Abstract
The research of drivers leading to plant extinction is a primary task in global biodiversity conservation. Despite Russia covering a large area, there is a lack of data on factors leading to plant extinction there, including orchids. We aimed to evaluate the anthropogenic drivers that threaten orchids included in the Russian Red Data Books. For this purpose, we generalized and systematized data on orchids included in all relevant (i.e., published during the last 10–11 years) regional Red Data Books available online on 31 December 2020. For each Red Data Book orchid, we identified threats, i.e., drivers leading to species extinction, according to the sections “Limiting factors” or “Limiting factors and threats” of the regional Red Data Books. We found the total taxonomic list of Red Data Book orchids in the analyzed regions of Russia. The similarity of the lists of orchid taxa in analyzed regions was established based on the Jaccard index. In regard to extinction drivers, we found which of them are the most serious threats to orchids in the regions of the Russian Federation. We believe that conducting a similar study for the whole array of threatened plants of Russia will provide highly valuable results demanded all over the world.
1. Introduction
The extinction of species is the leading problem in biodiversity conservation at national and global levels. While the extinction of animals can be more easily realized (e.g., [1]), we need more time to prove a plant extinction event [2]. Humphreys et al. (2019) [3] stated that plant extinction studies should undoubtedly come with caveats, indicating an underestimation of plant extinction rates, which could be explained by many unreported extinctions of poorly known taxa. Moreover, regional extinction events could be fixed more slowly at global assessments (e.g., global IUCN Red List). Therefore, they play an important role in the estimation of plant extinctions able to be achieved at a global scale through the regional one.
Although Russia covers about 11.0% of the total terrestrial area, there is a remarkable lack of biodiversity data from this country. This is well recordable in global reviews of biodiversity, including GBIF data [4], naturalized plants [5], ex situ plant conservation centers [6], and peatland vegetation conservation [7]. We believe that this derives from the low availability of biodiversity data written in Russian, which is not prepared according to international publication standards. In particular, this concerns data on the conservation and distribution of orchids. For instance, Khapugin (2020) [8] demonstrated that despite numerous studies of orchids in protected areas, there is a remarkable gap of data in Russia, even when taking into account the high number of protected areas in the country.
Notably, threats registered for each threatened plant could be recognized as extinction drivers. At the same time, various factors differently influence a certain plant species’ extinction. In this study, we aimed (i) to present the taxonomic composition of orchid species included in the recently published Red Data Books of Russia and (ii) to analyze the main threats leading to the extinction of orchids in Russia. We assumed that the extinction of orchids, as more attractive, vulnerable, and wider known plants, is more influenced by such factors as the direct elimination of the aboveground parts of the plants (bouquet gathering) and the disturbance of their habitats.
2. Materials and Methods
For the study, we analyzed the Red Data Books published and/or available online in 2010–2020. This period was selected to use the most modern and relevant data. As a result, we analyzed data on Red Data Book orchid taxa from 51 regions (Figure 1).
Figure 1.
Map of the Russian Federation, where the sampled regions are indicated. Empty regions refer to the absence of data on Red Data Book orchids (according to [9] with modifications).
To estimate drivers leading to the orchid taxa in Russia, we analyzed the sections “Limiting factors” or “Limiting factors and threats” in each Red Data Book. We did not assess and judge the completeness and reliability of the original data for each orchid included in each Red Data Book. In addition, we did not estimate the validity or reasons for the inclusion of each orchid in the Red Data Books of Russian regions. To establish a scheme of extinction drivers, we mainly applied IUCN—CMP Unified Classification of Direct Threats [10], with modifications based on some relevant publications [9,11,12]. As a result, we used 12 extinction drivers, applied previously in [9], namely, agriculture, fire, forestry, grazing, habitat degradation, habitat destruction, hydrological disturbance, invasion, mining, unknown, urbanization, and utilization.
To standardize the scheme of scientific names, we used the database Plants of the World Online (http://www.plantsoftheworldonline.org/, accessed on 1 November 2021). For this purpose, we applied a three-stage algorithm. In the first stage, we ordered all taxa alphabetically. In the second stage, the scientific names of the orchids were standardized according to the POWO database. In the third stage, we deleted duplicate taxa from species lists for further analysis.
Regarding the determination of the similarity of lists taxa from the regional Red Data Books using the Jaccard similarity index, Jaccard’s similarity index was calculated, where A = number of species in floristic list A; B = number of species in floristic list B; and C = number of species shared between two (A and B) floristic lists. For this purpose, we used the function “vegdist” in package “vegan” [13] in software R ver. 3.4.0 [14].
3. Results and Discussion
3.1. Taxonomic Diversity of Red Data Book Orchids
Originally, the accumulated sum of orchid taxa in 51 Red Data Books was 1070. After taxonomic standardization according to taxonomic systems, the total taxonomic list of Red Data Book plants in 51 Russian regions finally contained 120 taxa (Appendix A). The Red Data Book orchid species richness varied across the regions, ranging from 0 in the Astrakhan region to 44 in Krasnodarsky Krai. This is partially in accordance with Efimov (2020) [15], who demonstrated Krasnodarsky Krai as the region with the highest number of orchid taxa in Russia. The average number of Red Data Book species per region in Russia was 18 ± 8 (mean ± SD; median = 18). We also found no correlation between the region’s area and the number of Red Data Book orchids per region (r = 0.08, p < 0.95). The most widespread species were Cypripedium calceolus L. (48 regions) and Orchis militaris L. (46 regions), followed by Epipactis palustris (L.) Crantz (42 regions), Ponerorchis cucullata (L.) X.H.Jin, Schuit. & W.T.Jin (40 regions), Epipogium aphyllum Sw. (39 regions), Hammarbya paludosa (L.) Kuntze (38 regions), Liparis loeselii (L.) Rich. (35 regions), and Corallorhiza trifida Châtel. (33 regions) (Appendix A). Notably, orchids were demonstrated to be the most widespread among all Red Data Book plant species in Russia (see [9]). This is in accordance with data from other studies that demonstrated the high vulnerability of orchids in various regions around the world (e.g., [8,16,17]).
The 120 species belong to 39 genera. The highest number of taxa was found in Dactylorhiza (17 species), Orchis (11 species), Platanthera (11 species), and Neottia (10 species). Thirty-two genera were represented by one (e.g., Calypso, Chamorchis, Gastrodia, and Pogonia) to seven (Epipactis) species (Appendix A).
We conducted a cluster analysis (Ward method; Figure 2) of the similarity of taxonomic lists of orchids in Russian regions based on the Jaccard index similarity. The results reflected the biogeographical position of the regions, with some deviations caused by the number of Red Data Book orchids per region. In the obtained dendrogram, four groups (A, B, C, and D) are distinguishable. Thus, the large and complicated cluster A is represented by regions of the center of European Russia and regions of Urals (e.g., Sverdlovsk region and Chelyabinsk region) and West Siberia (e.g., Tyumen region and Omsk region). Cluster B includes regions of East Siberia (e.g., Krasnoyarsk region and Irkutsk region) and the northwest of European Russia (Novgorod region and Pskov region). Cluster C is certainly complicated, being represented by regions of the north of European Russia (Republic of Komi and Murmansk region) and West Siberia (Khanty-Mansi Autonomous Okrug), as well as the Russian Far East (e.g., Sakhalin region) and East Siberia (e.g., Republic of Yakutia). Finally, cluster D predominantly includes regions of the south of European Russia (e.g., Krasnodarsky Krai and Voronezh region). In this cluster, there is a highly different sub-cluster represented by the Republic of Kalmykia, Yamalo-Nenetskiy Autonomous Okrug, and Kaliningrad region based on the very low number of orchid species per region (i.e., one, four, and six taxa, respectively). These results are similar to the data demonstrated in [9], highlighting that the similarity of the lists of Red Data Book plants reflects the geographical position of regions.
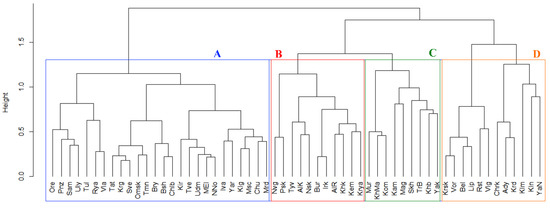
Figure 2.
Cluster tree (Ward method, Euclidian distance) showing the similarity of regions based on the Jaccard index similarity of the lists of Red Data Book orchid species in Russian regions. Designations: Ady—Republic of Adygea, AlK—Altaisky Krai, AlR—Republic of Altai, Bsh—Republic of Bashkiria, Bel—Belgorod region, Bry—Bryansk region, Bur—Republic of Buryatia, Chlb—Chelyabinsk region, Chrk—Republic of Karachay-Cherkessia, Chu—Republic of Chuvashia, Irk—Irkutsk region, Iva—Ivanovo region, Kln—Kaliningrad region, Klm—Republic of Kalmykia, Klg—Kaluga region, Kam—Kamchatsky Krai, Kem—Kemerovo region, Khb—Khabarovsk Krai, Khk—Republic of Khakassia, KhMa—Khanty-Mansi Autonomous Okrug, Kir—Kirov region, Kom—Republic of Komi, Krd—Krasnodarsky Krai, Krya—Krasnoyarsk Krai, Krg—Kurgan region, Krsk—Kursk region, Lip—Lipetsk region, Mag—Magadan region, MEl—Republic of Mari El, Mrd—Republic of Mordovia, Msc—Moscow region, Mur—Murmansk region, NNo—Nizhniy Novgorod region, Nvg—Novgorod region, Nsk—Novosibirsk region, Omsk—Omsk region, Ore—Orenburg region, Pnz—Penza region, Psk—Pskov region, Rst—Rostov region, Rya—Ryazan region, Skh—Sakhalin region, Sam—Samara region, Sve—Sverdlovsk region, Tat—Republic of Tatarstan, TrB—Zabaikalsky Krai, Tul—Tula region, Tve—Tver region, Tmn—Tyumen region, Tyv—Republic of Tyva, Udm—Republic of Udmurtia, Uly—Ulyanovsk region, Vla—Vladimir region, Vlg—Volgograd region, Vor—Voronezh region, Yak—Republic of Yakutia, YaN—Yamalo-Nenetskiy Autonomous Okrug, Yar—Yaroslavl region.
3.2. Extinction Drivers Leading to the Extinction of Red Data Book Orchid Species
To date, many studies have demonstrated that habitat destruction and ecosystem overexploitation are major extinction drivers of plants and animals [18]. Based on our results, it was found that the main drivers leading to the extinction of orchids are generally the same as those that were found for all plants in various regions of the world [9,12,19]. Undoubtedly, various factors cause a different influence on the possibility of orchid extinction. If we have these data, we may judge on measures counteracting the disappearance of plants in certain regions. In our study, we revealed that among the considered extinction drivers, habitat degradation (19.8%), hydrological disturbance (15.6%), urbanization (15.0%), and utilization (14.8%) play key roles in the extinction of Red Data Book orchid species in Russia resulting from multiple drivers (Figure 3).
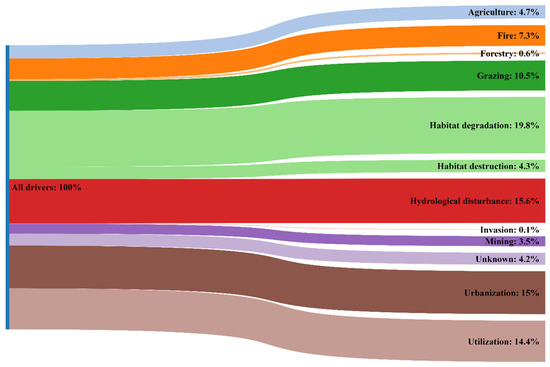
Figure 3.
Primary drivers leading to orchid extinctions in Russia according to lists of Red Data Books of Russian regions.
Notably, by having data on plant extinction in Russia [9] and worldwide [12], we may compare them with data on drivers causing orchid extinction in Russia. Thus, driver “unknown” is somewhat lower than for all Red Data Book plants in Russia (8.1%, see [9]) and even much lower than on a global scale (48.3%, see [12]). This could be explained by the high attractiveness and vulnerability of orchids around the world, which reflects the increased attention to these plants. In turn, the high attractiveness is a reason for drivers “urbanization” and especially “utilization” (including gathering for bouquets and/or attempts to introduce orchids into backyards) (e.g., [20,21]).
The high proportion of the driver “hydrological disturbance” is in accordance with data on the sensitivity of orchids to this factor around the world. Thus, it was found that the fruit set is influenced by precipitation amount [22,23]. The groundwater level (e.g., [24]), as well as drought conditions (e.g., [25]), is also crucial for several orchid species.
The most impactful driver was “habitat degradation”, which also coincides with data on orchids in various regions of the world (e.g., [21,26,27,28,29]). Despite the abovementioned drivers, other drivers could considerably influence various parameters of orchid populations, including “grazing” (e.g., [28,30]) and “fire” (e.g., [21,31]).
4. Conclusions
Our study of drivers leading to the extinction of orchids considerably contributes to the biodiversity conservation in Russia. Despite the high number of Russian-language publications on orchid distribution and conservation, there is a lack of data on factors causing orchid extinction in the whole of Russia. Our study could be considered as an attempt to estimate the drivers using internationally applied schemes (e.g., [9,12]) for orchids in Russia. Our results are considered as a basis for the creation of national classification of threats to plants.
The data on threats to Red Data Book orchids in Russian regions could fill the gaps in the global knowledge on drivers leading to the national extinction of orchid species. This is in accordance with data on extinction drivers on both Russian and global scales, where we found habitat degradation, hydrological disturbance, urbanization, and utilization to be leading factors threatening orchids in Russian regions. In comparison to data on plant extinction on a global and Russian scale, we found that orchids are more susceptible to the direct elimination of the aboveground parts of the orchids (utilization and grazing), disturbance of habitats (urbanization and habitat degradation), and changes in water regime (hydrological disturbance). As expected, the geographical position of regions is associated with the species composition of orchid floras in these regions. Based on our results, we suggest conducting more detailed studies of factors affecting or leading to species extinction of plants and particular plant groups (e.g., families and genera) in Russia or a wider area.
Funding
This study was performed within the framework of the state assignment FEWZ-2020-0009 from the Ministry of Education and Science of the Russian Federation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We are grateful to the anonymous reviewer for the helpful comments and suggestions that contributed to the improvement of the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
Appendix A

Table A1.
Taxonomic composition of Red Data Book orchids in the 51 studied regions of Russia.
Table A1.
Taxonomic composition of Red Data Book orchids in the 51 studied regions of Russia.
| Orchid Species | Number of Regions |
|---|---|
| Cypripedium calceolus L. | 48 |
| Orchis militaris L. | 46 |
| Epipactis palustris (L.) Crantz | 42 |
| Ponerorchis cucullata (L.) X.H.Jin, Schuit. & W.T.Jin | 40 |
| Epipogium aphyllum Sw. | 39 |
| Hammarbya paludosa (L.) Kuntze | 38 |
| Liparis loeselii (L.) Rich. | 35 |
| Corallorhiza trifida Châtel. | 33 |
| Cypripedium guttatum Sw. | 30 |
| Gymnadenia conopsea (L.) R.Br. | 30 |
| Neottia ovata (L.) Bluff & Fingerh. | 30 |
| Malaxis monophyllos (L.) Sw. | 27 |
| Cypripedium macranthon Sw. | 26 |
| Epipactis atrorubens (Hoffm.) Besser | 26 |
| Neottia cordata (L.) Rich. | 26 |
| Dactylorhiza majalis subsp. baltica (Klinge) H.Sund. | 26 |
| Dactylorhiza viridis (L.) R.M.Bateman, Pridgeon & M.W.Chase | 25 |
| Herminium monorchis (L.) R.Br. | 25 |
| Cephalanthera rubra (L.) Rich. | 23 |
| Calypso bulbosa (L.) Oakes | 22 |
| Neotinea ustulata (L.) R.M.Bateman, Pridgeon & M.W.Chase | 22 |
| Neottia nidus-avis (L.) Rich. | 22 |
| Platanthera bifolia (L.) Rich. | 22 |
| Dactylorhiza incarnata (L.) Soó | 20 |
| Platanthera chlorantha (Custer) Rchb. | 20 |
| Dactylorhiza maculata (L.) Soó | 19 |
| Dactylorhiza traunsteineri (Saut. ex Rchb.) Soó | 18 |
| Epipactis helleborine (L.) Crantz | 18 |
| Goodyera repens (L.) R.Br. | 18 |
| Dactylorhiza fuchsii (Druce) Soó | 17 |
| Dactylorhiza incarnata subsp. cruenta (O.F.Müll.) P.D.Sell | 17 |
| Cypripedium × ventricosum Sw. | 12 |
| Dactylorhiza russowii (Klinge) Holub | 12 |
| Cephalanthera longifolia (L.) Fritsch | 11 |
| Orchis mascula (L.) L. | 10 |
| Spiranthes sinensis (Pers.) Ames | 10 |
| Anacamptis coriophora (L.) R.M.Bateman, Pridgeon & M.W.Chase | 9 |
| Anacamptis palustris (Jacq.) R.M.Bateman, Pridgeon & M.W.Chase | 7 |
| Ophrys insectifera L. | 7 |
| Platanthera fuscescens (L.) Kraenzl. | 6 |
| Platanthera obtusata subsp. oligantha (Turcz.) Hultén | 5 |
| Cephalanthera damasonium (Mill.) Druce | 4 |
| Dactylorhiza fuchsii subsp. hebridensis (Wilmott) Soó | 4 |
| Neottia camtschatea (L.) Rchb.f. | 4 |
| Anacamptis pyramidalis (L.) Rich. | 3 |
| Dactylorhiza urvilleana (Steud.) H.Baumann & Künkele | 3 |
| Gymnadenia odoratissima (L.) Rich. | 3 |
| Limodorum abortivum (L.) Sw. | 3 |
| Neolindleya camtschatica (Cham.) Nevski | 3 |
| Neotinea tridentata (Scop.) R.M. Bateman, Pridgeon & M.W. Chase | 3 |
| Orchis picta Raf. | 3 |
| Pseudorchis albida (L.) Á.Löve & D.Löve | 3 |
| Traunsteinera sphaerica (M.Bieb.) Schltr. | 3 |
| Anacamptis morio (L.) R.M.Bateman, Pridgeon & M.W.Chase | 2 |
| Cypripedium shanxiense S.C.Chen | 2 |
| Cypripedium yatabeanum Makino | 2 |
| Dactylorhiza incarnata subsp. ochroleuca (Wüstnei ex Boll) P.F.Hunt & Summerh. | 2 |
| Dactylorhiza majalis (Rchb.) P.F.Hunt & Summerh. | 2 |
| Dactylorhiza romana subsp. georgica (Klinge) Soó ex Renz & Taubenheim | 2 |
| Dactylorhiza salina (Turcz. ex Lindl.) Soó | 2 |
| Dactylorhiza traunsteineri subsp. curvifolia (F.Nyl.) Soó | 2 |
| Eleorchis japonica (A.Gray) Maek. | 2 |
| Ephippianthus sachalinensis Rchb.f. | 2 |
| Gastrodia elata Blume | 2 |
| Gymnadenia × densiflora (Wahlenb.) A.Dietr. | 2 |
| Habenaria linearifolia Maxim. | 2 |
| Orchis pallens L. | 2 |
| Orchis purpurea Huds. | 2 |
| Orchis simia Lam. | 2 |
| Orchis spitzelii Saut. ex W.D.J.Koch | 2 |
| Oreorchis patens (Lindl.) Lindl. | 2 |
| Platanthera chorisiana (Cham.) Rchb.f. | 2 |
| Platanthera densa Freyn | 2 |
| Platanthera ophrydioides F.Schmidt | 2 |
| Platanthera tipuloides (L.f.) Lindl. | 2 |
| Pogonia japonica Rchb.f. | 2 |
| Ponerorchis chusua (D.Don) Soó | 2 |
| Steveniella satyrioides (Spreng.) Schltr. | 2 |
| Amitostigma kinoshitae (Makino) Schltr. | 1 |
| Cephalanthera cucullata Boiss. & Heldr. | 1 |
| Cephalanthera erecta (Thunb.) Blume | 1 |
| Cephalanthera longibracteata Blume | 1 |
| Chamorchis alpina (L.) Rich. | 1 |
| Cremastra appendiculata var. variabilis (Blume) I.D.Lund | 1 |
| Dactylorhiza fuchsii subsp. psychrophila (Schltr.) Holub | 1 |
| Dactylorhiza sambucina (L.) Soó | 1 |
| Dactylostalix ringens Rchb.f. | 1 |
| Epipactis condensata Boiss. ex D.P.Young | 1 |
| Epipactis microphylla (Ehrh.) Sw. | 1 |
| Epipactis papillosa Franch. & Sav. | 1 |
| Epipactis pontica Taubenheim | 1 |
| Galearis cyclochila (Franch. & Sav.) Soó | 1 |
| Goodyera henryi Rolfe | 1 |
| Goodyera schlechtendaliana Rchb.f. | 1 |
| Habenaria yezoensis H.Hara | 1 |
| Himantoglossum caprinum (M.Bieb.) Spreng. | 1 |
| Himantoglossum comperianum (Steven) P.Delforge | 1 |
| Liparis kumokiri F.Maek. | 1 |
| Liparis loeselii subsp. sachalinensis (Nakai) Efimov | 1 |
| Liparis makinoana Schltr. | 1 |
| Myrmechis japonica (Rchb.f.) Rolfe | 1 |
| Neottia acuminata Schltr. | 1 |
| Neottia convallarioides (Sw.) Rich. | 1 |
| Neottia krasnojarica Antipova | 1 |
| Neottia papilligera Schltr. | 1 |
| Neottia pinetorum (Lindl.) Szlach. | 1 |
| Neottia puberula (Maxim.) Szlach. | 1 |
| Ophrys apifera Huds. | 1 |
| Ophrys caucasica Woronow ex Grossh. | 1 |
| Ophrys sphegodes subsp. mammosa (Desf.) Soó ex E.Nelson | 1 |
| Orchis × colemanii Cortesi | 1 |
| Orchis × wulffiana Soó | 1 |
| Orchis provincialis Balb. ex Lam. & DC. | 1 |
| Orchis punctulata Steven ex Lindl. | 1 |
| Platanthera bifolia subsp. extremiorientalis (Nevski) Soó | 1 |
| Platanthera maximowicziana Schltr. | 1 |
| Platanthera sachalinensis F.Schmidt | 1 |
| Serapias vomeracea (Burm.f.) Briq. | 1 |
| Spiranthes spiralis (L.) Chevall. | 1 |
| Traunsteinera globosa (L.) Rchb. | 1 |

Table A2.
The number of taxa per genus of Red Data Book orchids in the 51 studied regions of Russia.
Table A2.
The number of taxa per genus of Red Data Book orchids in the 51 studied regions of Russia.
| Orchid Genus | Number of Species per Genus |
|---|---|
| Dactylorhiza | 17 |
| Orchis | 11 |
| Platanthera | 11 |
| Neottia | 10 |
| Epipactis | 7 |
| Cephalanthera | 6 |
| Cypripedium | 6 |
| Anacamptis | 4 |
| Liparis | 4 |
| Ophrys | 4 |
| Goodyera | 3 |
| Gymnadenia | 3 |
| Habenaria | 2 |
| Himantoglossum | 2 |
| Neotinea | 2 |
| Ponerorchis | 2 |
| Spiranthes | 2 |
| Traunsteinera | 2 |
| Amitostigma | 1 |
| Calypso | 1 |
| Chamorchis | 1 |
| Corallorhiza | 1 |
| Cremastra | 1 |
| Dactylostalix | 1 |
| Eleorchis | 1 |
| Ephippianthus | 1 |
| Epipogium | 1 |
| Galearis | 1 |
| Gastrodia | 1 |
| Hammarbya | 1 |
| Herminium | 1 |
| Limodorum | 1 |
| Malaxis | 1 |
| Myrmechis | 1 |
| Neolindleya | 1 |
| Oreorchis | 1 |
| Pogonia | 1 |
| Pseudorchis | 1 |
| Serapias | 1 |
| Steveniella | 1 |
References
- Burbidge, A.A.; Manly, F.J. Mammal extinctions on Australian islands: Causes and conservation implications. J. Biogeogr. 2002, 29, 465–473. [Google Scholar] [CrossRef]
- Cronk, Q. Plant extinctions take time. Science 2016, 353, 446–447. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, A.M.; Govaerts, R.; Ficinski, S.Z.; Nic Lughadha, E.; Vorontsova, M.S. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 2019, 3, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Yesson, C.; Brewer, P.W.; Sutton, T.; Caithness, N.; Pahwa, J.S.; Burgess, M.; Gray, W.A.; White, R.J.; Jones, A.C.; Bisby, F.A.; et al. How global is the global biodiversity information facility? PLoS ONE 2007, 2, e1124. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Pergl, J.; Essl, F.; Lenzner, B.; Dawson, W.; Kreft, H.; Weigelt, P.; Winter, M.; Kartesz, J.; Nishino, M.; et al. Naturalized alien flora of the world: Species diversity, taxonomic and phylogenetic patterns, geographic distribution and global hotspots of plant invasion. Preslia 2017, 89, 203–274. [Google Scholar] [CrossRef]
- Mounce, R.; Smith, P.; Brockington, S. Ex situ conservation of plant diversity in the world’s botanic gardens. Nat. Plants 2017, 3, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.G.; Grillas, P.; Fennessy, M.S.; Goodyer, E.; Graham, L.L.B.; Karofeld, E.; Lindsay, R.A.; Locky, D.A.; Ockendon, N.; Rial, A.; et al. A synthesis of evidence for the effects of interventions to conserve peatland vegetation: Overview and critical discussion. Mires Peat 2019, 24, 18. [Google Scholar]
- Khapugin, A.A. A global systematic review on orchid data in Protected Areas. Nat. Conserv. Res. 2020, 5 (Suppl. S1), 19–33. [Google Scholar] [CrossRef]
- Khapugin, A.A.; Kuzmin, I.V.; Silaeva, T.B. Anthropogenic drivers leading to regional extinction of threatened plants: Insights from regional Red Data Books of Russia. Biodivers. Conserv. 2020, 29, 2765–2777. [Google Scholar] [CrossRef]
- Salafsky, N.; Salzer, D.; Stattersfield, A.J.; Hilton-Taylor, C.; Neugarten, R.; Butchart, S.H.M.; Collen, B.; Cox, N.; Master, L.L.; O’Connor, S.; et al. A Standard Lexicon for Biodiversity Conservation: Unified Classifications of Threats and Actions. Conserv. Biol. 2008, 22, 897–911. [Google Scholar] [CrossRef]
- Aedo, C.; Medina, L.; Barberá, P.; Fernández-Albert, M. Extinctions of vascular plants in Spain. Nord. J. Bot. 2015, 33, 83–100. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent Anthropogenic Plant Extinctions Differ in Biodiversity Hotspots and Coldspots. Curr. Biol. 2019, 29, 2912–2918. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-3. 2019. Available online: https://CRAN.Rproject.org/package=vegan (accessed on 1 November 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 November 2021).
- Efimov, P.G. Orchids of Russia: Annotated checklist and geographic distribution. Nat. Conserv. Res. 2020, 5 (Suppl. S1), 1–18. [Google Scholar] [CrossRef]
- Khapugin, A.A.; Chugunov, G.G.; Vargot, E.V. Cypripedium calceolus (Orchidaceae) in Central Russia: A case study for its populations in two Protected Areas in the Republic of Mordovia (Russia). Lankesteriana 2017, 17, 403–417. [Google Scholar] [CrossRef][Green Version]
- Juiling, S.; Leon, S.K.; Jumian, J.; Tsen, S.; Lee, Y.L.; Khoo, E.; Sugau, J.B.; Nilus, R.; Pereira, J.T.; Damit, A.; et al. Conservation assessment and spatial distribution of endemic orchids in Sabah, Borneo. Nat. Conserv. Res. 2020, 5 (Suppl. S1), 136–144. [Google Scholar] [CrossRef]
- Pimm, S.; Raven, P.; Peterson, A.; Sekercioglu, C.H.; Ehrlich, P.R. Human impacts on the rates of recent, present, and future bird extinctions. Proc. Natl. Acad. Sci. USA 2006, 103, 10941–10946. [Google Scholar] [CrossRef]
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Perazza, G.; Decarli, M. Monitoring of Cypripedium calceolus (Orchidaceae) in the Adamello-Brenta Natural Park (Italy). Nat. Conserv. Res. 2020, 5 (Suppl. S1), 178–184. [Google Scholar] [CrossRef]
- Wraith, J.; Pickering, C. A continental scale analysis of threats to orchids. Biol. Conserv. 2019, 23, 7–17. [Google Scholar] [CrossRef]
- Kirillova, I.A.; Kirillov, D.V. Impact of weather conditions on seasonal development, population structure and reproductive success on Dactylorhiza traunsteineri (Orchidaceae) in the Komi Republic (Russia). Nat. Conserv. Res. 2020, 5 (Suppl. S1), 77–89. [Google Scholar] [CrossRef]
- Bleho, B.I.; Borkowsky, C.L.; Grantham, M.A.; Hamel, C.D. A 20 y Analysis of Weather and Management Effects on a Small White Lady’s-slipper (Cypripedium candidum) Population in Manitoba. Am. Midl. Nat. 2021, 185, 32–48. [Google Scholar] [CrossRef]
- Grootjans, A.; Shahrudin, R.; van de Craats, A.; Kooijman, A.; Oostermeijer, G.; Petersen, J.; Amatirsat, D.; Bland, C.; Stuyfzand, P. Window of opportunity of Liparis loeselii populations during vegetation succession on the Wadden Sea islands. J. Coast. Conserv. 2017, 21, 631–641. [Google Scholar] [CrossRef]
- Guevara-Perez, C.I.; Delgado-Sánchez, P.; Torres-Castillo, J.A.; Flores-Rivas, J.D.; Mendieta-Leiva, G.; Rosa-Manzano, E.D. Epiphytic orchids Stanhopea tigrina and Prosthechea cochleata are differentially affected by drought in a subtropical cloud forest. Photosynthetica 2019, 57, 1053–1065. [Google Scholar] [CrossRef]
- Lohani, N.; Tewari, L.M.; Joshi, G.C.; Kumar, R.; Kishor, K.; Upreti, B.M. Population assessment and threat categorization of endangered medicinal orchid Malaxis acuminata D. Don. from north-west Himalaya. Int. J. Conserv. Sci. 2013, 4, 483–492. [Google Scholar]
- De Beenhouwer, M.; Aerts, R.; Hundera, K.; Van Overtveld, K.; Honnay, O. Management intensification in Ethiopian coffee forests is associated with crown habitat contraction and loss of specialized epiphytic orchid species. Basic Appl. Ecol. 2015, 16, 592–600. [Google Scholar] [CrossRef]
- Tatarenko, I.; Dodd, M.; Wallace, H.; Bellamy, G.; Fleckney, A. Protecting small populations of rare species. Case study on Dactylorhiza viridis (Orchidaceae) in Fancott Woods and Meadows SSSI, Bedfordshire, UK. Nat. Conserv. Res. 2020, 5 (Suppl. S1), 165–171. [Google Scholar] [CrossRef]
- Kolanowska, M.; Jakubska-Busse, A. Is the lady’s-slipper orchid (Cypripedium calceolus) likely to shortly become extinct in Europe?—Insights based on ecological niche modelling. PLoS ONE 2020, 15, e0228420. [Google Scholar] [CrossRef]
- Romano, V.A.; Rosati, L.; Fascetti, S. Trends in population size of Ophrys argolica subsp. biscutella in the Appennino Lucano-Val d’Agri-Lagonegrese National Park (Italy). Nat. Conserv. Res. 2020, 5 (Suppl. S1), 155–164. [Google Scholar] [CrossRef]
- Brundrett, M.C. Using vital statistics and core-habitat maps to manage critically endangered orchids in the Western Australian wheatbelt. Aust. J. Bot. 2016, 64, 51–64. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).