Dynamics of Physiological Changes of Shiga Toxin-Producing Escherichia coli O157:H7 on Romaine Lettuce During Pre-Processing Cold Storage, and Subsequent Effects on Virulence and Stress Tolerance
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Greenhouse Propagation of Romaine Lettuce
2.1.2. STEC Strains
2.1.3. Selection of Rifampicin-Resistant Isolates
2.1.4. Spray Inoculation of Lettuce
2.1.5. Lettuce Harvesting
2.1.6. Cold Storage
2.2. Sample Collection
2.2.1. Culturable Cells
2.2.2. Injured Cells
2.2.3. Persister Cells
2.2.4. VBNC Cells
2.2.5. Acid Tolerance
2.2.6. Chlorine Tolerance
2.2.7. Virulence Assays
2.3. Industry Supplied Data
2.4. Data Analysis
2.4.1. Generating Standard Curve for the VBNC Cells
2.4.2. Statistical Analyses
3. Results
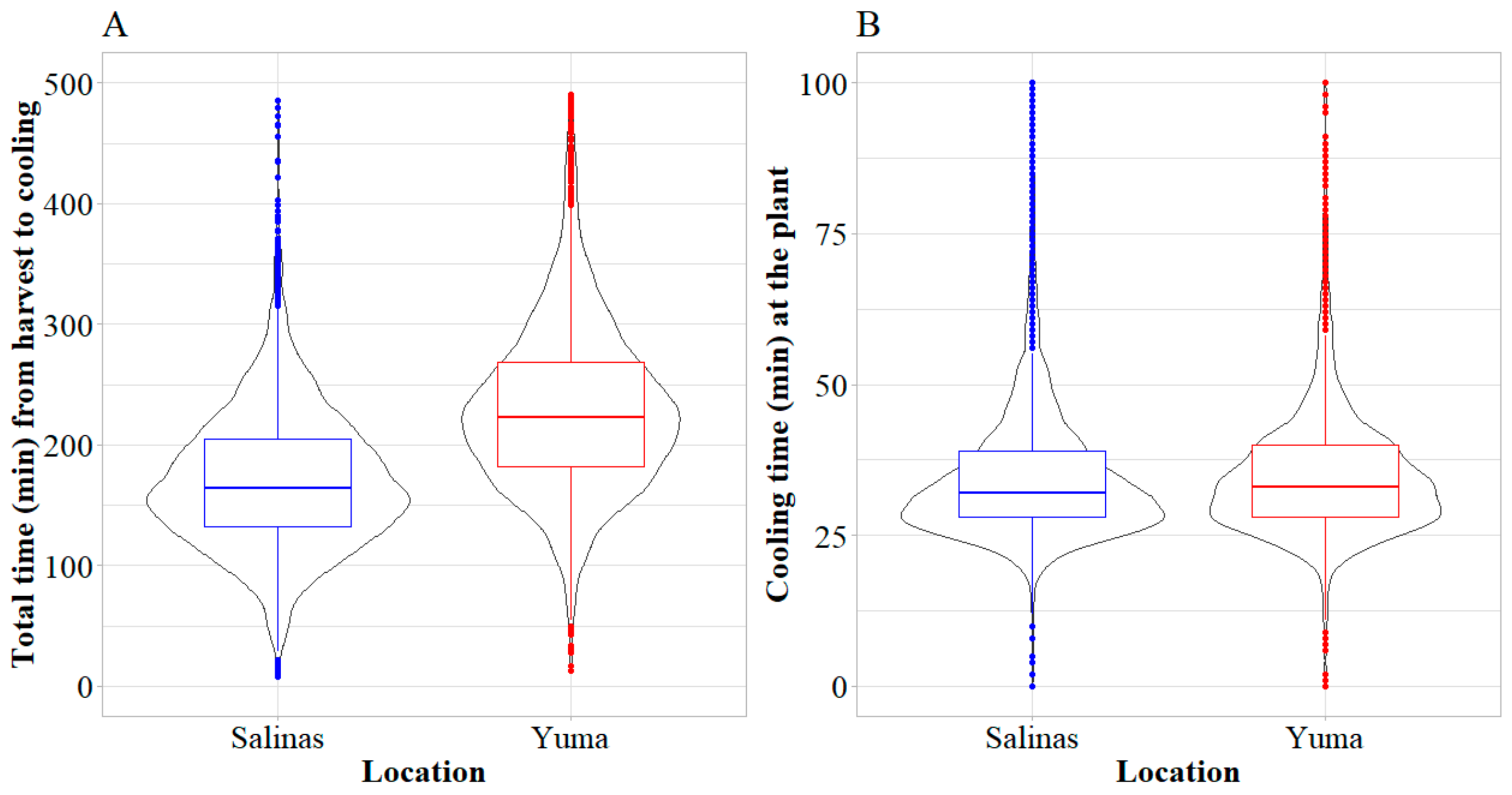
3.1. Harvest Temperatures and Cut-to-Cool Times of Romaine Lettuce from Salinas and Yuma Growing Regions
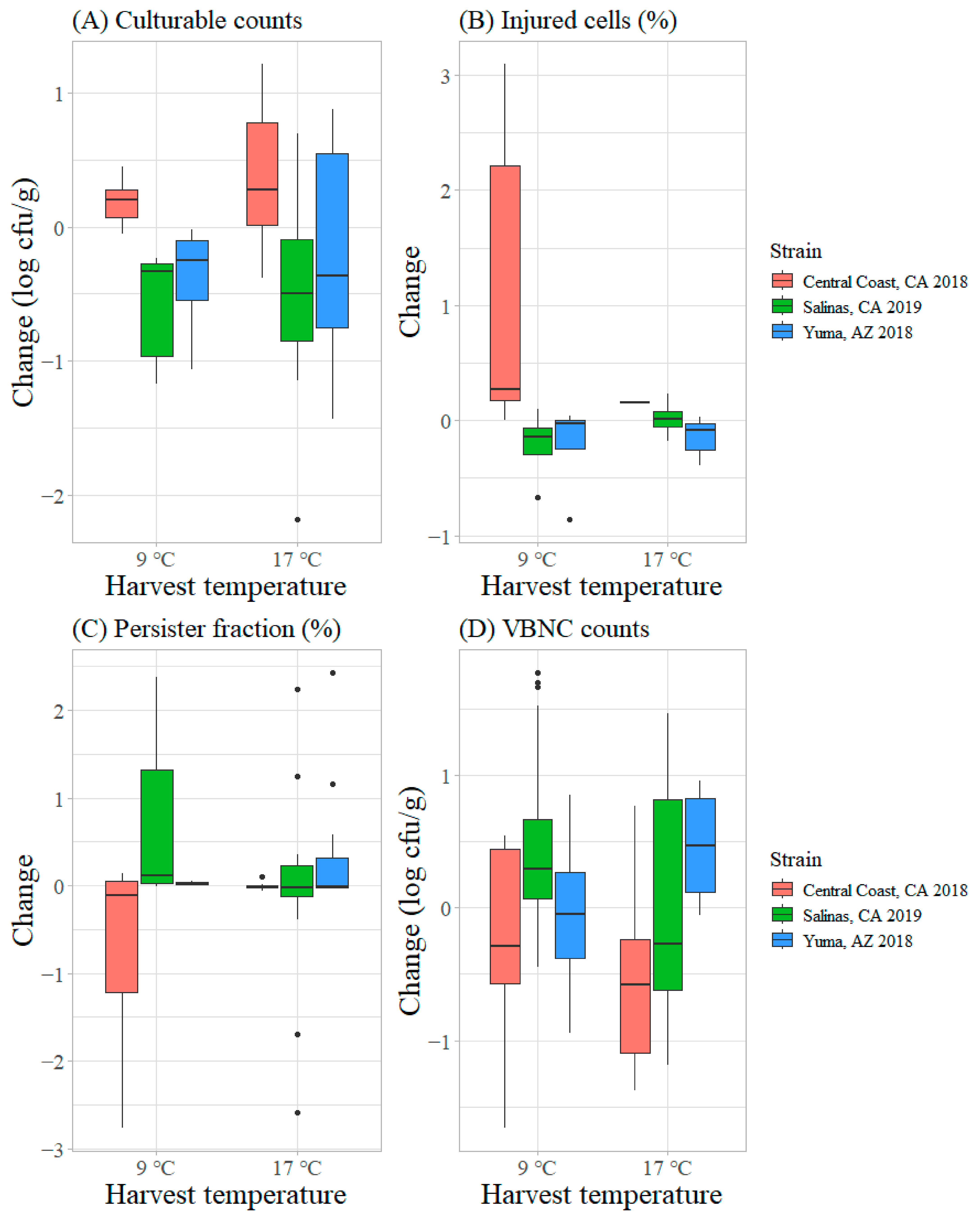
3.2. Behavior of STEC O157:H7 on Inoculated Romaine Lettuce at the Two Harvest Temperatures from Cut to Cool
3.2.1. Transitions in Physiological States of STEC O157:H7 on Inoculated Lettuce
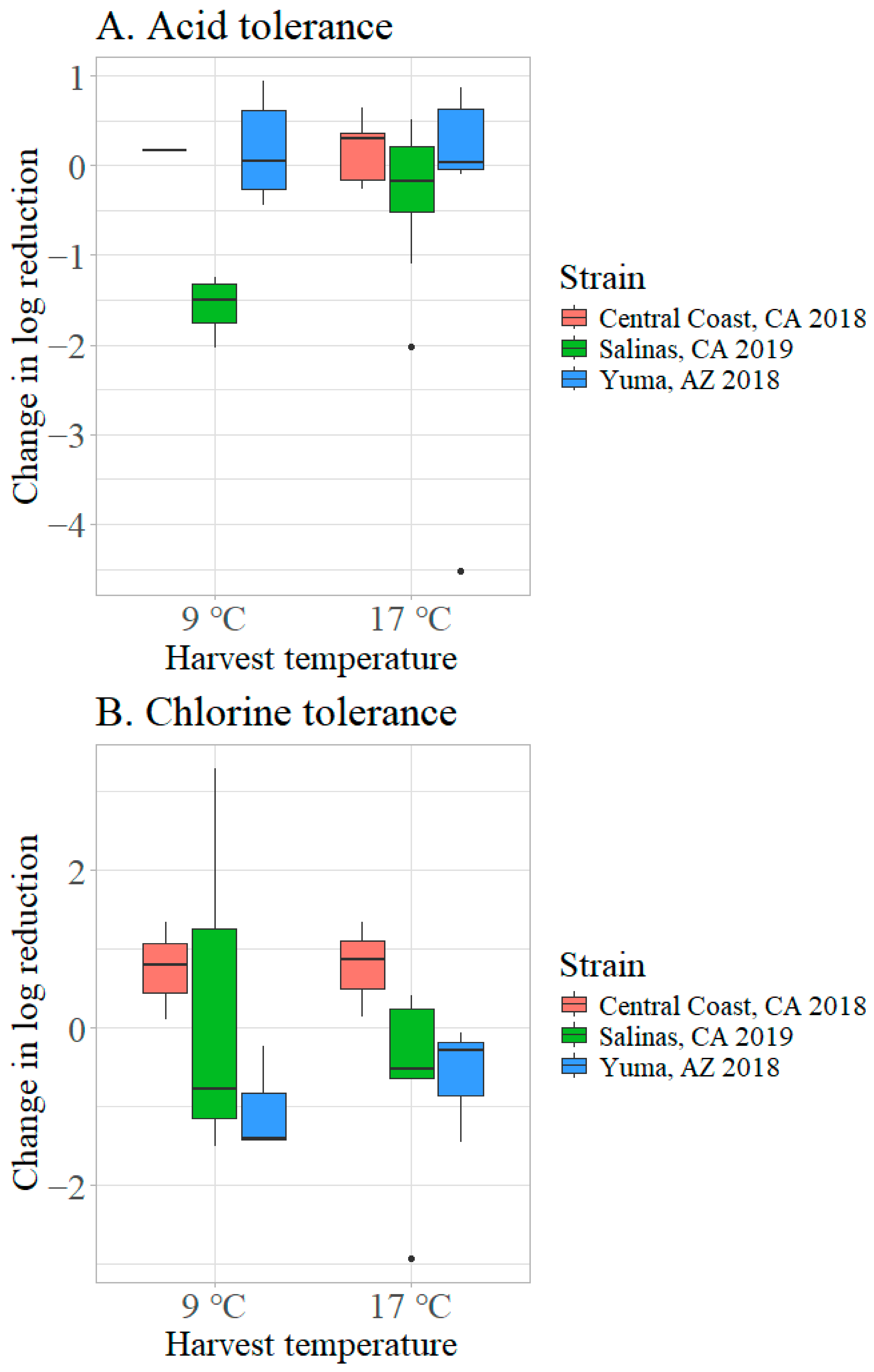
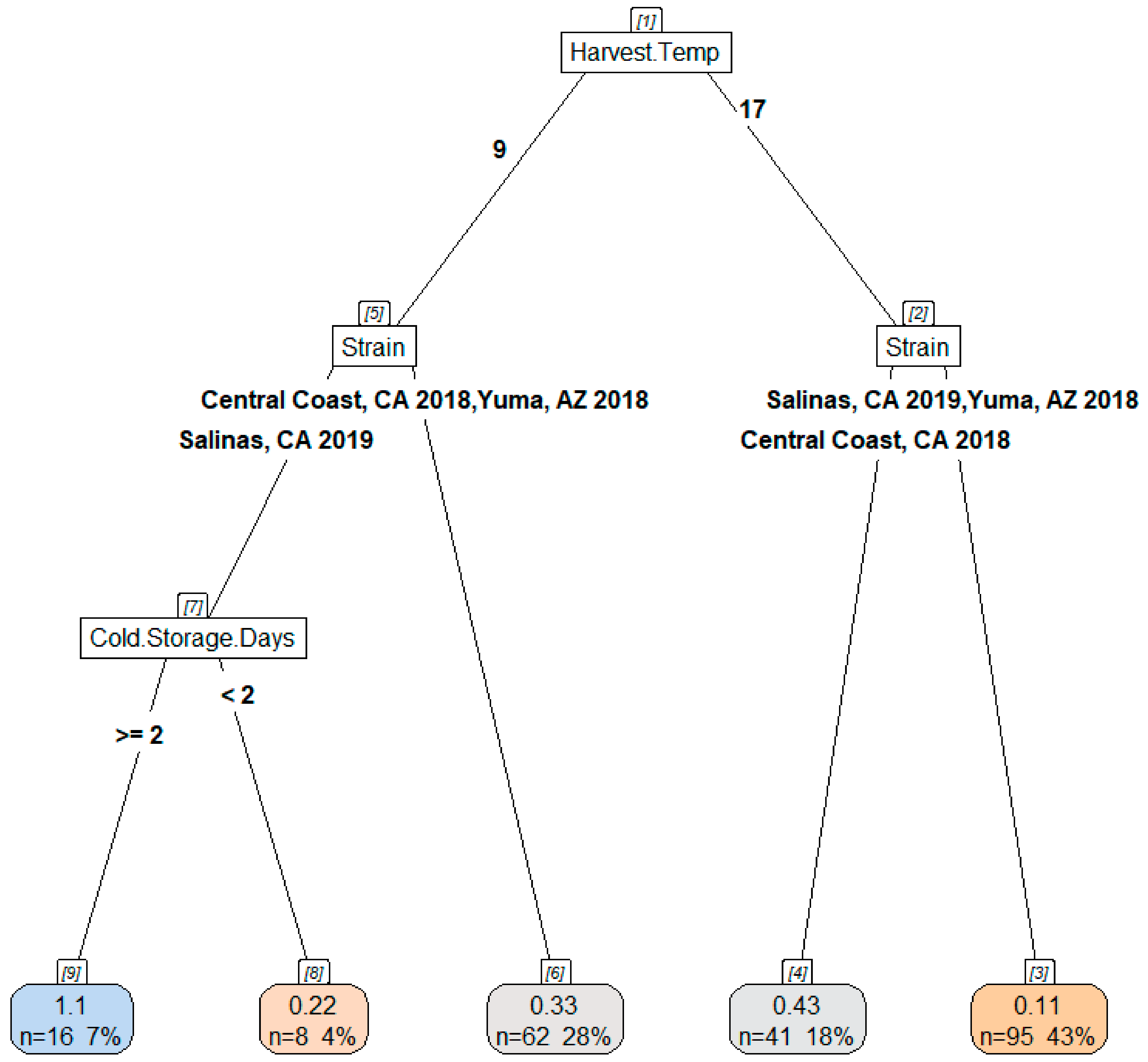
3.2.2. Effect of Harvest Temperature on Acid and Chlorine Tolerance of STEC O157:H7 on Inoculated Lettuce
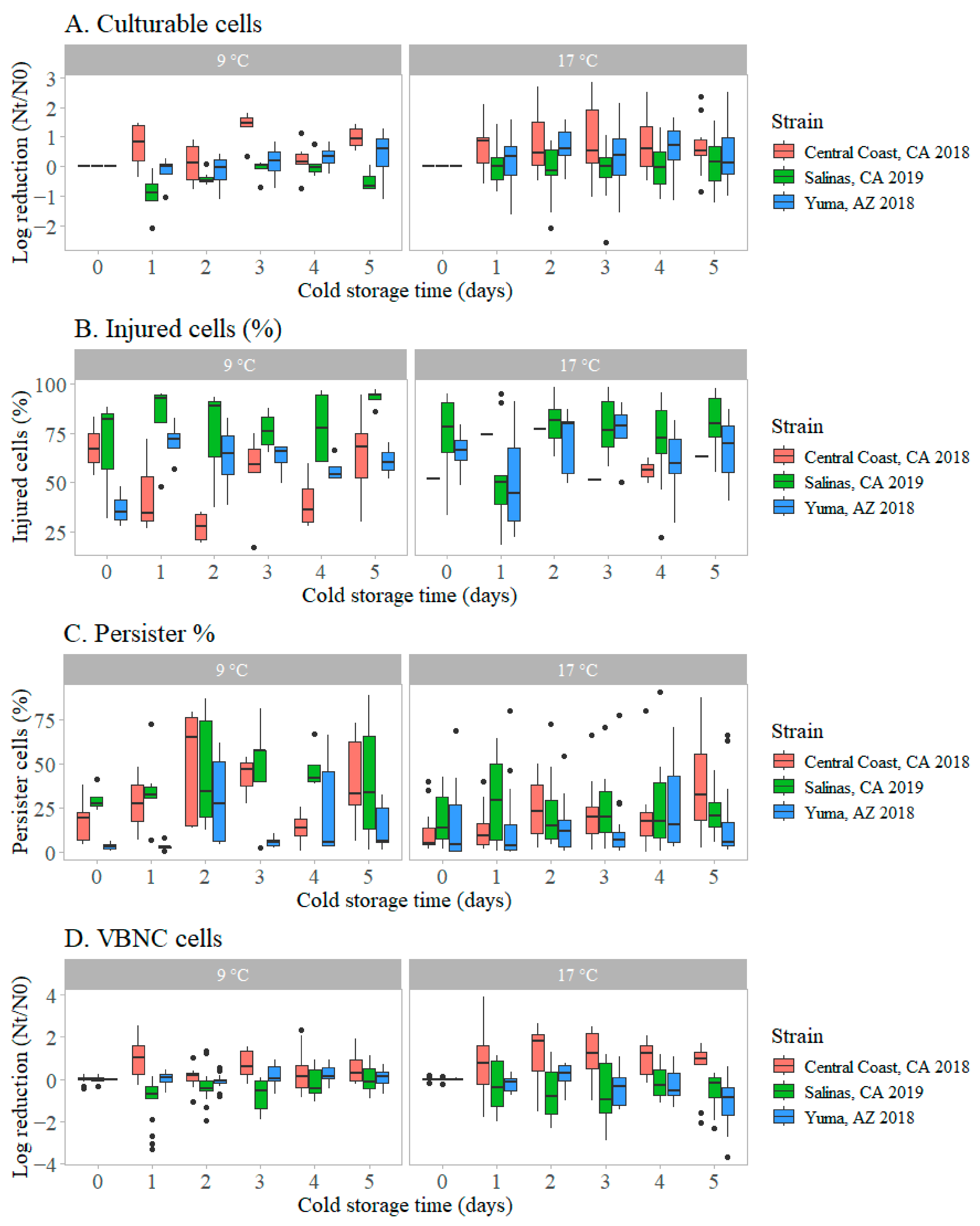
3.3. Transitions in Physiological States of STEC O157:H7 on Romaine Lettuce During Cold Storage
3.3.1. Changes in Culturable Cells During Cold Storage
3.3.2. Transition of STEC O157:H7 Cells to the Injured State During Cold Storage
3.3.3. Transition of Microbial Cells into the Persister State During Cold Storage
3.3.4. Transition of Microbial Cells to the VBNC State During Cold Storage
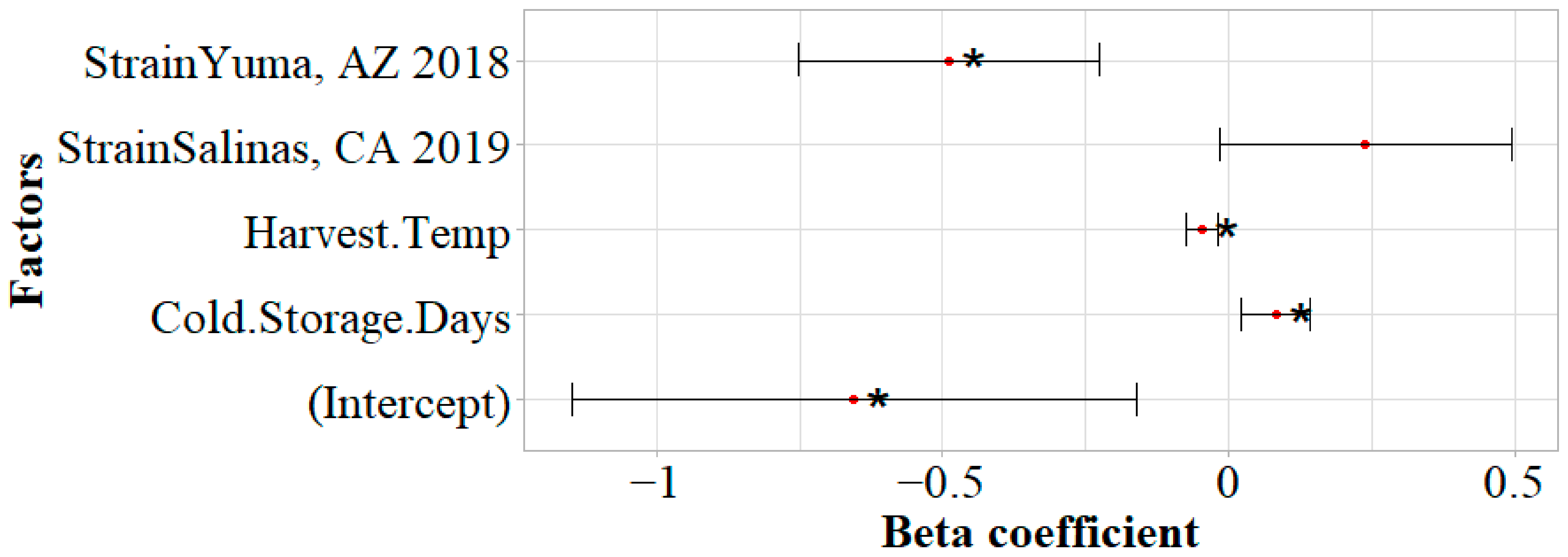
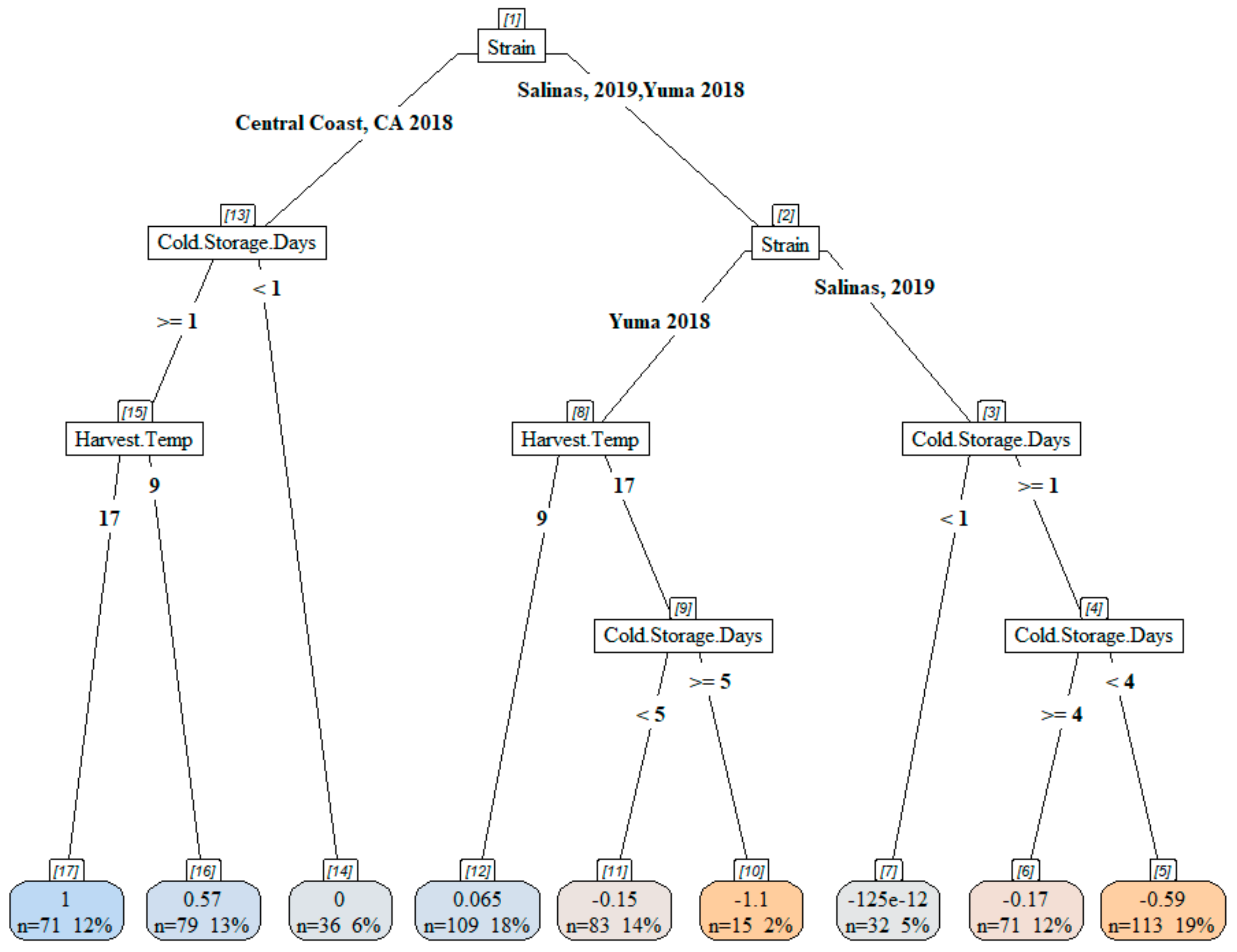
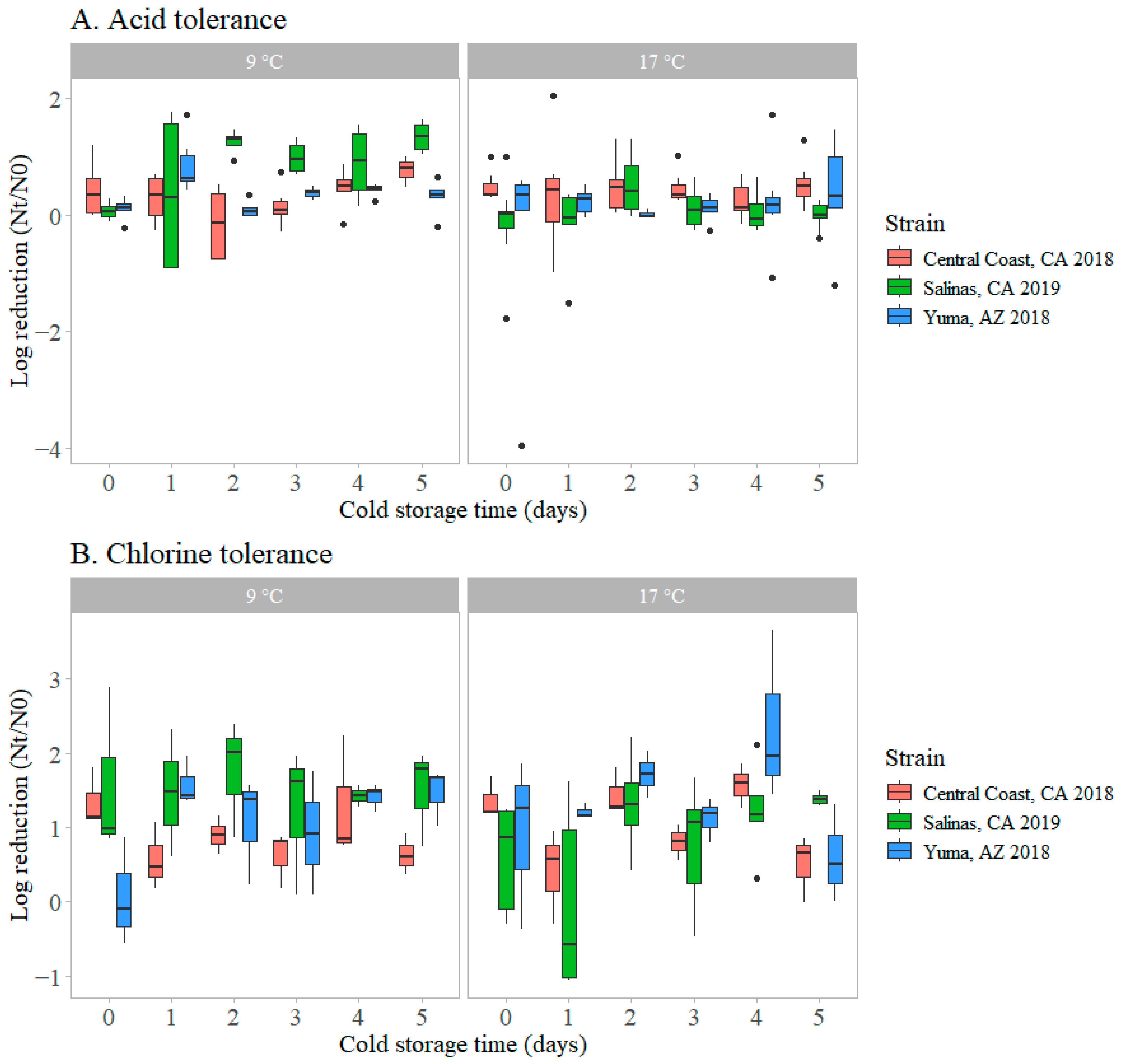
3.4. Acid and Chlorine Tolerance of STEC O157:H7 on Inoculated Lettuce During Cold Storage
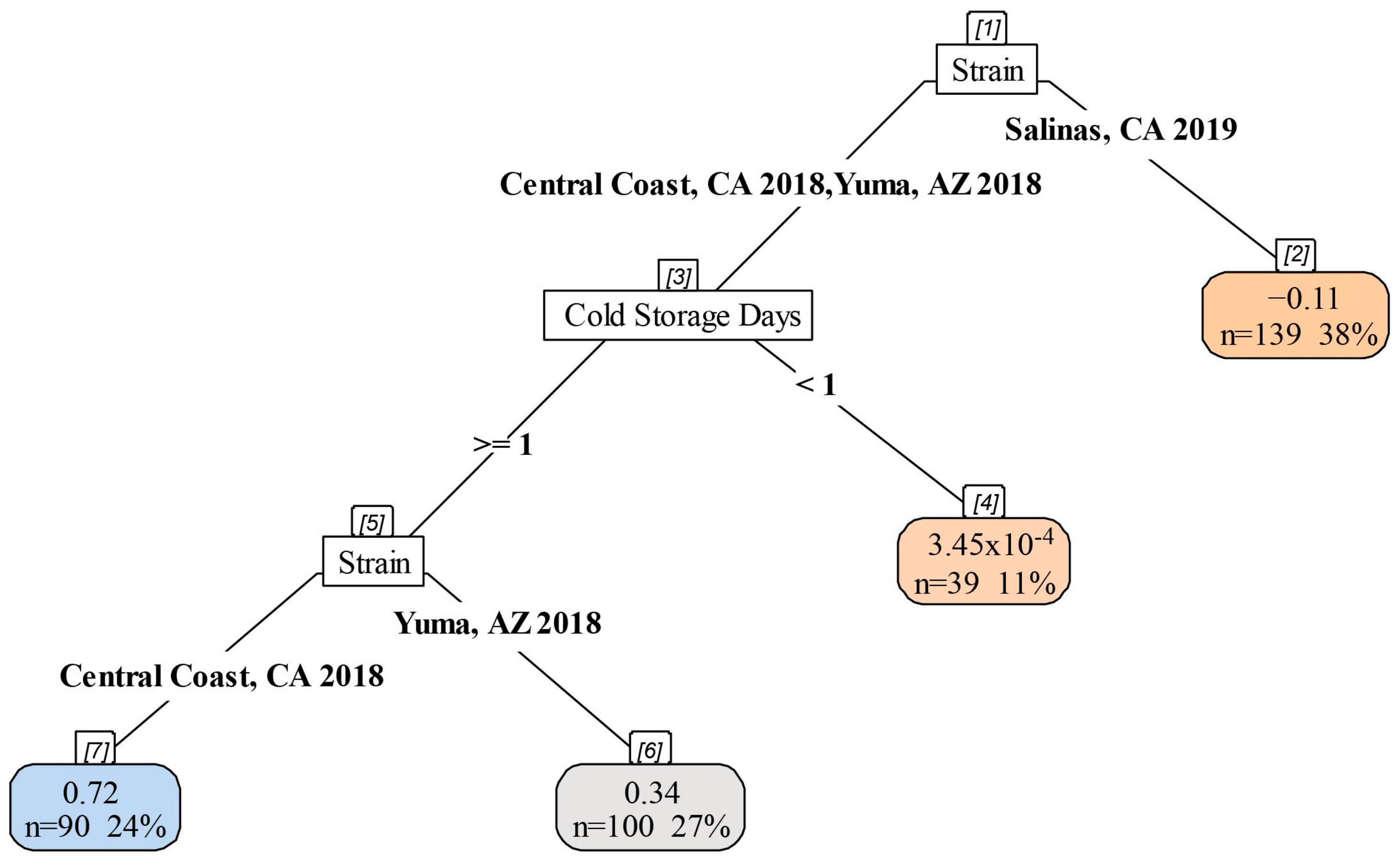
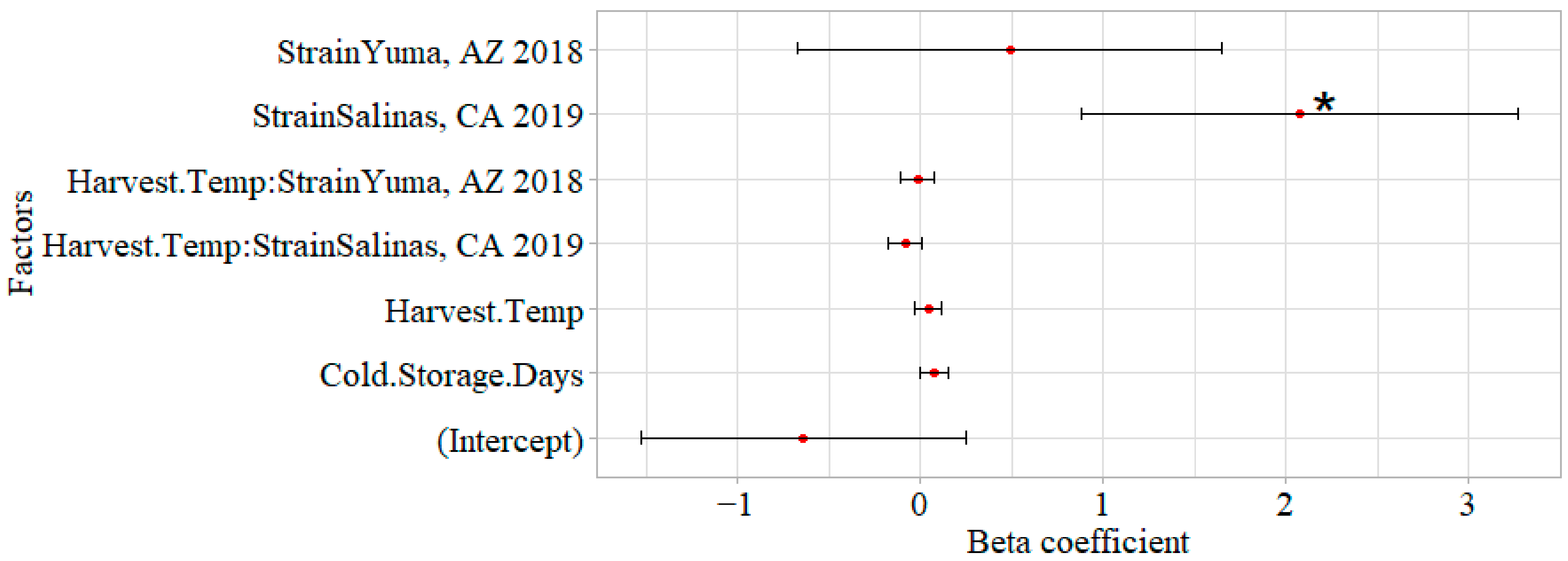
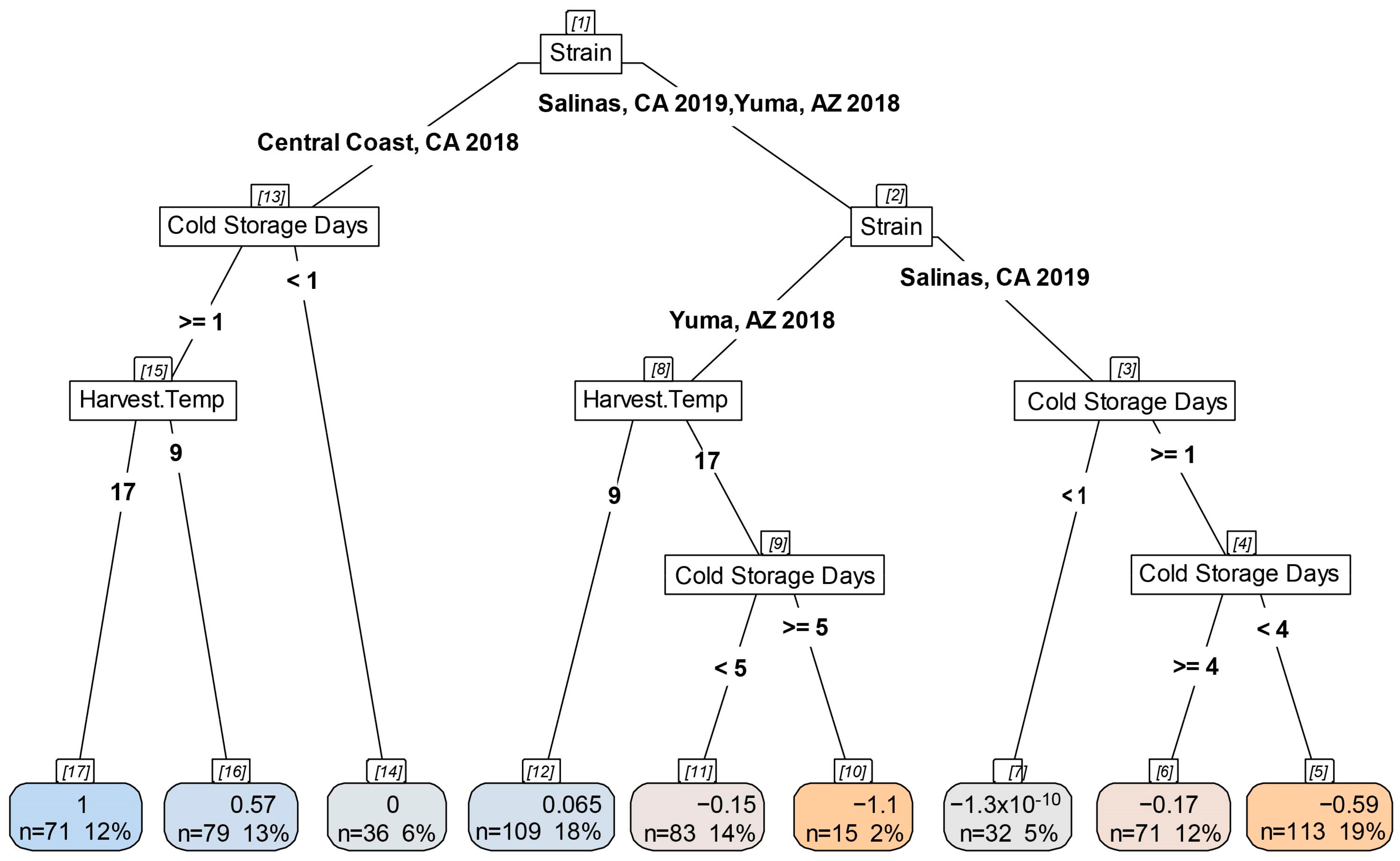
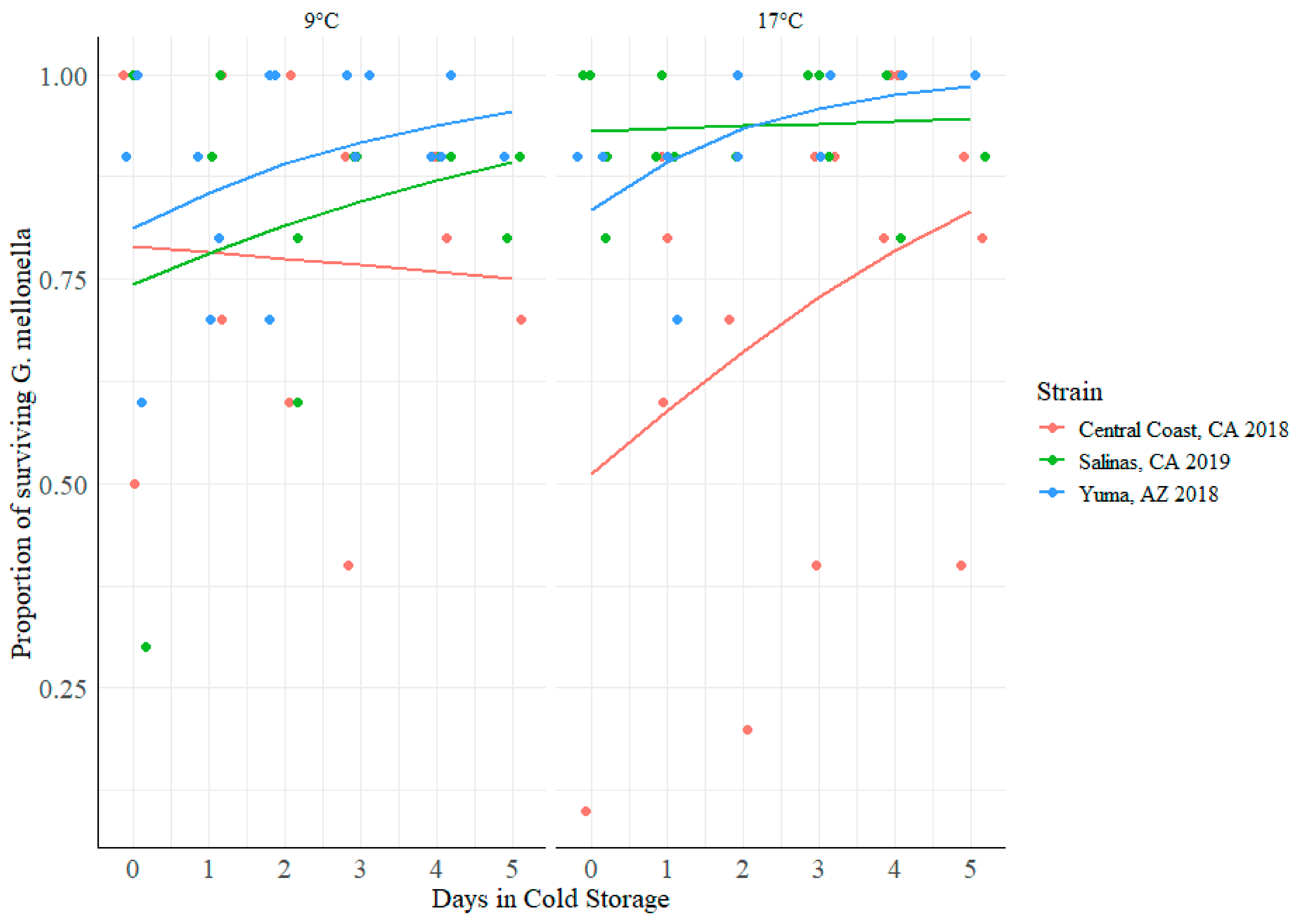
3.5. Impact of Cold Storage and Harvest Temperature on Virulence of STEC O157:H7
4. Discussion
4.1. Changes in Physiology During Cut-to-Cool Were Minimal, While Significant Differences in Physiology Occurred During Cold Storage
4.2. Increasing Cold Storage Time Led to Reduced Acid Tolerance
4.3. Increasing Cold Storage Time Did Not Influence Chlorine Tolerance
4.4. Increasing Cold Storage Time Led to Decreased Virulence in G. mellonella
4.5. Strains Affected the Variability in the Physiological, Tolerance, and Virulence Response of the Strains
4.6. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VBNC | Viable But Non-Culturable |
| STEC | Shiga toxin-producing E. coli |
| HUS | Hemolytic Uremic Syndrome |
| PAA | PeroxyAcetic Acid |
| MDHHS | Michigan Department of Health and Human Services |
| LB | Luria Bertani |
| TSA | Tryptic Soy Agar |
| Rif | Rifampicin |
| PBS | Phosphate Buffer Saline |
| MIC | Minimum Inhibitory Concentration |
| PMA | Propidium MonoAzide |
| MSU | Michigan State University |
| qpCR | Quantitative Polymerase Chain Reaction |
| SGF | Synthetic Gastric Fluid |
| Emmeans | Estimated Marginal Means |
| E. coli | Escherichia coli |
| CP | Complexity Parameter |
| xerror | Cross-validation Error |
| AICc | Akaike Information Criterion |
References
- Berger, C.N.; Sodha, S.V.; Shaw, R.K.; Griffin, P.M.; Pink, D.; Hand, P.; Frankel, G. Fresh Fruit and Vegetables as Vehicles for the Transmission of Human Pathogens. Environ. Microbiol. 2010, 12, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Scharff, R. Foodborne Illnesses from Leafy Greens in the United States: Attribution, Burden, and Cost. J. Food Prot. 2024, 87, 100275. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. Pathogenicity Assessment of Shiga Toxin-Producing Escherichia coli (STEC) and the Public Health Risk Posed by Contamination of Food with STEC. EFSA J. 2020, 18, e05967. [Google Scholar] [CrossRef]
- FDA. Factors Potentially Contributing to the Contamination of Romaine Lettuce Implicated in the Three Outbreaks of E. coli O157:H7 During the Fall of 2019. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/factors-potentially-contributing-contamination-romaine-lettuce-implicated-three-outbreaks-e-coli (accessed on 12 August 2024).
- Bottichio, L.; Keaton, A.; Thomas, D.; Fulton, T.; Tiffany, A.; Frick, A.; Mattioli, M.; Kahler, A.; Murphy, J.; Otto, M. Shiga Toxin–Producing Escherichia coli Infections Associated with Romaine Lettuce—United States, 2018. Clin. Infect. Dis. 2020, 71, e323–e330. [Google Scholar] [CrossRef]
- Chen, J.C.; Patel, K.; Smith, P.A.; Vidyaprakash, E.; Snyder, C.; Tagg, K.A.; Webb, H.E.; Schroeder, M.N.; Katz, L.S.; Rowe, L.A.; et al. Reoccurring Escherichia coli O157:H7 Strain Linked to Leafy Greens–Associated Outbreaks, 2016–2019—Volume 29, Number 9—September 2023—Emerging Infectious Diseases Journal—CDC. Emerg. Infect. Dis. 2023, 29, 1895–1899. [Google Scholar] [CrossRef]
- CDC. Multistate Outbreak of Shiga Toxin-Producing Escherichia coli O157:H7 Infections Linked to Leafy Greens (Final Update) | E. coli. Available online: https://archive.cdc.gov/www_cdc_gov/ecoli/2017/o157h7-12-17/index.html (accessed on 12 August 2024).
- Center for Disease Control CDC. E. coli Outbreak Linked to Romaine Lettuce B—Investigation Updates | E. coli Infections Linked to Romaine Lettuce | November 2018 | E. coli. 2018. Available online: https://archive.cdc.gov/www_cdc_gov/ecoli/2018/o157h7-11-18/updates.html (accessed on 23 July 2024).
- Center for Disease Control CDC. E. coli Outbreak Linked to Romaine Lettuce A—Advice | Investigation Notice: Multistate Outbreak of E. coli O157:H7 Infections April 2018 | E. coli. 2018. Available online: https://archive.cdc.gov/www_cdc_gov/ecoli/2018/o157h7-04-18/advice-consumers.html#print (accessed on 23 July 2024).
- Center for Disease Control CDC. E. coli Outbreak Linked to Romaine Lettuce—Investigation Updates | E. coli Infections | November 2019 | E. coli. 2019. Available online: https://archive.cdc.gov/www_cdc_gov/ecoli/2019/o157h7-11-19/updates.html (accessed on 23 July 2024).
- Sinkel, D. Good Agriculture Practices for Lettuce. Available online: https://www.nifa.usda.gov/sites/default/files/resource/Lettuce%20Good%20Agricultural%20Practices508.pdf (accessed on 30 April 2025).
- Lettuce Production and Trade—Rural Migration Blog. Migration Dialogue. Available online: https://migration.ucdavis.edu/rmn/blog/post/?id=2699 (accessed on 25 July 2024).
- USDA ERS USDA ERS—Chart Detail. Available online: https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=89157 (accessed on 25 July 2024).
- Smith, R.; Cruz, S.; Benito, S. Leaf Lettuce Production in California. Available online: https://anrcatalog.ucanr.edu/pdf/7216.pdf (accessed on 30 April 2025).
- Gombas, D.; Gorny, J. Commodity Specific Food Safety Guidelines for the Lettuce and Leafy Greens Supply Chain, 1st ed.; International Fresh-Cut Produce Association: Washington, DC, USA, 2006. Available online: https://www.fda.gov/media/77279/download?attachment (accessed on 30 April 2025).
- Brown, W.; Ryser, E.; Gorman, L.; Steinmaus, S.; Vorst, K. Transit Temperatures Experienced by Fresh-Cut Leafy Greens during Cross-Country Shipment. Food Control 2016, 61, 146–155. [Google Scholar] [CrossRef]
- Gu, G.; Ding, Q.; Redding, M.; Yang, Y.; O’Brien, R.; Gu, T.; Zhang, B.; Zhou, B.; Micallef, S.A.; Luo, Y. Differential Microbiota Shift on Whole Romaine Lettuce Subjected to Source or Forward Processing and on Fresh-Cut Products during Cold Storage. Int. J. Food Microbiol. 2024, 416, 110665. [Google Scholar] [CrossRef]
- Khalil, R.K.S. Effect of Abusive Storage Temperatures on Growth and Survival of Escherichia coli O157:H7 on Leafy Salad Vegetables in Egypt. LWT-Food Sci. Technol. 2016, 65, 954–962. [Google Scholar] [CrossRef]
- Choi, S.; Bang, J.; Kim, H.; Beuchat, L.R.; Ryu, J. Survival and Colonization of Escherichia coli O157:H7 on Spinach Leaves as Affected by Inoculum Level and Carrier, Temperature and Relative Humidity. J. Appl. Microbiol. 2011, 111, 1465–1472. [Google Scholar] [CrossRef]
- Owade, J.O.; Bergholz, T.M.; Mitchell, J. A Review of Conditions Influencing Fate of Shiga Toxin-producing Escherichia coli O157:H7 in Leafy Greens. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70013. [Google Scholar] [CrossRef]
- Luo, Y.; He, Q.; McEvoy, J.L. Effect of Storage Temperature and Duration on the Behavior of Escherichia coli O157:H7 on Packaged Fresh-cut Salad Containing Romaine and Iceberg Lettuce. J. Food Sci. 2010, 75, M390–M397. [Google Scholar] [CrossRef]
- Leonard, S.R.; Simko, I.; Mammel, M.K.; Richter, T.K.S.; Brandl, M.T. Seasonality, Shelf Life and Storage Atmosphere Are Main Drivers of the Microbiome and Escherichia coli O157:H7 Colonization of Post-Harvest Lettuce Cultivated in a Major Production Area in California. Environ. Microbiome 2021, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Ayrapetyan, M.; Williams, T.; Oliver, J.D. Relationship between the Viable but Nonculturable State and Antibiotic Persister Cells. J. Bacteriol. 2018, 200, 1–15. [Google Scholar] [CrossRef] [PubMed]
- King, A.D.; Magnuson, J.A.; Török, T.; Goodman, N. Microbial Flora and Storage Quality of Partially Processed Lettuce. J. Food Sci. 1991, 56, 459–461. [Google Scholar] [CrossRef]
- Baur, S.; Klaiber, R.; Hammes, W.P.; Carle, R. Sensory and Microbiological Quality of Shredded, Packaged Iceberg Lettuce as Affected by Pre-Washing Procedures with Chlorinated and Ozonated Water. Innov. Food Sci. Emerg. Technol. 2004, 5, 45–55. [Google Scholar] [CrossRef]
- USDA. Peracetic Acid Processing. 2000. Available online: https://www.ams.usda.gov/sites/default/files/media/Peracetic%20Acid%20Technical%20Report%20Handling.pdf (accessed on 30 April 2025).
- National Agricultural Library. Use of Ozone to Enhance the Safety of Produce. Available online: https://www.nal.usda.gov/research-tools/food-safety-research-projects/use-ozone-enhance-safety-produce (accessed on 2 September 2024).
- Davidson, G.R.; Kaminski-Davidson, C.N.; Ryser, E.T. Persistence of Escherichia coli O157:H7 during Pilot-Scale Processing of Iceberg Lettuce Using Flume Water Containing Peroxyacetic Acid-Based Sanitizers and Various Organic Loads. Int. J. Food Microbiol. 2017, 248, 22–31. [Google Scholar] [CrossRef]
- Davidson, G.R.; Buchholz, A.L.; Ryser, E.T. Efficacy of Commercial Produce Sanitizers against Nontoxigenic Escherichia coli O157:H7 during Processing of Iceberg Lettuce in a Pilot-Scale Leafy Green Processing Line. J. Food Prot. 2013, 76, 1838–1845. [Google Scholar] [CrossRef]
- Saldaña, Z.; Sánchez, E.; Xicohtencatl-Cortes, J.; Puente, J.L.; Girón, J.A. Surface Structures Involved in Plant Stomata and Leaf Colonization by Shiga-Toxigenic Escherichia coli O157:H7. Front. Microbiol. 2011, 2, 119. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Sun, Y.; Li, C.; Li, Y.; Zhang, Q.; Wu, Z. Reduction of Escherichia coli O157:H7 and Naturally Present Microbes on Fresh-Cut Lettuce Using Lactic Acid and Aqueous Ozone. RSC Adv. 2019, 9, 22636–22643. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, Y.; Wang, F.; Yang, J.; Pan, Z.; Huang, M.; Shen, K.; Shen, C. Multidrug-Resistant Enteroaggregative Escherichia coli (EAEC) Enters Dormant State during Heat Treatment: A Potential Hazard in Municipal Sludge. Environ. Pollut. 2022, 305, 119312. [Google Scholar] [CrossRef]
- Leinberger, F.H.; Cassidy, L.; Edelmann, D.; Schmid, N.E.; Oberpaul, M.; Blumenkamp, P.; Schmidt, S.; Natriashvili, A.; Ulbrich, M.H.; Tholey, A. Protein Aggregation Is a Consequence of the Dormancy-Inducing Membrane Toxin TisB in Escherichia coli. mSystems 2024, 9, e01060-24. [Google Scholar] [CrossRef] [PubMed]
- Munther, D.S.; Carter, M.Q.; Aldric, C.V.; Ivanek, R.; Brandl, M.T. Formation Escherichia coli O157:H7 Persister Cells in the Lettuce Phyllosphere and Application of Differential Equation Models To Predict Their Prevalence on Lettuce Plants in the Field. Appl. Environ. Microbiol. 2020, 86, e01602-19. [Google Scholar] [CrossRef] [PubMed]
- Thao, S.; Brandl, M.T.; Carter, M.Q. Enhanced Formation of Shiga Toxin-Producing Escherichia coli Persister Variants in Environments Relevant to Leafy Greens Production. Food Microbiol. 2019, 84, 103241. [Google Scholar] [CrossRef]
- Dinu, L.D.; Bach, S. Induction of Viable but Nonculturable Escherichia coli O157:H7 in the Phyllosphere of Lettuce: A Food Safety Risk Factors. Appl. Environ. Microbiol. 2011, 77, 8295–8302. [Google Scholar] [CrossRef]
- Truchado, P.; Gómez-Galindo, M.; Gil, M.I.; Allende, A. Cross-Contamination of Escherichia coli O157:H7 and Listeria monocytogenes in the Viable but Non-Culturable (VBNC) State during Washing of Leafy Greens and the Revival during Shelf-Life. Food Microbiol. 2023, 109, 104155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gross, C.A. Cold Shock Response in Bacteria. Annu. Rev. Genet. 2021, 55, 377–400. [Google Scholar] [CrossRef]
- Phadtare, S.; Inouye, M. Genome-Wide Transcriptional Analysis of the Cold Shock Response in Wild-Type and Cold-Sensitive, Quadruple-Csp-Deletion Strains of Escherichia coli. J. Bacteriol. 2004, 186, 7007–7014. [Google Scholar] [CrossRef]
- Keto-Timonen, R.; Hietala, N.; Palonen, E.; Hakakorpi, A.; Lindström, M.; Korkeala, H. Cold Shock Proteins: A Minireview with Special Emphasis on Csp-Family of Enteropathogenic Yersinia. Front. Microbiol. 2016, 7, 1151. [Google Scholar] [CrossRef]
- White-Ziegler, C.A.; Um, S.; Pérez, N.M.; Berns, A.L.; Malhowski, A.J.; Young, S. Low Temperature (23 °C) Increases Expression of Biofilm-, Cold-Shock- and RpoS-Dependent Genes in Escherichia coli K-12. Microbiology 2008, 154, 148–166. [Google Scholar] [CrossRef]
- King, T.; Kocharunchitt, C.; Gobius, K.; Bowman, J.P.; Ross, T. Global Genome Response of Escherichia coli O157:H7 Sakai during Dynamic Changes in Growth Kinetics Induced by an Abrupt Temperature Downshift. PLoS ONE 2014, 9, e99627. [Google Scholar] [CrossRef]
- Kocharunchitt, C.; King, T.; Gobius, K.; Bowman, J.P.; Ross, T. Integrated Transcriptomic and Proteomic Analysis of the Physiological Response of Escherichia coli O157:H7 Sakai to Steady-State Conditions of Cold and Water Activity Stress. Mol. Cell. Proteom. 2012, 11, M111.009019. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hou, Y.; Inouye, M. CspA, the Major Cold-Shock Protein Escherichia coli, Is an RNA Chaperone. J. Biol. Chem. 1997, 272, 196–202. [Google Scholar] [CrossRef]
- Crozier, L.; Hedley, P.E.; Morris, J.; Wagstaff, C.; Andrews, S.C.; Toth, I.; Jackson, R.W.; Holden, N.J. Whole-Transcriptome Analysis of Verocytotoxigenic Escherichia coli O157:H7 (Sakai) Suggests Plant-Species-Specific Metabolic Responses on Exposure to Spinach and Lettuce Extracts. Front. Microbiol. 2016, 7, 1088. [Google Scholar]
- Kennedy, N.M.; Mukherjee, N.; Banerjee, P. Escherichia coli O157:H7 Cells Exposed to Lettuce Leaf Lysate in Refrigerated Conditions Exhibit Differential Expression of Selected Virulence and Adhesion-Related Genes with Altered Mammalian Cell Adherence. J. Food Prot. 2016, 79, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Fang, L.; Inouye, M. The CspA Family in Escherichia coli: Multiple Gene Duplication for Stress Adaptation. Mol. Microbiol. 1998, 27, 247–255. [Google Scholar] [CrossRef]
- Kim, Y.; Wang, X.; Zhang, X.; Grigoriu, S.; Page, R.; Peti, W.; Wood, T.K. Escherichia coli Toxin/Antitoxin Pair MqsR/MqsA Regulate Toxin CspD. Environ. Microbiol. 2010, 12, 1105–1121. [Google Scholar] [CrossRef]
- Sharma, M.; Lakshman, S.; Ferguson, S.; Ingram, D.T.; Luo, Y.; Patel, J. Effect of Modified Atmosphere Packaging on the Persistence and Expression of Virulence Factors of Escherichia coli O157:H7 on Shredded Iceberg Lettuce. J. Food Prot. 2011, 74, 718–726. [Google Scholar] [CrossRef]
- Carey, C.M.; Kostrzynska, M.; Thompson, S. Escherichia coli O157:H7 Stress and Virulence Gene Expression on Romaine Lettuce Using Comparative Real-Time PCR. J. Microbiol. Methods 2009, 77, 235–242. [Google Scholar] [CrossRef]
- Sharma, D.; Kraft, A.L.; Owade, J.O.; Milicevic, M.; Yi, J.; Bergholz, T.M. Impact of Biotic and Abiotic Factors on Listeria monocytogenes, Salmonella enterica, and Enterohemorrhagic Escherichia coli in Agricultural Soil Extracts. Microorganisms 2024, 12, 1498. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.Q. Real-Time PCR Methodology for Selective Detection of Viable Escherichia coli O157:H7CELLS by Targeting Z3276 as a Genetic Marker. Appl. Environ. Microbiol. 2012, 78, 5297–5304. [Google Scholar] [CrossRef]
- Beumer, R.R.; De Vries, J.; Rombouts, F.M. Campylobacter Jejuni Non-Culturable Coccoid Cells. Int. J. Food Microbiol. 1992, 15, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Alvarez, M.X.; Townsend Ramsett, M.K.; Malekmohammadi, S.; Bergholz, T.M. Evidence of Hypervirulence in Listeria monocytogenes Clonal Complex 14. J. Med. Microbiol. 2019, 68, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Scalfaro, C.; Iacobino, A.; Nardis, C.; Franciosa, G. Galleria Mellonella as an in Vivo Model for Assessing the Protective Activity of Probiotics against Gastrointestinal Bacterial Pathogens. FEMS Microbiol. Lett. 2017, 364, fnx064. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J.; Stevens, W.L. A Table for the Calculation of Working Probits and Weights in Probit Analysis. Biometrika 1948, 35, 191–201. [Google Scholar] [CrossRef]
- The CRAN Team. Community Ecology Package. 2022. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 30 April 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation. R Package Version 1.1.2. Computer Software. 2023. Available online: https://dplyr.tidyverse.org/ (accessed on 30 April 2025).
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population Marginal Means in the Linear Model: An Alternative to Least Squares Means. Am. Stat. 1980, 34, 216–221. [Google Scholar] [CrossRef]
- Therneau, T.; Atkinson, B.; Ripley, B. Rpart: Recursive Partitioning and Regression Trees. R Package Version 4.1-15. Available online: https://cran.r-project.org/web/packages/rpart/rpart.pdf (accessed on 30 April 2025).
- James, S.J.; James, C.; Evans, J.A. Modelling of Food Transportation Systems—A Review. Int. J. Refrig. 2006, 29, 947–957. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Xu, X. Application and Research Progress of Cold Storage Technology in Cold Chain Transportation and Distribution. J. Therm. Anal. Calorim. 2020, 139, 1419–1434. [Google Scholar] [CrossRef]
- Qi, T.; Ji, J.; Zhang, X.; Liu, L.; Xu, X.; Ma, K.; Gao, Y. Research Progress of Cold Chain Transport Technology for Storage Fruits and Vegetables. J. Energy Storage 2022, 56, 105958. [Google Scholar] [CrossRef]
- Russell, N.J. Bacterial Membranes: The Effects of Chill Storage and Food Processing. An Overview. Int. J. Food Microbiol. 2022, 79, 27–34. [Google Scholar] [CrossRef]
- Strocchi, M.; Ferrer, M.; Timmis, K.N.; Golyshin, P.N. Low Temperature-Induced Systems Failure in Escherichia coli: Insights from Rescue by Cold-Adapted Chaperones. Proteomics 2006, 6, 193–206. [Google Scholar] [CrossRef]
- Barria, C.; Malecki, M.; Arraiano, C.M. Bacterial Adaptation to Cold. Microbiology 2013, 159, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K. Cold Shock Response in Escherichia coli. J. Mol. Microbiol. Biotechnol. 1999, 1, 193–202. [Google Scholar]
- Vidovic, S.; Mangalappalli-Illathu, A.K.; Korber, D.R. Prolonged Cold Stress Response of Escherichia coli O157 and the Role of RpoS. Int. J. Food Microbiol. 2011, 146, 163–169. [Google Scholar] [CrossRef] [PubMed]
- King, T.; Vockler, C.J.; Allnutt, T.R.; Fegan, N. Transcriptomic Response of Escherichia coli O157 Isolates on Meat: Comparison between a Typical Australian Isolate from Cattle and a Pathogenic Clinical Isolate. Food Microbiol. 2019, 82, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.N.; Oxford, L.; O’Byrne, C.P. Survival of Low-PH Stress by Escherichia coli O157:H7: Correlation between Alterations in the Cell Envelope and Increased Acid Tolerance. Appl. Environ. Microbiol. 1999, 65, 3048–3055. [Google Scholar] [CrossRef]
- Tosun, H.; Gönül, Ş.A. The Effect of Acid Adaptation Conditions on Acid Tolerance Response of Escherichia coli O157:H7. Turk. J. Biol. 2005, 29, 197–202. [Google Scholar]
- Choi, S.H.; Baumler, D.J.; Kaspar, C.W. Contribution of Dps to Acid Stress Tolerance and Oxidative Stress Tolerance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2000, 66, 3911–3916. [Google Scholar] [CrossRef]
- Elhanafi, D.; Leenanon, B.; Bang, W.; Drake, M.A. Impact of Cold and Cold-Acid Stress on Poststress Tolerance and Virulence Factor Expression of Escherichia coli O157:H7. J. Food Prot. 2004, 67, 19–26. [Google Scholar] [CrossRef]
- Tyagi, D.; Kraft, A.L.; Smith, S.L.; Roof, S.E.; Sherwood, J.S.; Wiedmann, M.; Bergholz, T.M. Pre-Harvest Survival and Post-Harvest Chlorine Tolerance of Enterohemorrhagic Escherichia coli on Lettuce. Toxins 2019, 11, 675. [Google Scholar] [CrossRef]
- Garcia, A.; Mount, J.R.; Davidson, P.M. Ozone and Chlorine Treatment of Minimally Processed Lettuce. J. Food Sci. 2003, 68, 2747–2751. [Google Scholar] [CrossRef]
- Abnavi, M.D. Chlorine Decay and Pathogen Cross Contamination Dynamics in Fresh Produce Washing Process; Cleveland State University: Cleveland, OH, USA, 2021; ISBN 979-8-53557-758-6. [Google Scholar]
- Ramarao, N.; Nielsen-Leroux, C.; Lereclus, D. The Insect Galleria mellonella as a Powerful Infection Model to Investigate Bacterial Pathogenesis. J. Vis. Exp. 2012, 70, e4392. [Google Scholar] [CrossRef]
- Allen, K.J.; Lepp, D.; McKellar, R.C.; Griffiths, M.W. Examination of Stress and Virulence Gene Expression in Escherichia coli O157:H7 Using Targeted Microarray Analysis. Foodborne Pathog. Dis. 2008, 5, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.K.; Liu, Y.; Juneja, V.; Huang, L.; Hwang, C.-A.; Desk, S. Effects of Stresses on the Growth and Cytotoxicity of Shiga-Toxin Producing Escherichia coli in Ground Beef and Spinach. SDRP J. Food Sci. Technol. 2016, 1, 37–43. [Google Scholar]
- Ali, M.G.; Abdelhamid, A.G.; Yousef, A.E. How Colonizing Alfalfa Sprouts Modulates the Virulence of Shiga Toxin-Producing Escherichia coli. Int. J. Food Microbiol. 2025, 428, 110972. [Google Scholar] [CrossRef]
- Carter, M.Q.; Carychao, D.; Bono, J.L. Comparative Analyses of Persistence Traits in Escherichia coli O157:H7 Strains Belonging to Different Clades Including REPEXH01 and REPEXH02 Strains. Front. Microbiol. 2024, 15, 1501956. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Haley, B.J.; Li, C.; Nou, X. Genomic Comparison of Reoccurring, Emerging, and Persistent (REP) Shiga Toxin-Producing Escherichia Coli O157:H7. Foodborne Pathog. Dis. 2024. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Redding, M.; Gu, G.; Luo, Y.; Patel, J.; Nou, X. Biofilm Formation of Escherichia coli O157:H7 Strains Associated with Recent Reoccurring Lettuce Outbreaks. Food Microbiol. 2025, 128, 104728. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.Y.; Geornaras, I.; Byelashov, O.A.; Paik, H.; Kim, K.; Sofos, J.N. Thermotolerance of Rifampicin-Resistant Escherichia coli O 157: H 7 Derivatives and Their Parental Strains in a Broth System. J. Food Saf. 2016, 36, 3–10. [Google Scholar] [CrossRef]
- Akhtar, M.; Maserati, A.; Diez-Gonzalez, F.; Sampedro, F. Does Antibiotic Resistance Influence Shiga-Toxigenic Escherichia coli O26 and O103 Survival to Stress Environments? Food Control 2016, 68, 330–336. [Google Scholar] [CrossRef]
- Michino, H.; Araki, K.; Minami, S.; Takaya, S.; Sakai, N.; Miyazaki, M.; Ono, A.; Yanagawa, H. Massive Outbreak of Escherichia coli 0157:H7 Infection in Schoolchildren in Sakai City, Japan, Associated with Consumption of White Radish Sprouts. Am. J. Epidemiol. 1999, 150, 787–796. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Abram, F.; Brennan, F.; Gaffney, M.; Burgess, C.M. Survival of Escherichia coli and Listeria Innocua on Lettuce after Irrigation with Contaminated Water in a Temperate Climate. Foods 2021, 10, 2072. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, D.; Owade, J.O.; Kamphuis, C.J.; Evans, A.; Rump, E.S.; Catur, C.; Mitchell, J.; Bergholz, T.M. Dynamics of Physiological Changes of Shiga Toxin-Producing Escherichia coli O157:H7 on Romaine Lettuce During Pre-Processing Cold Storage, and Subsequent Effects on Virulence and Stress Tolerance. Appl. Microbiol. 2025, 5, 45. https://doi.org/10.3390/applmicrobiol5020045
Sharma D, Owade JO, Kamphuis CJ, Evans A, Rump ES, Catur C, Mitchell J, Bergholz TM. Dynamics of Physiological Changes of Shiga Toxin-Producing Escherichia coli O157:H7 on Romaine Lettuce During Pre-Processing Cold Storage, and Subsequent Effects on Virulence and Stress Tolerance. Applied Microbiology. 2025; 5(2):45. https://doi.org/10.3390/applmicrobiol5020045
Chicago/Turabian StyleSharma, Dimple, Joshua O. Owade, Corrine J. Kamphuis, Avery Evans, E. Shaney Rump, Cleary Catur, Jade Mitchell, and Teresa M. Bergholz. 2025. "Dynamics of Physiological Changes of Shiga Toxin-Producing Escherichia coli O157:H7 on Romaine Lettuce During Pre-Processing Cold Storage, and Subsequent Effects on Virulence and Stress Tolerance" Applied Microbiology 5, no. 2: 45. https://doi.org/10.3390/applmicrobiol5020045
APA StyleSharma, D., Owade, J. O., Kamphuis, C. J., Evans, A., Rump, E. S., Catur, C., Mitchell, J., & Bergholz, T. M. (2025). Dynamics of Physiological Changes of Shiga Toxin-Producing Escherichia coli O157:H7 on Romaine Lettuce During Pre-Processing Cold Storage, and Subsequent Effects on Virulence and Stress Tolerance. Applied Microbiology, 5(2), 45. https://doi.org/10.3390/applmicrobiol5020045






