA | Excluding the null value | Not large | Moderate |
| War-related stress | <500 | NA | Excluding the null value | NA | Low |
| Stress | >1000 | <0.05 | Excluding the null value | Not large | Moderate |
| Influenza vaccine | <500 | >0.05 | Including the null value | Not large | Low |
| Serum vitamin D | >1000 | >0.05 | Excluding the null value | Large | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrim, S.; Apostolos-Pereira, S.L.; Santillan, T.I.V.; Adoni, T.; Callegaro, D.; Silva, G.D. Risk Factors for Relapses in Multiple Sclerosis Beyond Disease-Modifying Therapy: An Umbrella Review of Systematic Reviews and Meta-Analyses. Sclerosis 2025, 3, 41. https://doi.org/10.3390/sclerosis3040041
Terrim S, Apostolos-Pereira SL, Santillan TIV, Adoni T, Callegaro D, Silva GD. Risk Factors for Relapses in Multiple Sclerosis Beyond Disease-Modifying Therapy: An Umbrella Review of Systematic Reviews and Meta-Analyses. Sclerosis. 2025; 3(4):41. https://doi.org/10.3390/sclerosis3040041
Chicago/Turabian StyleTerrim, Sara, Samira Luisa Apostolos-Pereira, Thiago Ivan Vilchez Santillan, Tarso Adoni, Dagoberto Callegaro, and Guilherme Diogo Silva. 2025. "Risk Factors for Relapses in Multiple Sclerosis Beyond Disease-Modifying Therapy: An Umbrella Review of Systematic Reviews and Meta-Analyses" Sclerosis 3, no. 4: 41. https://doi.org/10.3390/sclerosis3040041
APA StyleTerrim, S., Apostolos-Pereira, S. L., Santillan, T. I. V., Adoni, T., Callegaro, D., & Silva, G. D. (2025). Risk Factors for Relapses in Multiple Sclerosis Beyond Disease-Modifying Therapy: An Umbrella Review of Systematic Reviews and Meta-Analyses. Sclerosis, 3(4), 41. https://doi.org/10.3390/sclerosis3040041






