Abstract
Background: Renal arteriovenous malformations (rAVMs) are rare vascular anomalies that may lead to hematuria, anemia, or acute kidney injury (AKI). Although endovascular embolization is the treatment of choice, post-procedural complications such as new-onset proteinuria may occur and require long-term management. Case Presentation: A 56-year-old man with recurrent gross hematuria and elevated serum creatinine (128 μmol/L) was diagnosed with a right rAVM and underwent successful selective embolization. Despite recovery of renal function, follow-up revealed new-onset proteinuria (2.2 g/24 h). Results: Introduction of an angiotensin-converting enzyme inhibitor (ACEi) resulted in partial improvement of proteinuria, while subsequent addition of a sodium–glucose cotransporter-2 inhibitor (SGLT2i) achieved almost complete resolution of proteinuria (0.33 g/24 h) and stable renal function (serum creatinine 93 μmol/L) after 12 months. Conclusions: This case highlights the occurrence of post-embolization proteinuria and illustrates the synergistic renoprotective effect of combined ACEi and SGLT2i therapy in a non-diabetic patient with vascular kidney disease.
1. Introduction
Renal arteriovenous malformations (rAVMs) are rare vascular anomalies characterized by abnormal direct communications between arteries and veins without intervening capillaries, resulting in significant hemodynamic disturbances [1]. Although their prevalence is estimated to be below 0.04% in the general population, rAVMs represent an important differential diagnosis in patients with unexplained hematuria or acute kidney injury (AKI) [2,3]. The clinical presentation is variable and depends on the size and location of the lesion, as well as the degree of shunting. Patients may remain asymptomatic for years or present with gross hematuria, flank pain, anemia, hypertension and, in rare cases, high-output cardiac failure [4,5,6]. Because the vascular etiology of hematuria is often overlooked, affected patients may undergo prolonged urological evaluation for malignancy or urolithiasis before a definitive diagnosis is established [7].
Diagnosis of rAVMs relies on imaging modalities. Ultrasonography with color Doppler can provide the first clue, while computed tomography (CT) angiography and digital subtraction angiography remain the gold standards for confirming the presence and extent of the vascular malformation [8,9]. Early detection and treatment are essential to prevent recurrent bleeding, progressive renal dysfunction and potentially life-threatening complications [8,9].
Therapeutic strategies are determined by the severity of symptoms, anatomical characteristics of the lesion and overall renal function. Endovascular embolization is the current treatment of choice for symptomatic AVMs due to its minimally invasive nature, high efficacy and renal parenchyma preservation [10,11]. However, post-procedural complications, such as impaired renal function or proteinuria, may occur. These can be particularly challenging in patients with preexisting hypertension or single kidney function, raising important considerations for long-term management and renoprotection [12,13].
To our knowledge, the sequential use of angiotensin-converting enzyme inhibitor (ACEi) and sodium-glucose cotransporter-2 inhibitor (SGLT2i) in the management of post-embolization proteinuria has not been previously reported. Here, we present a case of a 56-year-old man with recurrent hematuria and AKI secondary to rAVM, successfully treated with embolization but complicated by subsequent proteinuria. We highlight the beneficial role of ACEi and SGLT2-i in resolving post-procedural renal dysfunction, emphasizing their potential renoprotective role in complex vascular kidney disease.
2. Case Presentation
This case study was approved by the Ethical Committee of the University Clinical Centre of Serbia (approval number: 890/8) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained.
A 56-year-old man was admitted to the Clinic of Nephrology, University Clinical Centre of Serbia, with painless gross hematuria. The first episode had occurred five years earlier, with several recurrent episodes since, but without a definitive diagnosis. One month prior to admission, he experienced another episode, accompanied by low-grade fever (37.4 °C).
CT urography revealed a 26 × 16 mm aneurysm of the right renal artery and two smaller aneurysms in the same vessel, together with a prominent vascular arborization in the upper pole, suggestive of an AVM. The right kidney demonstrated reduced contrast secretion and excretion, while the left kidney appeared normal. Ultrasound performed on admission confirmed an enlarged right kidney with a dilated pelvicalyceal system and microcalculi.
The patient had a 20-year history of hypertension under treatment. On examination, blood pressure was 130/70 mmHg and heart rate 73/min, with otherwise unremarkable findings. Laboratory tests showed hemoglobin ranging from 109 to 102 g/L (reference 138–175 g/L) and elevated serum creatinine up to 128 μmol/L (reference 59–104 μmol/L), with otherwise normal biochemistry. Urine sediment revealed numerous erythrocytes. There was no history of renal trauma, biopsy, or surgery and he denied anticoagulant use, drug abuse, smoking, or family history of bleeding disorders. The detailed biochemical and hematological results at admission and during follow-up are summarized in Table 1.

Table 1.
Laboratory parameters at admission and during follow-up after rAVM embolization.
During hospitalization, he received antibiotics and underwent cystoscopy with evacuation of bladder clots. CT angiography confirmed the AVM (Figure 1a) and selective angioembolization was performed via a left femoral approach (Figure 1b). Post-procedure angiography demonstrated complete occlusion of the AVM nidus (Figure 2a) with preserved perfusion of the remaining renal vasculature (Figure 2b). At discharge, he was clinically stable, with normalized creatinine and clear urine. The administered therapy during hospitalization and subsequent follow-up is outlined in Table 2. After discharge, the patient was prescribed fosinopril (Monopril) 10 mg twice daily, titrated to the maximally tolerated dose and empagliflozin 10 mg once daily. Follow-up visits were scheduled at 6 and 12 months after embolization as determined by the attending nephrologist, consistent with routine monitoring intervals for renal recovery and therapy adjustment in clinically stable patients.
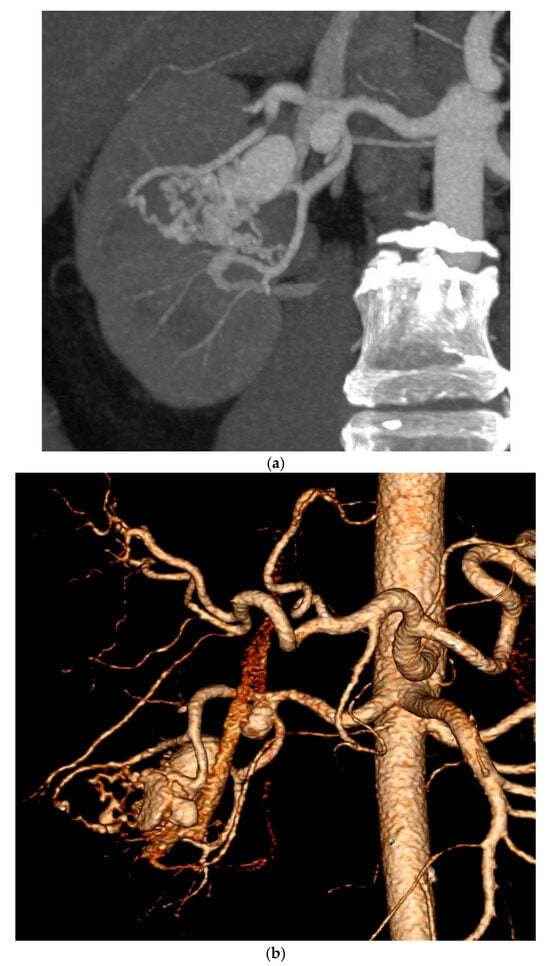
Figure 1.
(a) Maximum intensity projection, axial plane of CT renal angiography in arterial phase, showing aneurysmal dilatation of a segmental branch of the right renal artery with early opacification of the renal vein, suggestive of arteriovenous malformation. (b) Selective renal angiography confirming the presence of a large AVM. Selective cannulation of arterial feeders was performed using a Rebar-18 microcatheter (Medtronic/EV3 Neurovascular—Irvine, California, USA), followed by coil placement and Onyx-18/34 glue embolization of the AVM nidus.
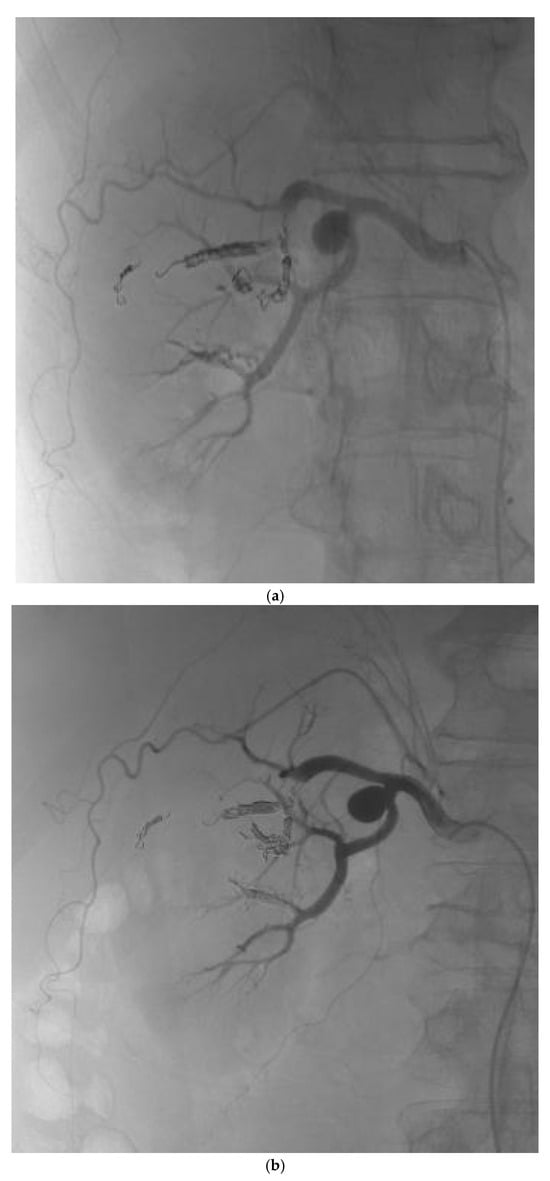
Figure 2.
(a) Post-procedure angiogram of the right renal artery showing complete occlusion of the AVM nidus. (b) Post-procedure angiogram demonstrating preserved blood flow in the remaining arterial vasculature of the right kidney.

Table 2.
Administrated therapy list.
3. Discussion
rAVMs are uncommon vascular lesions that may lead to hematuria and renal impairment, often complicating diagnosis and management. Endovascular embolization is the treatment of choice for symptomatic cases because of its minimally invasive nature and renal-sparing potential, although complications such as infarction, hypertension, or new-onset proteinuria may occur [6,9,14,15,16,17,18].
Most published cases have focused on procedural success and bleeding control, with limited discussion of renal outcomes or renoprotective therapy. Mukendi et al. reported favorable post-embolization results without commenting on proteinuria [19], while Becker and Hinrichs [20] described a high-flow rAVF treated by Fogarty-assisted embolization, where mild proteinuria persisted. García Rojo et al. [21] similarly reported preserved renal function after embolization but no pharmacologic intervention. In contrast, our case demonstrates almost complete remission of proteinuria following sequential therapy with ACEi and SGLT2i, representing, to our knowledge, the first documented instance of complete recovery after rAVM embolization. The mechanisms of post-embolization proteinuria are not well defined. In this patient, post-embolization proteinuria may have resulted from localized overflow and hyperfiltration in the remaining functional glomeruli after occlusion of the AVM nidus. This hemodynamic imbalance likely increased single-nephron filtration load rather than reflecting systemic or metabolic injury, distinguishing it from diabetic glomerulopathy. Hemodynamic remodeling, increased glomerular capillary pressure and hyperfiltration in remaining nephrons, as well as perinecrotic microinjury, are all possible contributors. In this setting, use of antiproteinuric therapy is rational. ACEi reduces intraglomerular pressure by dilating the efferent arteriole, thereby lowering glomerular hypertension and limiting proteinuria, which appear early and contribute to long-term renal preservation [22,23]. In this case, ACEi was selected primarily due to local availability and the patient’s good tolerance profile. Although angiotensin II receptor antagonists (ARBs) provide comparable renoprotective effects and may be preferred in individuals prone to cough or with metabolic syndrome, ACEi therapy was clinically appropriate given the absence of adverse reactions and the established efficacy in reducing post-ischemic proteinuria. The resolution of proteinuria observed after ACE inhibition in this case aligns with these known mechanisms. Unlike the glomerular injury typical of diabetic chronic kidney disease (CKD), where proteinuria reflects chronic metabolic and inflammatory damage, post-embolization proteinuria in this case likely resulted from localized hemodynamic remodeling and nephron hyperfiltration rather than diffuse glomerulosclerosis [24]. Therefore, the observed renoprotective response to ACE and SGLT2 inhibition shows their efficacy even in a non-diabetic, structurally mediated setting.
The addition of an SGLT2 inhibitor provided complementary renoprotection through improved tubuloglomerular feedback, reduced intraglomerular pressure and anti-inflammatory and antifibrotic effects, consistent with large outcome trials such as CREDENCE and DAPA-CKD [25,26]. The remission observed after sequential ACE and SGLT2 inhibition aligns with these mechanisms, suggesting a potential synergistic effect even in non-diabetic, structurally mediated kidney injury.
The timing of therapy was also crucial. Embolization corrected the structural lesion, but residual hyperfiltration likely contributed to persistent proteinuria. Sequential initiation of ACE inhibition followed by SGLT2 inhibition provided complementary hemodynamic and structural benefits. The favorable 12-month course supports the hypothesis that dual renoprotective therapy may reverse post-ischemic glomerular stress, though confirmation requires larger studies.
This report, however, has several important limitations. As a single case, spontaneous remission cannot be excluded, and the durability of remission remains uncertain. Dual therapy may not be tolerated in patients with borderline perfusion or blood pressure. Further, no histological or biomarker confirmation was available, so conclusions rely on clinical response. The absence of a renal biopsy, urinary biomarkers and extended follow-up limits the ability to confirm the underlying mechanism of proteinuria resolution. The improvement was inferred from clinical parameters rather than histopathological evidence and therefore, should be interpreted cautiously. As acknowledged, the causal link between dual therapy and almost complete remission of proteinuria cannot be confirmed based on a single case. Larger case series or prospective studies would be necessary to reach statistical significance and validate this observation.
4. Conclusions
rAVMs are rare vascular anomalies that may cause hematuria and acute kidney injury, with endovascular embolization serving as the preferred therapeutic approach. Despite technical success, post-procedural complications such as proteinuria can emerge and influence long-term renal outcomes. In this case, almost complete resolution of proteinuria was observed during sequential therapy with an ACEi and a SGLT2i. Although this remission coincided with dual therapy, causality cannot be established from a single observation. Larger studies are needed to confirm whether the potential synergistic renoprotective effect of combined ACEi and SGLT2i observed here can be generalized to similar patients.
Author Contributions
Conceptualization—A.M., A.B., J.P., V.C., B.L. and M.B.; methodology—A.M. and M.B.; software—A.M., V.C., B.L., A.S. and M.B.; validation—J.P., V.C., B.L., A.B. and M.B.; formal analysis—A.B., S.G., K.F., I.M., A.S., N.T. and M.B.; investigation—A.M., J.P., V.C., B.L., A.B., S.G., K.F., N.T. and M.B.; resources V.C., B.L., A.B., K.F., S.G., N.T. and M.B.; writing—original draft preparation—A.M., J.P., K.F., N.T., I.M. and M.B.; writing—review and editing, A.B., S.G., A.S. and M.B.; visualization, V.C., B.L. and A.S.; project administration—M.B.; data curation—A.M., V.C., B.L. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Ethical Committee of the University Clinical Centre of Serbia (approval number: 890/8, approval date: 21 December 2018).
Informed Consent Statement
Informed consent was obtained from the patient in this study.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, X.; Zhao, Z. Renal Arteriovenous Malformation Causing Hematuria: Case Report and Review of the Literature. Medicine 2023, 102, e34547. [Google Scholar] [CrossRef]
- Banthia, R.; Kumar, A.; Prasad, R.; Lal, H. Congenital Renal Arteriovenous Malformation: A Rare Cause of Visible Haematuria. BMJ Case Rep. 2021, 14, e242347. [Google Scholar] [CrossRef]
- Hsu, J.T.; Hsieh, S.J.; Chang, C.-Y. Renal Arteriovenous Malformation: A Rare and Potentially Life-Threatening Cause of Hematuria. Asian J. Surg. 2024, 47, 716–717. [Google Scholar] [CrossRef]
- Carrafiello, G.; Laganà, D.; Peroni, G.; Mangini, M.; Fontana, F.; Mariani, D.; Piffaretti, G.; Fugazzola, C. Gross Hematuria Caused by a Congenital Intrarenal Arteriovenous Malformation: A Case Report. J. Med. Case Rep. 2011, 5, 510. [Google Scholar] [CrossRef]
- Hermans, B.; Uvin, P.; Vanhoucke, J.-L.; Goeman, L.; Verhamme, L.; Boel, K.; Van Der Eecken, H.; Ryckaert, T.; Marrannes, J.; Joniau, S.; et al. Incidentally Detected Renal Arteriovenous Malformation: A Case Report and Review of the Literature. EMJ Urol. 2017, 5, 71–75. [Google Scholar] [CrossRef]
- Hatzidakis, A.; Rossi, M.; Mamoulakis, C.; Kehagias, E.; Orgera, G.; Krokidis, M.; Karantanas, A. Management of Renal Arteriovenous Malformations: A Pictorial Review. Insights Imaging 2014, 5, 523–530. [Google Scholar] [CrossRef]
- Meunier-Geleng, L.; Choffel, L.; Simon, G.; Frontczak, A.; Kleinclauss, F. Symptomatic Renal Arteriovenous Malformation before and after Rupture—Endovascular and Surgical Approach. Urol. Case Rep. 2025, 61, 103106. [Google Scholar] [CrossRef]
- Lv, S.P.; Qin, L.L.; Mou, H.; Huang, T.; Wang, K.Q. Multimodal Imaging Techniques for the Diagnosis of Congenital Left Renal Arteriovenous Fistula: A Case Report. World J. Clin. Cases 2025, 13, 104062. [Google Scholar] [CrossRef]
- Agarwal, D.; Gupta, S.; Das, C.J.; Hatimota, P.; Gadwal, S.K. Imaging and Endovascular Interventions in Renal Arteriovenous Shunts. Br. J. Radiol. 2025, 98, 1556–1572. [Google Scholar] [CrossRef]
- Alyami, A.; Alothman, A.; Balaraj, F.; Alomairi, M.; Ghazwani, Y.; Albqami, N. Post-Traumatic Renal Arteriovenous Malformation Failed Endovascular Embolization. J. Surg. Case Rep. 2024, 2024, rjae302. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Keshava, S.N.; Lenin, A.; Mukha, R. Endovascular Management of a Patient with Massive Renal Arteriovenous Fistula: Challenges and Tricks. BMJ Case Rep. 2021, 14, e236358. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.B.A.; Bishara, S.; Charitopoulos, K.; Ellis, D.; Nikolinakos, P.; Poushi, M.Z.; Donkov, I. Renal Atrophy Following Selective Transcatheter Arterial Embolization for Angiomyolipoma as an Uncommon but Significant Complication: A Case Report and Literature Review. Cureus 2025, 17, e81968. [Google Scholar] [CrossRef]
- Para, S.A.; Mehdi, S.; Malik, S.; Wani, S.; Bhat, A.; Choh, N. Safety and Efficacy of Selective Renal Artery Embolization in the Management of Postprocedural Acute Renal Bleeding: Experience of a Tertiary Care Center. J. Urol. Surg. 2023, 10, 326–333. [Google Scholar] [CrossRef]
- Waybill, P.; Fischer, J.L. Percutaneous Management of a Large Renal AVM. Endovasc. Today 2013, Apr. Supplement. Available online: https://evtoday.com/articles/2013-apr-supplement/percutaneous-management-of-a-large-renal-avm (accessed on 2 October 2025).
- Öcal, O. Endovascular Treatment of Renal Bleeding: Technical Success, Clinical Tolerability and Overall Success. Ph.D. Dissertation, Ludwig-Maximilians-Universität München, München, Germany, 2022. Available online: https://edoc.ub.uni-muenchen.de/30568/7/Oecal_Osman.pdf (accessed on 2 October 2025).
- Boyer, N.; Eldridge, J.; Prowle, J.R.; Forni, L.G. Postoperative Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2022, 17, 1535–1545. [Google Scholar] [CrossRef]
- Longhitano, E.; Calabrese, V.; Casuscelli, C.; Di Carlo, S.; Maltese, S.; Romeo, A.; Calanna, M.; Conti, G.; Santoro, D. Proteinuria and Progression of Renal Damage: The Main Pathogenetic Mechanisms and Pharmacological Approach. Medicina 2024, 60, 1821. [Google Scholar] [CrossRef]
- Gahan, J.C.; Gaitonde, M.; Wadskier, L.; Cadeddu, J.A.; Trimmer, C. Renal Function Outcomes Following Selective Angioembolization for Iatrogenic Vascular Lesions after Partial Nephrectomy. J. Endourol. 2013, 27, 1516–1519. [Google Scholar] [CrossRef]
- Mukendi, A.M.; Rauf, A.; Doherty, S.; Mahlobo, F.; Afolayan, P.; Dawadi, S. Renal arteriovenous malformation: An unusual pathology. S. Afr. J. Radiol. 2019, 23, a1704. [Google Scholar] [CrossRef]
- Becker, L.S.; Hinrichs, J.B. Fogarty-assisted transcatheter embolization of a large renal high-flow arteriovenous fistula. CVIR Endovasc. 2022, 5, 19. [Google Scholar] [CrossRef]
- García Rojo, E.; García Villalón, Á.; Gutiérrez Sánchez, Á.; de Haro García, Á.; Martínez Jiménez, C. Management of a Giant Renal Arteriovenous Malformation with Endovascular Approach: A Case Report. Clin. Case Rep. Int. 2023, 7, 249. [Google Scholar]
- Sarafidis, P. Use of ACEi/ARBs, SGLT2 Inhibitors and MRAs Can Help Us Reach the Therapeutic Ceiling in CKD. Clin. Kidney J. 2024, 17, sfae014. [Google Scholar] [CrossRef]
- van den Belt, S.M.; Heerspink, H.J.L.; Gracchi, V.; de Zeeuw, D.; Wühl, E.; Schaefer, F.; ESCAPE Trial Group. Early Proteinuria Lowering by Angiotensin-Converting Enzyme Inhibition Predicts Renal Survival in Children with CKD. J. Am. Soc. Nephrol. 2018, 29, 2225–2233. [Google Scholar] [CrossRef]
- Srivastava, T.; Hariharan, S.; Alon, U.S.; McCarthy, E.T.; Sharma, R.; El-Meanawy, A.; Savin, V.J.; Sharma, M. Hyperfiltration-Mediated Injury in the Remaining Kidney of a Transplant Donor. Transplantation 2018, 102, 1624–1635. [Google Scholar] [CrossRef]
- UK Kidney Association. UKKA Guideline: SGLT2i in Adults with Kidney Disease, v1, 20 October 2021. Available online: https://www.ukkidney.org/sites/renal.org/files/UKKA%20guideline_SGLT2i%20in%20adults%20with%20kidney%20disease%20v1%2020.10.21.pdf (accessed on 2 October 2025).
- Wang, M.; Zuo, L. Cardiorenal Benefits of SGLT2 Inhibitors in Patients with Chronic Kidney Disease and Concomitant Hypertension. Cardiorenal Med. 2025, 15, 496–509. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).