Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the ongoing coronavirus disease 2019 (COVID-19) pandemic. Despite the fact that it affects all ages and profiles, some evidence shows that patients with comorbidities, including diabetes, obesity, and hypertension, are likely to be most affected. For chronic diseases, such as human immunodeficiency virus (HIV), information is scarce, with a few studies from high-income countries. This study reports predictors of SARS-CoV-2 seropositivity (IgG + IgM) among unvaccinated people living with HIV attending Bugando Medical Centre in Mwanza, Tanzania. Methodology: A cross-sectional, hospital-based study involving 150 HIV-infected patients was conducted at Bugando Medical Centre between June and July 2022. A pretested questionnaire was used to collect the participants’ information. Blood samples were collected and used for the detection of SARS-CoV-2 (IgM/IgG) antibodies by immunochromatographic assay. Data were analyzed using STATA version 15. Results: The mean age of the enrolled participants was 46.4 ± 11.1 years. The overall seropositivity (IgG + IgM) of SARS-CoV-2 antibodies was 79/150 (52.7%) [95% CI: 44.4–60.9]. Seropositivity of IgM only was 8/150 (5.3) [95% CI: 1.7–8.8], while that of IgG only was 61/150 (40.6%) [95% CI: 32.7–48.4]. Seropositivity of both IgG and IgM was observed in 10/150 (6.7%) patients. In a multivariate logistic regression analysis, history of sore throat (OR: 6.34, 95% CI: 2.305–18.351, p < 0.001), low CD4 count (OR: 0.99, 95% CI: 0.995–0.999, p = 0.004), and use of pit latrines (OR: 2.30, 95% CI: 1.122–4.738, p = 0.023) were independently associated with SARS-CoV-2 seropositivity. Conclusion: HIV-infected individuals with history of sore throat, low CD4 count, and use of pit latrines were more likely to be SARS-CoV-2 IgG seropositive. There is a need to routinely screen for SARS-CoV-2 infection among HIV-infected individuals to obtain comprehensive data regarding the interactions of the pathogens.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has recently emerged as an important viral infection causing human-to-human transmission with global health impacts. The WHO declared COVID-19 a Public Health Emergency of International Concern on 30 January 2020 and a pandemic on 11 March 2020. As of 16 March 2023, a total of 676 m cases were reported, with 6.8 m deaths reported worldwide. In addition, a total of 13.3 billion doses of COVID-19 vaccines were administered (https://coronavirus.jhu.edu/map.html, accessed on 16 March 2023).
SARS-CoV-2 infection activates innate and adaptive immune responses, such as induction of virus-specific T and B cells. Moreover, it also causes dysfunctional immune responses, including inflammatory cytokine storms, which have been linked to severe forms of COVID-19 [1]. As has been well established, CD4 T cells play a pivotal role in coordinating immune responses by inducing antibody production by B cells. Moreover, they also promote effector activity of CD8 T cells and the establishment of B and T cell memory [2]. SARS-CoV-2 and HIV viruses have distinct virological characteristics despite sharing CD4 T cell lymphopenia. Recent studies have reported that people living with HIV (PLHIV) were found to have a significant high risk of SARS-CoV-2 infection [3]. However, the widely use of antiretroviral drugs has resulted to a balance of the immune system that is important in protecting individuals from other infections, including COVID-19, by the mounting of proper immune responses.
A recent systematic review demonstrated that HIV-positive patients co-infected with SARS-CoV-2 have high comorbidities, especially hypertension and diabetes mellitus [4]. Regarding symptoms, it has been reported that about two-thirds of PLHIV presented with mild to moderate symptoms, with the majority having fever and cough. Among PLHIV, deaths have been observed more frequently in males over 50 years old with multiple comorbidities [5]. These observations underscore a need to regularly screen HIV patients for SARS-CoV-2 infections during the COVID-19 pandemic.
The prevalence of SARS-CoV-2 has been largely estimated by the number of confirmed clinical cases and by reverse transcriptase polymerase chain reaction (RT-PCR). However, the actual burden of the disease is unclear because not every infected individual shows the signs and symptoms of COVID-19, necessitating the need for seroepidemiological studies [6]. Population-based seroepidemiological studies provide reliable estimates of exposure to infection and can be used to assess the level of humoral immunity if quantitative assays are used [7].
Globally, a systematic review reported that about 6 out of 10 individuals were SARS-CoV-2 seropositive by September 2021. Seroprevalence was found to increase significantly in 2021, from 26.6% to 86.7%, in Africa. In addition, a significant increase was also observed in high-income countries and was linked to increase in vaccination coverage. It was further documented that, using pre-vaccination data, stringent public health and social measures were associated with low seroprevalence. The global prevalence of HIV among COVID-19 cases was 2%, with the highest prevalence observed in developing countries, especially those in sub-Saharan Africa [8], while in Italy, by October 2020, the seroprevalence of SARS-CoV-2 among PLHIV was 0.72% [9]. High SARS-CoV-2 seropositivity (IgG only + IgM only + (IgG and IgM)) among HIV-infected individuals was reported in Pune, India, with an observed seroprevalence of 54.6% [10].
Several factors such as age, sex, metabolic disorders, etc. have been established to increase the risk of SARS-CoV-2 seropositivity. However, the role of immunodeficiencies has not been well elucidated [11]. PLHV and co-infection with other viruses have increased risk of acquiring comorbidities that may lead to increased risk of mortality. The burden might be greater in sub-Saharan African due to the fact that more than 25.6 million individuals are living with HIV in sub-Saharan Africa, accounting for two-thirds of world HIV infections [12]. In Tanzania, the prevalence of HIV was reported to be 4.8% among the population aged 15 years and above [13]. Despite the high HIV burden in sub-Saharan Africa, most of the studies on SARS-CoV-2 in the HIV population have been reported from high-income countries. In Tanzania, data regarding the burden of SARS-CoV-2 in different populations, including the HIV-infected population, are limited. However, in general populations, SARS-CoV-2 seroprevalence before the emergence of the SARS-CoV-2 Omicron variant was 58.9% out of 2051 sera tested [14] in Zanzibar and 50.4% out of 805 sera tested [15] in a community in Mwanza 20 months after the first COVID 19 case was declared in Tanzania. Currently, SARS-CoV-2 is routinely tested among travelers as a prerequisite for the issuance of travel permits and among those who present with signs and symptoms of SARS-CoV-2 infection; therefore, the estimated number from case surveillance is smaller than the actual number because not all infected individuals present with symptoms. This study estimated the burden of SARS-CoV-2 among unvaccinated HIV-infected patients; the information that might be useful as baseline data for further studies in this special group to understand the interactions of the two pathogens.
2. Materials and Methods
2.1. Study Design, Study Area, Study Population, Sample Size Estimation, and Sampling Procedures
A cross-sectional, hospital-based study was conducted between June and July 2022 at Bugando Medical Centre (BMC) in Mwanza, Tanzania, with about 2000 patients attending care and treatment clinics (CTCs) in a month. All individuals who were SARS-CoV-2 unvaccinated and HIV seropositive, regardless of age and sex, and who agreed to participate in the study were enrolled. Sample size was estimated by Kish Leslie, using a prevalence of 3.1% [16]. The minimum sample size was 47 samples; however, a total of 150 participants were enrolled using a simple random sampling method.
2.2. Data Collection Procedure
Participant information was collected using a generic protocol for a population-based, age- and gender-stratified serosurvey for SARS-CoV-2 recommended by the Africa Centres for Disease Control and Prevention [17], which included sociodemographic and clinical data. As per protocol, the period covers the time since the emergence of SARS-CoV-2 to the date of data collection. Blood samples were then collected by a qualified phlebotomist in EDTA tubes (Becton & Dickson Co., Ltd., Nairobi, Kenya), following aseptic procedures. After the blood sample collection, centrifugation of the blood samples was performed, and plasma was obtained and stored at −20 °C for further processing.
2.3. Laboratory Processing
Each sample was tested for the presence of specific antibodies (IgM/IgG) of SARS-CoV-2 spike proteins using a rapid immunochromatographic test as per the manufacturer’s instructions (RADI COVID-19 IgM/IgG, KHMedical, Seoul, Republic of Korea). The kit has a sensitivity of 90% and a specificity of 100% [18]. Positive IgM and IgG was defined by the appearance of a red band in the test window indicating the presence of Ig or IgM together with a red band appearing in the control window as the quality control of the assay. The appearance of the three red bands for IgG, IgM, and the control indicated the presence of both IgG and IgM. The results were considered negative when no red lines appeared in the test window with the presence of a red band in the control window. With the presence of a red band(s) without a control red band, the results were considered invalid.
2.4. Data Analysis
Samples were labelled with unique identification numbers indicated on data collection forms. Laboratory results were recorded on laboratory sheets, then sorted and entered into Microsoft Excel spreadsheets for cleaning and coding. Data analysis was performed using STATA version 15. Categorical variables, such as sex, location, toilet type, etc., were summarized as percentages and fractions, while means (SDs) were used to summarize continuous variables (age, viral load, and CD4 count). Univariate and multivariate regression analyses were performed to assess the factors associated with overall seropositivity (IgG only + IgM only + both IgG and IgM) for SARS-CoV-2 antibodies among unvaccinated PLHIV attending BMC CTC in Mwanza. All variables were tested in the univariate analysis; the variables with p-values less than 0.05 were subjected to the multivariate regression model controlled by age. A p-value <0.05 was considered statistically significant.
3. Results
3.1. Sociodemographic and Clinical Characteristics of Patients
A total of 150 patients aged between 18 and 72 years were recruited in the study. The mean age of the study participants was 46.4 ± 11.1 years. More than two-thirds (104 (69.3%)) of the participants were female, and more than three-quarters (129 (86.0%)) were from urban areas. More than one-third (59 (39.3%)) never attended school, and the majority (130 (86.7%)) used tap water. More than half (81 (54%)) used modern toilets, while the majority (133 (88.7%)) lived in brick/iron houses (Table 1).

Table 1.
Sociodemographic characteristics of enrolled participants.
In this study, all 150 HIV patients who were enrolled were on antiretroviral therapy, with a median CD4 counts of 490 (IQR: 363–666) cells per cubic millimeter. Furthermore, more than a half 84 (56%) reported history of cough and 79 (52.7%) reported history of fever. Only 32 (21.3%) participants reported history of sore throat (Table 2).

Table 2.
Clinical characteristics of HIV patients.
3.2. Seropositivity of SARS-CoV-2 Antibodies among Unvaccinated HIV/AIDS Patients Attending Bugando Medical Center
The overall seropositivity of SARS-CoV-2 antibodies (IgM only + IgG only + both IgG and IgM) was 79/150 (52.7%) [95% CI: 44.4–60.9]. Seropositivity of IgM only was 8/150 (5.3%) [95% CI: 1.7–8.8], while IgG-only seropositivity was 61/150 (40.6%) [95% CI: 32.7–48.4]. Seropositivity of both IgG and IgM was observed in 10 (6.7%, 95% CI: 2.6–10.7) patients (Figure 1).
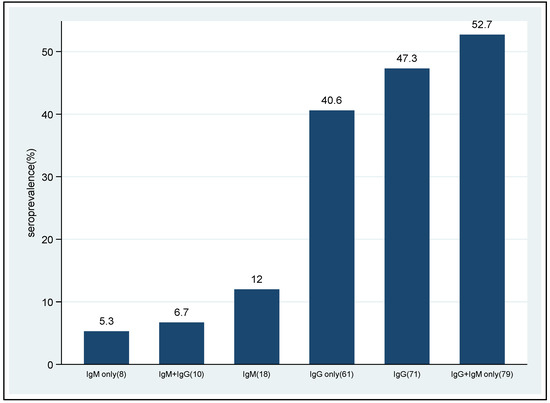
Figure 1.
Seropositivity of SARS-CoV-2 antibodies among non-vaccinated HIV patients.
3.3. Factors Associated with Overall SARS-CoV-2 Seropositivity (IgG + IgM only) among HIV Patients
In the univariate logistic regression analysis, use of pit latrines (OR: 2.07, 95% CI: 1.071–3.981, p = 0.030) and history of sore throat (OR: 5.314, 95% CI: 2.037–13.865, p = 0.001) were significantly associated with SARS-CoV-2 seropositivity. Furthermore, as CD4 count increased, SARS-CoV-2 seropositivity decreased (OR: 0.99, 95% CI: 0.996–0.999, p = 0.007). In the multivariate logistic regression analysis, use of pit latrines (OR: 2.30, 95% CI: 1.122–4.738, p = 0.23) and history of sore throat (OR: 6.34, 95% CI: 2.305–18.351, p < 0.001) remained significantly associated with SARS-CoV-2 seropositivity, while increased CD4 counts was found to be protective (OR: 0.995, 95% CI: 0.995–0.999, p = 0.004) (Table 3).

Table 3.
Univariate and multivariate logistic regression analyses of the factors associated with overall SARS-CoV-2 seropositivity.
4. Discussion
People living with HIV (PLHIV) are generally considered to be at higher risk of SARS-CoV-2 infection than those without HIV infection. The majority of PLHIV are in sub-Saharan Africa. Different governments in Africa have been implementing various strategies to control the COVID-19 pandemic. These strategies might have severely interrupted HIV care delivery among several other healthcare services due to the fact that resources, both consumables and health staff, might have been directed to control the spread of SARS-CoV-2 infections. Few studies from high-income countries (HIC) have documented higher case fatality rates for COVID-19 disease among PLHIV than among the general population [19], underscoring the need for more studies in developing countries.
Data on SARS-CoV-2 seropositivity among different populations are important to estimate the actual burden of SARS-CoV-2 infections, especially in developing countries, including Tanzania. This study documents the seropositivity of SARS-CoV-2 among PLHIV in Tanzania, a country which implemented no strict SARS-CoV-2 preventive measures. It should be noted that Tanzania is one among several developing countries that have high HIV burdens, with an estimate of 1.7 million PLHIV [20]. In this study, it was observed that more than half of the participants living with HIV were seropositive for SARS-CoV-2 antibodies, which is comparable to a previous study conducted in Pune, India, that reported seropositivity of 54.6% among HIV-infected individuals [10]. The overall seropositivity in this study is significantly higher than in previous reports from Western Kenya and Metropolitan Detroit, which reported seropositivity of 3.1% and 10.3%, respectively [16,21]. The differences could be explained by the fact that the studies in Kenya and Metropolitan Detroit were performed in the early stages of the COVID-19 pandemic, while in this study enrolment was carried out during the period in which the Omicron variants were in circulation. In addition, Tanzania did not take strict actions regarding protection against COVID-19; for example, Tanzania did not implement complete lockdowns, which might have contributed to more exposure to SARS-CoV-2 in the community than in communities with lockdowns. As in many other studies reporting SARS-CoV-2 seropositivity, IgG seropositivity was higher than that of IgM, indicating that the majority of participants had past infections. In addition, most of the participants reported being asymptomatic with respect to COVID-19-like symptoms, suggesting that SARS-CoV-2 infection is asymptomatic in the majority of exposed individuals, underscoring the importance of seroepidemiological studies to estimate the magnitude of the disease.
The overall (IgG and IgM) seropositivity in this study was 52.7%. This seropositivity is almost similar to the result of a study carried out in Mwanza, Tanzania, among non-HIV participants, which reported a seropositivity of 50.4% (15). The similarity in seropositivity could be due to the fact that Tanzania did not implement stringent COVID-19 preventive measures in the communities and across all populations; thus, everyone had equal exposure to SARS-CoV-2 infection.
Among the factors analyzed in this study, the use of pit latrines showed a significant association with SARS-CoV-2 seropositivity, which corroborates previous findings by L Liu et al. [22]. This association may be due to the fact that SARS-CoV-2 can be transmitted via fecal–oral routes, especially in underprivileged societies because of their poor wastewater infrastructures and overpopulation. The use of pit latrines has been often linked to poor hygiene conditions, including improper hand hygiene, which may expose individuals to SARS-CoV-2 infection.
History of sore throat was significantly associated with SARS-CoV-2 seropositivity in the current study, which is in agreement with a previous study by Lovato et al. [23]. This may be due to the fact that SARS-CoV-2 affects mainly the respiratory tract, resulting in sore throat, as in many other respiratory diseases. Sore throat has been documented among the most common otorhinolaryngologic symptoms in COVID-19 patients. Generally, the most commonly reported symptoms have been cough, fever, fatigue, headache, loss of smell, nasal obstruction, cough, rhinorrhea, and sore throat. A previous study in Mwanza [15], Tanzania, among non-HIV participants observed that history of runny nose and history of loss of taste were associated with SARS-CoV-2 seropositivity, indicating that different populations might have different predominant symptoms of SARS-CoV-2.
Furthermore, it was observed that low CD4 counts was significantly associated with SARS-CoV-2 seropositivity, indicating that, as CD4 counts increases, the chance of being seropositive decreases. Low immunity and reduced immune response to SARS-CoV-2 among PLHIV have been found to increase risk of SARS-CoV-2 infection and severity of COVID-19 due to the fact that CD4 cells play a key role in the pathogenesis of the viral infections [24]. This may be explained by the fact that HIV causes immunosuppression by depleting CD4 T cells, thus reducing the capacity of the individual to defend against bacterial, fungal, parasitic, and viral infections, such as SARS-CoV-2. This is supported by the findings from the current study which showed that the participants had an overall median CD4 count of 490 (363–666), indicating well-functioning immune systems, and those with low CD4 counts were more likely to be seropositive. The high median CD4 counts could be explained by the fact that these patients were on antiretroviral medications that improved their immune status; hence, there was less chance of them being seropositive.
Limitations to the study include possible recall biases in some responses to the questionnaire.
5. Conclusions
This study has reported high overall SARS-CoV-2 seropositivity among HIV-infected individuals on ART. Seropositivity was found to be predicted by low CD4 counts, use of pit latrines, and history of sore throat. Further longitudinal studies should focus on the clinical outcomes of COVID-19 among HIV patients and the general interactions between HIV and SARS-CoV-2, especially in HIV-endemic areas.
Author Contributions
H.A.N., J.S.M., B.N. and M.M.M. participated in the design of the study; F.C., P.C.L. and B.M. participated in the data/sample curation; F.C., P.C.L., H.A.N. and B.M. participated in the laboratory analysis of the samples; H.A.N., N.M., M.M. and S.E.M. performed the data analysis; M.M.M. and S.E.M. participated in the data interpretation; H.A.N., M.M.M. and F.C. wrote the first draft of the manuscript; S.E.M. undertook the critical review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Fogarty International Centre of the National Institutes of Health under Award Number R25 TW011227 as a fellowship grant to Community of Young Research Peers (CYRP) aimed at Transforming Health Professions Education in Tanzania (THET). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
Ethical clearance to conduct this study was requested from the joint CUHAS/BMC Research Ethics and Review Committee (CREC) with clearance number CREC/2387/2022. The permission to conduct this study was sought from the General Director of the Bugando Medical Center and the manager of the CUHAS Laboratory. Participation was voluntary, and patients were free to participate or to refuse. After explaining the importance of the study and all procedures, if he/she was willing to participate in the study, the patient signed a consent form. Each patient was required to answer some questions about the topics in the questionnaire, and a blood sample was collected. The test results were kept confidential, and the names of the patients were not used when reporting the study results. The risks and benefits were explained clearly to each patient before participating in the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated/analyzed in the study are included in the manuscript.
Acknowledgments
The authors acknowledge the support provided by the Department of Microbiology and Immunology of the Catholic University of Health and Allied Sciences, BMC-accredited laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F. The trinity of 467 COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Ssentongo, P.; Heilbrunn, E.S.; Ssentongo, A.E.; Advani, S.; Chinchilli, V.M.; Nunez, J.J.; Du, P. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 6283. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Asadi, A.; Noorbakhsh, N.; Dashtbin, S.; Asadollahi, P.; Dranbandi, A.; Navidifar, T.; Ghanavati, R. COVID-19 in HIV-positive patients: A systematic review of case reports and case series. J. Clin. Lab. Anal. 2022, 36, e24308. [Google Scholar] [CrossRef]
- Mirzaei, H.; McFarland, W.; Karamouzian, M.; Sharifi, H. COVID-19 among People Living with HIV: A Systematic Review. AIDS Behav. 2021, 25, 85–92. [Google Scholar] [CrossRef]
- Sah, P.; Fitzpatrick, M.C.; Zimmer, C.F.; Abdollahi, E.; Juden-Kelly, L.; Moghadas, S.M.; Singer, B.H.; Galvani, A.P. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc. Natl. Acad. Sci. USA 2021, 118, e2109229118. [Google Scholar] [CrossRef] [PubMed]
- Bergeri, I.; Whelan, M.G.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022, 19, e1004107. [Google Scholar] [CrossRef]
- Oyelade, T.; Alqahtani, J.S.; Hjazi, A.M.; Li, A.; Kamila, A.; Raya, R.P. Global and Regional Prevalence and Outcomes of COVID-19 in People Living with HIV: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 22. [Google Scholar] [CrossRef]
- Lombardi, F.; Ricci, R.; Belmonti, S.; Fabbiani, M.; Borghetti, A.; Baldin, G.; Ciccullo, A.; Tamburrini, E.; Visconti, E.; Sanguinetti, M. Seroprevalence of SARS-CoV-2 antibodies in hiv-infected patients in rome, italy during the COVID-19 outbreak. Diagnostics 2021, 11, 1154. [Google Scholar] [CrossRef]
- Ghate, M.; Shidhaye, P.; Gurav, S.; Gadhe, K.; Kale, V.; Jain, P.; Thakar, M. Seroprevalence of Anti-SARS-CoV-2 IgG Antibodies among HIV Infected Individuals Attending ART Centre at Pune: A Cross-Sectional Study. J. Int. Assoc. Provid. AIDS Care (JIAPAC) 2022, 21, 23259582221077943. [Google Scholar] [CrossRef]
- Rosenthal, E.M.; Rosenberg, E.S.; Patterson, W.; Ferguson, W.P.; Gonzalez, C.; DeHovitz, J.; Udo, T.; Rajulu, D.T.; Hart-Malloy, R.; Tesoriero, J. Factors associated with SARS-CoV-2-related hospital outcomes among and between persons living with and without diagnosed HIV infection in New York State. PLoS ONE 2022, 17, e0268978. [Google Scholar] [CrossRef] [PubMed]
- Amuche, N.J.; Emmanuel, E.I.; Innocent, N.E. HIV/AIDS in sub-Saharan Africa: Current status, challenges and prospects. Asian Pac. J. Trop. Dis. 2017, 7, 239–256. [Google Scholar] [CrossRef]
- Zanzibar, A. Tanzania HIV Impact Survey (THIS) 2016–2017; CDC: Atlanta, GA, USA, 2017. [Google Scholar]
- Salum, S.S.; Sheikh, M.A.; Hebestreit, A.; Kelm, S. Anti SARS-CoV-2 seroprevalence in Zanzibar in 2021 before the Omicron wave. IJID Reg. 2022, 4, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Nyawale, H.A.; Moremi, N.; Mohamed, M.; Njwalila, J.; Silago, V.; Krone, M.; Konje, E.T.; Mirambo, M.M.; Mshana, S.E. High Seroprevalence of SARS-CoV-2 in Mwanza, Northwestern Tanzania: A Population-Based Survey. Int. J. Environ. Res. Public Health 2022, 19, 11664. [Google Scholar] [CrossRef]
- Crowell, T.A.; Daud, I.I.; Maswai, J.; Owuoth, J.; Sing’Oei, V.; Imbach, M.; Dear, N.; Sawe, F.; Eller, L.A.; Polyak, C.S.; et al. Severe acute respiratory syndrome coronavirus-2 antibody prevalence in people with and without HIV in rural Western Kenya, January to March. Aids 2021, 35, 2401. [Google Scholar] [CrossRef] [PubMed]
- Africa, C. Generic Protocol for a Population-Based, Age-and Gender-Stratified Sero-Survey Study for SARS-CoV-2; Africa CDC: Addis Ababa, Ethiopia, 2020. [Google Scholar]
- Kish, L. Sampling Organizations and Groups of Unequal Sizes. Am. Sociol. Rev. 1965, 30, 564. [Google Scholar] [CrossRef]
- Kanwugu, O.N.; Adadi, P. HIV/SARS-CoV-2 coinfection: A global perspective. J. Med. Virol. 2021, 93, 726–732. [Google Scholar] [CrossRef]
- UNICEF. AIDS HIV and AIDS; UNICEF: New York, NY, USA, 2020; pp. 1–6. [Google Scholar]
- Gudipati, S.; Lee, M.; Scott, M.; Yaphe, S.; Huisting, J.; Yared, N.; Brar, I.; Markowitz, N. The seroprevalence of COVID-19 in patients living with HIV in metropolitan Detroit. Int. J. STD AIDS 2022, 33, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, J.; Hou, Y.; Tao, Z.; Chen, Z.; Chen, K. Pit latrines may be a potential risk in rural China and low-income countries when dealing with COVID-19. Sci. Total. Environ. 2021, 761, 143283. [Google Scholar] [CrossRef]
- Lovato, A.; Rossettini, G.; de Filippis, C. Sore throat in COVID-19: Comment on “Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis”. J. Med. Virol. 2020, 92, 714. [Google Scholar] [CrossRef]
- Sun, J.; Patel, R.C.; Zheng, Q.; Madhira, V.; Olex, A.L.; Islam, J.Y.; French, E.; Chiang, T.P.-Y.; Akselrod, H.; Moffitt, R. COVID-19 disease severity among people with HIV infection or solid organ transplant in the United States: A nationally-representative, multicenter, observational cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).