Inactivation Kinetics of Escherichia coli and Staphylococcus aureus Using Ultrasound in a Model Parenteral Emulsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Media and Chemicals
2.2. Emulsion Preparation
2.3. Bacterial Inoculation
2.4. Ultrasound (US) Treatments
2.5. Effect of Temperature
2.6. Synergistic Effect of US and Temperature
2.7. Inactivation Kinetics Modeling
2.7.1. Linear Model
2.7.2. Weibull Model
2.7.3. Model Evaluation
2.8. Statistical Analysis
3. Results and Discussion
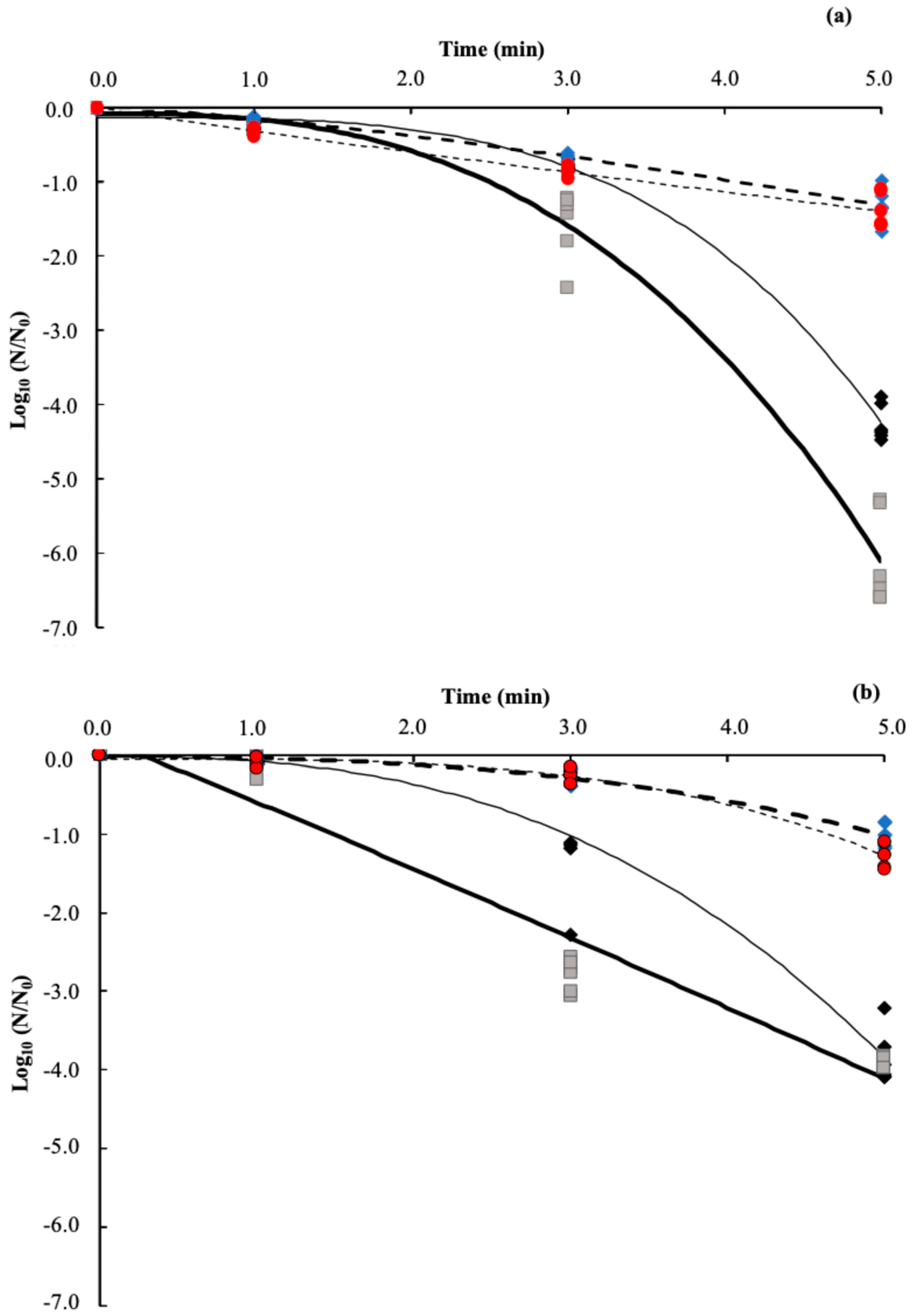
3.1. Inactivation of the Microorganisms by the US
3.2. Effect of Temperature
3.3. Linear Model
3.4. Non-Linear Weibull Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tota, A.; Serra, A.; Raoul, P.; Gasbarrini, A.; Rinninella, E.; Mele, M.C. Lipid-Enriched Parenteral Nutrition and Bloodstream Infections in Hospitalized Patients: Is It a Real Concern? Medicina 2022, 58, 885. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.D.; Hand, K.S.; Elia, M. Systematic Review and Meta-Analyses of the Effect of Lipid Emulsion on Microbial Growth in Parenteral Nutrition. J. Hosp. Infect. 2016, 94, 307–319. [Google Scholar] [CrossRef]
- Almaduri, S.; Septiani, A.M.; Pujilestari, D.; Wiyatami, M. Fungal Contamination in Lipid Emulsions of Parenteral Nutrition: A Review. J. Indones. Sehat Healthy Indones. J. 2022, 1, 1–6. [Google Scholar]
- Dao, H.; Lakhani, P.; Police, A.; Kallakunta, V.; Ajjarapu, S.S.; Wu, K.W.; Ponkshe, P.; Repka, M.A.; Narasimha Murthy, S. Microbial Stability of Pharmaceutical and Cosmetic Products. AAPS PharmSciTech 2018, 19, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Nyuydze, C.; Martínez-Monteagudo, S.I. Role of Soy Lecithin on Emulsion Stability of Dairy Beverages Treated by Ultrasound. Int. J. Dairy Technol. 2021, 74, 84–94. [Google Scholar] [CrossRef]
- Bao, G.; Niu, J.; Li, S.; Zhang, L.; Luo, Y. Effects of Ultrasound Pretreatment on the Quality, Nutrients and Volatile Compounds of Dry-Cured Yak Meat. Ultrason. Sonochem. 2022, 82, 105864. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. High-Pressure Homogenization: Principles and Applications beyond Microbial Inactivation. Food Eng. Rev. 2021, 13, 490–508. [Google Scholar] [CrossRef]
- Salleh-Mack, S.Z.; Roberts, J.S. Ultrasound Pasteurization: The Effects of Temperature, Soluble Solids, Organic Acids and PH on the Inactivation of Escherichia Coli ATCC 25922. Ultrason. Sonochem. 2007, 14, 323–329. [Google Scholar] [CrossRef]
- Huu, C.N.; Rai, R.; Yang, X.; Tikekar, R.V.; Nitin, N. Synergistic Inactivation of Bacteria Based on a Combination of Low Frequency, Low-Intensity Ultrasound and a Food Grade Antioxidant. Ultrason. Sonochem. 2021, 74, 105567. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Suriya, E.; Muppidathi Keerthana, R.; Kamatchi, M.; Kumar, N.M.; Anbarasan, T.; Jeyanthi, J. Ultrasound Aided Extraction of Yellow Pigment from Tecoma Castanifolia Floral Petals: Optimization by Response Surface Method and Evaluation of the Antioxidant Activity. Ind. Crop. Prod. 2019, 130, 467–477. [Google Scholar] [CrossRef]
- Costello, K.M.; Velliou, E.; Gutierrez-Merino, J.; Smet, C.; El Kadri, H.; Impe, J.F.V.; Bussemaker, M. The Effect of Ultrasound Treatment in Combination with Nisin on the Inactivation of Listeria Innocua and Escherichia Coli. Ultrason. Sonochem. 2021, 79, 105776. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, M.; Pandur, Ž.; Stepišnik Perdih, T.; Stopar, D.; Petkovšek, M.; Dular, M. Effects of Cavitation on Different Microorganisms: The Current Understanding of the Mechanisms Taking Place Behind the Phenomenon. A Review and Proposals for Further Research. Ultrason. Sonochem. 2019, 57, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Roohi, R.; Hashemi, S.M.B.; Zarrinpour Balaei, M.R. Synergist Effect of Thermosonication and NaCl on Inactivation of Staphylococcus Aureus and Shigella Flexneri in Lettuce: The Effect of Acoustic Field and Reaction Kinetics. Ultrason. Sonochem. 2025, 112, 107161. [Google Scholar] [CrossRef]
- Ninomiya, K.; Hosoi, H. Effect of Ultrafine Bubbles on Ultrasound-Induced Microbial Inactivation. Chem. Eng. Sci. 2025, 306, 121233. [Google Scholar] [CrossRef]
- Mustapha, A.T.; Wahia, H.; Ji, Q.; Fakayode, O.A.; Zhang, L.; Zhou, C. Multiple-Frequency Ultrasound for the Inactivation of Microorganisms on Food: A Review. J. Food Process. Eng. 2024, 47, e14587. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ascanio, G.; Calderón-Santoyo, M.; Brito-Bazán, E.; Brito-de la Fuente, E.; Ragazzo-Sánchez, J.A. Modeling the Inactivation of Aspergillus Niger Spores in a Model Parenteral Emulsion by High Hydrostatic Pressure and Its Effect on the Emulsion Droplet Size. High Press. Res. 2023, 43, 106–120. [Google Scholar] [CrossRef]
- Bulut, S.; Karatzas, K.A.G. Inactivation of Escherichia Coli K12 in Phosphate Buffer Saline and Orange Juice by High Hydrostatic Pressure Processing Combined with Freezing. LWT Food Sci. Technol. 2021, 136, 110313. [Google Scholar] [CrossRef]
- He, Q.; Liu, D.; Ashokkumar, M.; Ye, X.; Jin, T.Z.; Guo, M. Antibacterial Mechanism of Ultrasound against Escherichia Coli: Alterations in Membrane Microstructures and Properties. Ultrason. Sonochem. 2021, 73, 105509. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sheen, S.; Sommers, C.; Sheen, L.Y. Modeling the Survival of Escherichia Coli O157:H7 under Hydrostatic Pressure, Process Temperature, Time and Allyl Isothiocyanate Stresses in Ground Chicken Meat. Front. Microbiol. 2018, 14, 1871. [Google Scholar] [CrossRef]
- Chuang, S.; Sheen, S.; Sommers, C.H.; Sheen, L.Y. Modeling the Effect of Simultaneous Use of Allyl Isothiocyanate and Cinnamaldehyde on High Hydrostatic Pressure Inactivation of Uropathogenic and Shiga Toxin-Producing Escherichia Coli in Ground Chicken. J. Sci. Food Agric. 2021, 101, 1193–1201. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impe, J.F. GInaFiT, a Freeware Tool to Assess Non-Log-Linear Microbial Survivor Curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On Calculating Sterility in Thermal Preservation Methods: Application of the Weibull Frequency Distribution Model. Acta Hortic. 2001, 566, 107–114. [Google Scholar] [CrossRef]
- van Boekel, M.A.J.S. On the Use of the Weibull Model to Describe Thermal Inactivation of Microbial Vegetative Cells. Int. J. Food Microbiol. 2002, 74, 139–159. [Google Scholar] [CrossRef] [PubMed]
- John, D.; Ramaswamy, H.S. Comparison of Pulsed Light Inactivation Kinetics and Modeling of Escherichia Coli (ATCC-29055), Clostridium Sporogenes (ATCC-7955) and Geobacillus Stearothermophilus (ATCC-10149). Curr. Res. Food Sci. 2020, 3, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Kaavya, R.; Pandiselvam, R.; Abdullah, S.; Sruthi, N.U.; Jayanath, Y.; Ashokkumar, C.; Chandra Khanashyam, A.; Kothakota, A.; Ramesh, S.V. Emerging Non-Thermal Technologies for Decontamination of Salmonella in Food. Trends Food Sci. Technol. 2021, 112, 400–418. [Google Scholar] [CrossRef]
- Nehring, P.; Lorenzo, J.M.; Santos, S.P.; Wagner, R.; de Menezes, C.R.; dos Santos, B.A.; Barin, J.S.; Campagnol, P.C.B.; Cichoski, A.J. Effect of Ultrasound Application on the Growth of S. Xylosus Inoculated in by-Products from the Poultry Industry. Curr. Res. Food Sci. 2022, 5, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Evaluation of Ultrasoundinduced Damage to Escherichia Coli and Staphylococcus Aureus by Flow Cytometry and Transmission Electron Microscopy. Appl. Environ. Microbiol. 2016, 82, 1828–1837. [Google Scholar] [CrossRef]
- Yang, R.; Guan, J.; Sun, S.; Sablani, S.S.; Tang, J. Understanding Water Activity Change in Oil with Temperature. Curr. Res. Food Sci. 2020, 3, 158–165. [Google Scholar] [CrossRef]
- National Advisory Committee on Microbiological Criteria for Foods. Parameters for Determining Inoculated Pack/Challenge. J. Food Prot. 2010, 73, 140–202. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Calderón-Santoyo, M.; Ascanio, G.; Ragazzo-Calderón, F.Z.; Parra-Saldívar, R.; Ragazzo-Sánchez, J.A. Harnessing Emerging Technologies to Obtain Biopolymer from Agro-Waste: Application into the Food Industry. Biomass Convers. Biorefinery 2023, 14, 29265–29282. [Google Scholar] [CrossRef]
- Titikshya, S.; Sahoo, M.; Kumar, V.; Naik, S.N. Microbial Inactivation with Heat Treatments. In Thermal Food Engineering Operations; Wiley-Scrivener: Hoboken, NJ, USA, 2022; pp. 45–74. [Google Scholar]
- Stringer, S.C.; George, S.M.; Peck, M.W. Thermal Inactivation of Escherichia Coli O157:H7. J. Appl. Microbiol. Symp. Suppl. 2000, 88, 79S–89S. [Google Scholar] [CrossRef]
- Kennedy, J.; Blair, I.S.; McDowell, D.A.; Bolton, D.J. An Investigation of the Thermal Inactivation of Staphylococcus Aureus and the Potential for Increased Thermotolerance as a Result of Chilled Storage. J. Appl. Microbiol. 2005, 99, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tikekar, R.V.; Ding, Q.; Gilbert, A.R.; Wimsatt, S.T. Inactivation of Foodborne Pathogens by the Synergistic Combinations of Food Processing Technologies and Food-Grade Compounds. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2110–2138. [Google Scholar] [CrossRef] [PubMed]
- Nikparvar, B.; Subires, A.; Capellas, M.; Hernandez-Herrero, M.; Crauwels, P.; Riedel, C.U.; Bar, N. A Diffusion Model to Quantify Membrane Repair Process in Listeria Monocytogenes Exposed to High Pressure Processing Based on Fluorescence Microscopy Data. Front. Microbiol. 2021, 12, 598739. [Google Scholar] [CrossRef]
- Benjamin, K.N.; Goyal, A.; Nair, R.V.; Endy, D. Genome-Wide Transcription Response of Staphylococcus Epidermidis to Heat Shock and Medically Relevant Glucose Levels. Front. Microbiol. 2024, 15, 1408796. [Google Scholar] [CrossRef]
- Gomez-Gomez, A.; Brito-de la Fuente, E.; Gallegos, C.; Garcia-Perez, J.V.; Quiles, A.; Benedito, J. Microbial Inactivation by Means of Ultrasonic Assisted Supercritical CO2. Effect on Cell Ultrastructure. J. Supercrit. Fluids 2022, 179, 105407. [Google Scholar] [CrossRef]
- Gomez-Gomez, A.; Brito-de la Fuente, E.; Gallegos, C.; Garcia-Perez, J.V.; Benedito, J. Non-Thermal Pasteurization of Lipid Emulsions by Combined Supercritical Carbon Dioxide and High-Power Ultrasound Treatment. Ultrason. Sonochem. 2020, 67, 105138. [Google Scholar] [CrossRef]
- Tola, Y.B.; Ramaswamy, H.S. Combined Effects of High Pressure, Moderate Heat and PH on the Inactivation Kinetics of Bacillus Licheniformis Spores in Carrot Juice. Food Res. Int. 2014, 62, 50–58. [Google Scholar] [CrossRef]
- Ngnitcho, P.F.K.; Tango, C.N.; Khan, I.; Daliri, E.B.M.; Chellian, R.; Oh, D.H. The Applicability of Weibull Model for the Kinetics Inactivation of Listeria Monocytogenes and Escherichia coli O157: H7 on Soybean Sprouts Submitted to Chemical Sanitizers in Combination with Ultrasound at Mild Temperatures. LWT 2018, 91, 573–579. [Google Scholar] [CrossRef]


| Media | Temperature Condition | Temperature (°C) | ||
|---|---|---|---|---|
| US Treatment Time (min) | ||||
| 1 | 3 | 5 | ||
| Saline solution | WC | 37.5 ± 2.9 Ac | 55.9 ± 2.8 Ab | 69.0 ± 5.7 Aa |
| C | 23.8 ± 1.1 Bb | 27.0 ± 1.4 Ba | 27.8 ± 0.4 Ba | |
| Emulsion | WC | 37.5 ± 1.9 Ac | 56.3 ± 1.9 Ab | 75.3 ± 5.6 Aa |
| C | 25.0 ± 0.7 Bb | 27.8 ± 0.4 Ba | 28.0 ± 1.4 Ba | |
| Microorganism | Media | Temperature (°C) | Time (min) | Reduction (Log10 CFU/mL) | Synergistic Effect (Log10 CFU/mL) |
|---|---|---|---|---|---|
| Escherichia coli | Saline solution | 37.5 ± 2.0 | 1 | - | - |
| 25.0 ± 0.7 | - | - | |||
| Emulsion | 37.5 ± 2.0 | - | - | ||
| 25.0 ± 0.7 | - | - | |||
| Staphylococcus aureus | Saline solution | 37.5 ± 2.0 | - | - | |
| 25.0 ± 0.7 | - | - | |||
| Emulsion | 37.5 ± 2.0 | - | - | ||
| 25.0 ± 0.7 | - | - | |||
| E. coli | Saline solution | 55.5 ± 2.0 | 3 | 0.10 ± 0.05 d | 0.03 ± 0.01 f |
| 27.0 ± 1.5 | - | - | |||
| Emulsion | 55.5 ± 2.0 | 0.33 ± 0.08 *bc | 0.21 ± 0.08 e | ||
| 27.0 ± 1.5 | - | - | |||
| S. aureus | Saline solution | 55.5 ± 2.0 | 0.57 ± 0.10 *c | 0.12 ± 0.04 e | |
| 27.0 ± 1.5 | - | - | |||
| Emulsion | 55.5 ± 2.0 | 0.86 ± 0.12 *b | 0.51 ± 0.13 d | ||
| 27.0 ± 1.5 | - | - | |||
| E. coli | Saline solution | 69.0 ± 2.5 | 5 | 2.28 ± 0.12 *a | 0.66 ± 0.19 cd |
| 27.0 ± 1.5 | - | - | |||
| Emulsion | 75.5 ± 1.5 | 2.93 ± 0.18 *a | 1.77 ± 0.23 a | ||
| 28.0 ± 1.5 | - | - | |||
| S. aureus | Saline solution | 69.0 ± 2.5 | 2.02 ± 0.32 *a | 0.82 ± 0.07 c | |
| 27.0 ± 1.5 | - | - | |||
| Emulsion | 75.5 ± 1.5 | 1.62 ± 0.58 *a | 1.03 ± 0.15 b | ||
| 28.0 ± 1.5 | - | - |
| Microorganism | Media | Temperature Condition | Linear Model | Weibull Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Equation | D-Value (min) | R2 | RMSE | α (min−β) | β | td (min) | R2 | RMSE | |||
| Escherichia coli | Saline solution | WC | 1.47 ± 0.07 f | 0.8670 | 0.7705 | 3.37 ± 0.09 d* | 3.58 ± 0.24 | 5.28 | 0.9609 | 0.9924 | |
| C | 4.04 ± 0.23 c | 0.9681 | 0.3060 | 4.07 ± 0.19 c | 1.36 ± 0.17 | 13.29 | 0.9575 | 0.1166 | |||
| Emulsion | WC | 1.00 ± 0.19 g | 0.8813 | 0.7758 | 2.58 ± 0.17 f* | 2.71 ± 0.26 | 4.67 | 0.9781 | 0.3969 | ||
| C | 3.53 ± 0.30 d | 0.9814 | 0.2804 | 3.49 ± 0.26 de | 0.92 ± 0.11 | 20.07 | 0.9543 | 0.1236 | |||
| Staphylococcus aureus | Saline solution | WC | 1.56 ± 0.23 f | 0.8711 | 0.7455 | 2.99 ± 0.25 ef* | 2.62 ± 0.40 | 5.53 | 0.9423 | 0.4125 | |
| C | 5.87 ± 0.13 a | 0.8965 | 0.3565 | 5.02 ± 0.09 a* | 2.56 ± 0.33 | 9.41 | 0.9575 | 0.0908 | |||
| Emulsion | WC | 1.25 ± 0.25 fg | 0.9705 | 0.5815 | 1.25 ± 0.23 g | 1.05 ± 0.13 | 5.79 | 0.9496 | 0.4982 | ||
| C | 4.84 ± 0.09 b | 0.8677 | 0.4234 | 4.70 ± 0.07 b | 3.36 ± 0.42 | 7.59 | 0.9694 | 0.0963 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iñiguez-Moreno, M.; Calderón-Santoyo, M.; Ascanio, G.; Brito-Bazán, E.; Córdova-Aguilar, M.S.; Brito-de la Fuente, E.; Ragazzo-Sánchez, J.A. Inactivation Kinetics of Escherichia coli and Staphylococcus aureus Using Ultrasound in a Model Parenteral Emulsion. Appl. Microbiol. 2025, 5, 34. https://doi.org/10.3390/applmicrobiol5010034
Iñiguez-Moreno M, Calderón-Santoyo M, Ascanio G, Brito-Bazán E, Córdova-Aguilar MS, Brito-de la Fuente E, Ragazzo-Sánchez JA. Inactivation Kinetics of Escherichia coli and Staphylococcus aureus Using Ultrasound in a Model Parenteral Emulsion. Applied Microbiology. 2025; 5(1):34. https://doi.org/10.3390/applmicrobiol5010034
Chicago/Turabian StyleIñiguez-Moreno, Maricarmen, Montserrat Calderón-Santoyo, Gabriel Ascanio, Estefanía Brito-Bazán, María Soledad Córdova-Aguilar, Edmundo Brito-de la Fuente, and Juan Arturo Ragazzo-Sánchez. 2025. "Inactivation Kinetics of Escherichia coli and Staphylococcus aureus Using Ultrasound in a Model Parenteral Emulsion" Applied Microbiology 5, no. 1: 34. https://doi.org/10.3390/applmicrobiol5010034
APA StyleIñiguez-Moreno, M., Calderón-Santoyo, M., Ascanio, G., Brito-Bazán, E., Córdova-Aguilar, M. S., Brito-de la Fuente, E., & Ragazzo-Sánchez, J. A. (2025). Inactivation Kinetics of Escherichia coli and Staphylococcus aureus Using Ultrasound in a Model Parenteral Emulsion. Applied Microbiology, 5(1), 34. https://doi.org/10.3390/applmicrobiol5010034









