Plant Growth-Promoting Microorganisms as Biocontrol Agents: Mechanisms, Challenges, and Future Prospects
Abstract
1. Introduction
2. Study Selection Criteria
3. Plant Growth-Promoting Rhizobacteria (PGPR), Filamentous Fungi, Yeasts, and Rhizobacteria as Promising Biocontrol Agents
3.1. Plant Growth-Promoting Rhizobacteria (PGPR)
3.2. Fungi as Biocontrol Agents
3.3. Yeasts as Biocontrol Agents
| Yeast Species | Plant-Beneficial Effect(s) | Reference |
|---|---|---|
| Saccharomyces cerevisiae | Auxin production, biofilm formation, antifungal metabolite production | [19] |
| Candida oleophila | Nutrient competition, secretion of chitinases and glucanases, colonization of plant surfaces | [55] |
| Metschnikowia fructicola | VOC production, inhibition of Botrytis cinerea, postharvest disease control | [56] |
| Pichia anomala | Mycoparasitism, production of antifungal enzymes, enhancement of plant immunity | [57] |
3.4. Rhizobacteria and the Phytomicrobiome
4. Biocontrol Mechanisms of PGPMs
5. Challenges of Employing PGPR, Filamentous Fungi, Yeasts, and Rhizobacteria as Biocontrol Agents
5.1. Challenges of Employing Fungi
5.2. Challenges of Employing Yeasts
5.3. Challenges of Employing Rhizobacteria
5.4. Proposed Solutions and Future Directions
- Formulation Advancements: Encapsulation techniques, such as alginate beads and biochar-based carriers, can enhance microbial survival during storage and improve field persistence. These formulations provide protection from environmental stress and enable controlled release of microbes [74].
- Consortia Development: Employing microbial consortia instead of single strains can broaden functional range, improve colonization, and provide synergistic effects on plant growth and disease suppression. Combining bacteria with fungi or yeasts helps ensure performance across variable soil and crop conditions [73].
- Genomics and Omics Technologies: Whole-genome sequencing and transcriptomics allow for the identification of genes responsible for biocontrol and plant growth-promoting traits. These tools can guide the selection or genetic improvement of strains with enhanced adaptability and effectiveness [83].
- Precision Agriculture and Microbiome Engineering: Advances in precision agriculture enable targeted application of PGPMs based on soil health indicators and crop phenology. Additionally, microbiome engineering techniques, such as synthetic microbial communities, are being developed to shape plant-associated microbiota for improved outcomes [84].
- Regulatory Streamlining and Policy Support: Harmonizing global regulatory frameworks and recognizing microbial products under integrated pest and nutrient management policies would reduce commercialization barriers. Europe’s shift toward recognizing microbial biostimulants separately from PPPs marks a significant step, but broader implementation and clarity are required [77].
6. Rhizosphere Competence and Biocontrol Mechanisms
| Microorganism Group | Mechanisms of Antagonism | References |
|---|---|---|
| PGPR | Antibiosis: Producing antibiotics and secondary metabolites to inhibit pathogens. | [66] |
| Siderophore production: Depriving pathogens of iron through chelation. | [62] | |
| ISR: Activating jasmonic acid and ethylene signaling pathways in plants. | [67] | |
| Nutrient competition: Rapidly colonizing the rhizosphere to outcompete pathogens. | [93] | |
| Volatile organic compounds: Releasing antimicrobial and growth-promoting compounds. | [94] | |
| Filamentous Fungi | Mycoparasitism: Secreting enzymes like chitinases and glucanases to degrade pathogens. | [43] |
| Competition: Occupying space and resources to exclude pathogens. | [44] | |
| ISR: Triggering plant defenses via salicylic acid and jasmonic acid pathways. | [95] | |
| Antimicrobial compounds: Producing bioactive substances like gliotoxin to suppress pathogens. | [55] | |
| Nutrient enhancement: Solubilizing phosphorus and improving root nutrient uptake. | [38] | |
| Yeasts | Nutrient and space competition: Colonizing plant surfaces to block pathogens. | [56] |
| Antifungal metabolites: Producing enzymes like β-1,3-glucanase and organic acids. | [57] | |
| Induced host resistance: Enhancing plant immune responses to combat pathogens. | [69] | |
| Biofilm formation: Creating physical barriers on plant surfaces. | [19] | |
| Mycoparasitism: Occasionally degrading fungal pathogens directly. | [70] | |
| Rhizobacteria | Biofilm formation: Protecting plant roots and creating a competitive microenvironment. | [58] |
| Secretion of plant hormones: Enhancing root growth and overall plant health. | [61] | |
| Quorum-sensing interference: Disrupting pathogen communication to inhibit virulence. | [71] | |
| Toxin degradation: Neutralizing harmful substances produced by pathogens. | [72] | |
| Nutrient mobilization: Solubilizing phosphorus and enhancing nitrogen fixation. | [66] |
7. Integration of Rhizosphere Competence into Biocontrol Mechanisms
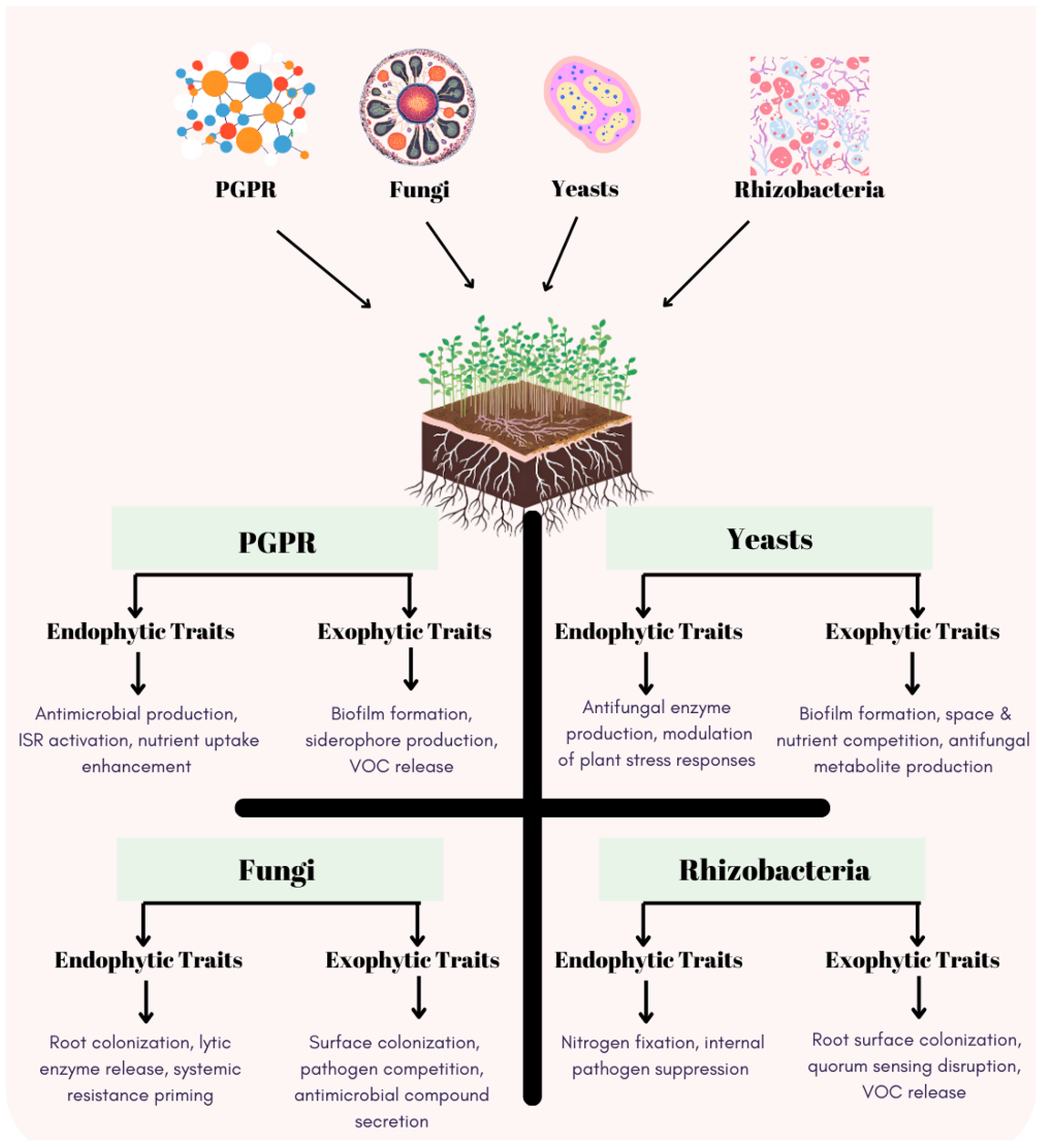
8. Endophytic and Exophytic Traits of Microorganisms in Biocontrol
9. Yeasts and Their Role in Plant Growth Promotion
10. Conclusions
11. Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adedibu, P.A. Ecological Problems of Agriculture: Impacts and Sustainable Solutions. Sci. Prepr. 2023. [Google Scholar] [CrossRef]
- Mitra, B.; Chowdhury, A.R.; Dey, P.; Hazra, K.K.; Sinha, A.K.; Hossain, A.; Meena, R.S. Use of Agrochemicals in Agriculture: Alarming Issues and Solutions. In Input Use Efficiency for Food and Environmental Security; Bhatt, R., Meena, R.S., Hossain, A., Eds.; Springer Nature Singapore: Singapore, 2021; pp. 85–122. ISBN 978-981-16-5198-4. [Google Scholar]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Abrol, D.P.; Shankar, U. Pesticides, Food Safety and Integrated Pest Management. In Integrated Pest Management; Pimentel, D., Peshin, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 167–199. ISBN 978-94-007-7795-8. [Google Scholar]
- Dhawan, A.K.; Peshin, R. Integrated Pest Management: Concept, Opportunities and Challenges. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 51–81. ISBN 978-1-4020-8991-6. [Google Scholar]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to Global Food Security from Emerging Fungal and Oomycete Crop Pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef]
- Koskey, G.; Mburu, S.W.; Awino, R.; Njeru, E.M.; Maingi, J.M. Potential Use of Beneficial Microorganisms for Soil Amelioration, Phytopathogen Biocontrol, and Sustainable Crop Production in Smallholder Agroecosystems. Front. Sustain. Food Syst. 2021, 5, 606308. [Google Scholar] [CrossRef]
- Dixit, R.; Kamat, S.; Srivastava, A.; Kumari, M. Molecular Basis of Plant-PGPM Interactions During Amelioration of Biotic Stress. In Microbial Biocontrol: Food Security and Post Harvest Management; Kumar, A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 129–165. ISBN 978-3-030-87288-5. [Google Scholar]
- Kour, D.; Kour, H.; Khan, S.S.; Khan, R.T.; Bhardwaj, M.; Kailoo, S.; Kumari, C.; Rasool, S.; Yadav, A.N.; Sharma, Y.P. Biodiversity and Functional Attributes of Rhizospheric Microbiomes: Potential Tools for Sustainable Agriculture. Curr. Microbiol. 2023, 80, 192. [Google Scholar] [CrossRef]
- Ayaz, M.; Li, C.-H.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Shafiq, M.; Ali, F.; Yu, X.-Y.; Yu, Q.; Zhao, J.-T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Panda, S.K.; Das, S. Potential of Plant Growth-Promoting Microbes for Improving Plant and Soil Health for Biotic and Abiotic Stress Management in Mangrove Vegetation. Rev. Environ. Sci. Biotechnol. 2024, 23, 801–837. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Babalola, O.O. Fungi That Promote Plant Growth in the Rhizosphere Boost Crop Growth. J. Fungi 2023, 9, 239. [Google Scholar] [CrossRef]
- Chaffai, R.; Ganesan, M.; Cherif, A. Plant Growth-Promoting Rhizobacteria (PGPR) and Plant Growth-Promoting Fungi (PGPF) for Alleviating Abiotic Stress in Plants. In Plant Adaptation to Abiotic Stress: From Signaling Pathways and Microbiomes to Molecular Mechanisms; Springer Nature: Singapore, 2024; pp. 457–496. ISBN 978-981-97-0671-6. [Google Scholar]
- Harun-Or-Rashid, M.; Khan, A.; Hossain, M.T.; Chung, Y.R. Induction of Systemic Resistance against Aphids by Endophytic Bacillus velezensis YC7010 via Expressing PHYTOALEXIN DEFICIENT4 in Arabidopsis. Front. Plant Sci. 2017, 8, 211. [Google Scholar] [CrossRef]
- Tarroum, M.; Romdhane, W.B.; Al-Qurainy, F.; Ali, A.A.M.; Al-Doss, A.; Fki, L.; Hassairi, A. A Novel PGPF Penicillium olsonii Isolated from the Rhizosphere of Aeluropus littoralis Promotes Plant Growth, Enhances Salt Stress Tolerance, and Reduces Chemical Fertilizers Inputs in Hydroponic System. Front. Microbiol. 2022, 13, 996054. [Google Scholar] [CrossRef]
- Botha, A. The Importance and Ecology of Yeasts in Soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Sivasithamparam, K. Potential of Yeasts as Biocontrol Agents of Soil-Borne Fungal Plant Pathogens and as Plant Growth Promoters. Mycoscience 2006, 47, 25–35. [Google Scholar] [CrossRef]
- Kapoor, D.; Karnwal, A. Yeast as Plant Growth Promoter and Biocontrol Agent. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 429–457. ISBN 978-0-12-821394-0. [Google Scholar]
- Mukherjee, A.; Verma, J.P.; Gaurav, A.K.; Chouhan, G.K.; Patel, J.S.; Hesham, A.E.-L. Yeast a Potential Bio-Agent: Future for Plant Growth and Postharvest Disease Management for Sustainable Agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 1497–1510. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal Evolution: Major Ecological Adaptations and Evolutionary Transitions. Biol. Rev. 2019, 94, 1443–1476. [Google Scholar] [CrossRef]
- Yarzábal Rodríguez, L.A.; Álvarez Gutiérrez, P.E.; Gunde-Cimerman, N.; Ciancas Jiménez, J.C.; Gutiérrez-Cepeda, A.; Ocaña, A.M.F.; Batista-García, R.A. Exploring Extremophilic Fungi in Soil Mycobiome for Sustainable Agriculture amid Global Change. Nat. Commun. 2024, 15, 6951. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Hariprasad, P.; Nayak, S.C.; Niranjana, S.R. Application of Rhizobacteria Antagonistic to Fusarium oxysporum f. sp. lycopersici for the Management of Fusarium Wilt in Tomato. Rhizosphere 2016, 2, 72–74. [Google Scholar] [CrossRef]
- Afrouz, M.; Sayyed, R.Z.; Fazeli-Nasab, B.; Piri, R.; Almalki, W.; Fitriatin, B.N. Seed Bio-Priming with Beneficial Trichoderma Harzianum Alleviates Cold Stress in Maize. PeerJ 2023, 11, e15644. [Google Scholar] [CrossRef]
- Nunes, C.A. Biological Control of Postharvest Diseases of Fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major Biological Control Strategies for Plant Pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef]
- Huang, X.; Liu, X.; Li, Z. Bile Acids and Coronavirus Disease 2019. Acta Pharm. Sin. B 2024, 14, 1939–1950. [Google Scholar] [CrossRef]
- Arora, N.K.; Tewari, S.; Singh, R. Multifaceted Plant-Associated Microbes and Their Mechanisms Diminish the Concept of Direct and Indirect PGPRs. In Plant Microbe Symbiosis: Fundamentals and Advances; Arora, N.K., Ed.; Springer: New Delhi, India, 2013; pp. 411–449. ISBN 978-81-322-1286-7. [Google Scholar]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying Mechanics of Plant Growth Promoting Rhizobacteria (PGPR): A Review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Nelson, L.M. Plant Growth Promoting Rhizobacteria (PGPR): Prospects for New Inoculants. Crop Manag. 2004, 3, 1–7. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Rhizosphere Colonization Determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Plant Growth-Promoting Rhizobacteria (PGPR) and Their Action Mechanisms in Availability of Nutrients to Plants. In Phyto-Microbiome in Stress Regulation; Kumar, M., Kumar, V., Prasad, R., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2020; pp. 147–203. ISBN 978-981-15-2575-9. [Google Scholar]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR Mediated Alterations in Root Traits: Way Toward Sustainable Crop Production. Front. Sustain. Food Syst. 2021, 4, 618230. [Google Scholar] [CrossRef]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in Plant Growth and Development: Roles in Abiotic Stress Tolerance and Secondary Metabolites Secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef]
- Lyu, D.; Backer, R.; Smith, D. Plant Growth-Promoting Rhizobacteria (PGPR) as Plant Biostimulants in Agriculture. In Biostimulants for Sustainable Crop Production; Burleigh Dodds Science Publishing: Sawston, UK, 2020; ISBN 978-1-003-04786-5. [Google Scholar]
- Naz, R.; Khushhal, S.; Asif, T.; Mubeen, S.; Saranraj, P.; Sayyed, R.Z. Inhibition of Bacterial and Fungal Phytopathogens Through Volatile Organic Compounds Produced by Pseudomonas sp. In Secondary Metabolites and Volatiles of PGPR in Plant-Growth Promotion; Sayyed, R.Z., Uarrota, V.G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 95–118. ISBN 978-3-031-07558-2. [Google Scholar]
- Saraf, M.; Pandya, U.; Thakkar, A. Role of Allelochemicals in Plant Growth Promoting Rhizobacteria for Biocontrol of Phytopathogens. Microbiol. Res. 2014, 169, 18–29. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen Biocontrol Using Plant Growth-Promoting Bacteria (PGPR): Role of Bacterial Diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Rabinal, C.; Bhat, S. Identification of Differentially Expressed Genes in Trichoderma koningii IABT1252 During Its Interaction with Sclerotium rolfsii. Curr. Microbiol. 2020, 77, 396–404. [Google Scholar] [CrossRef]
- Hossain, M.d.M.; Sultana, F.; Islam, S. Plant Growth-Promoting Fungi (PGPF): Phytostimulation and Induced Systemic Resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 135–191. ISBN 978-981-10-6592-7. [Google Scholar]
- Hernández-Fernández, M.; Cordero-Bueso, G.; Ruiz-Muñoz, M.; Cantoral, J.M. Culturable Yeasts as Biofertilizers and Biopesticides for a Sustainable Agriculture: A Comprehensive Review. Plants 2021, 10, 822. [Google Scholar] [CrossRef]
- Kowalska, J.; Krzymińska, J.; Tyburski, J. Yeasts as a Potential Biological Agent in Plant Disease Protection and Yield Improvement—A Short Review. Agriculture 2022, 12, 1404. [Google Scholar] [CrossRef]
- Reichling, J. Plant-Microbe Interactions and Secondary Metabolites with Antibacterial, Antifungal and Antiviral Properties. In Functions and Biotechnology of Plant Secondary Metabolites; Wink, M., Ed.; Wiley-Blackwell: Oxford, UK, 2010; pp. 214–347. ISBN 978-1-4443-1887-6. [Google Scholar]
- Azcón, R.; Medina, A.; Aroca, R.; Ruiz-Lozano, J.M. Abiotic Stress Remediation by the Arbuscular Mycorrhizal Symbiosis and Rhizosphere Bacteria/Yeast Interactions. In Molecular Microbial Ecology of the Rhizosphere; De Bruijn, F.J., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 991–1002. ISBN 978-1-118-29617-2. [Google Scholar]
- Ge, J.; Li, D.; Ding, J.; Xiao, X.; Liang, Y. Microbial Coexistence in the Rhizosphere and the Promotion of Plant Stress Resistance: A Review. Environ. Res. 2023, 222, 115298. [Google Scholar] [CrossRef]
- Vidal, C.; González, F.; Santander, C.; Pérez, R.; Gallardo, V.; Santos, C.; Aponte, H.; Ruiz, A.; Cornejo, P. Management of Rhizosphere Microbiota and Plant Production under Drought Stress: A Comprehensive Review. Plants 2022, 11, 2437. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: Utilization of Antagonistic Yeasts to Manage Postharvest Fungal Diseases of Fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef]
- Sui, Y.; Wisniewski, M.; Droby, S.; Liu, J. Responses of Yeast Biocontrol Agents to Environmental Stress. Appl. Environ. Microbiol. 2015, 81, 2968–2975. [Google Scholar] [CrossRef]
- Villa, F.; Cappitelli, F.; Cortesi, P.; Kunova, A. Fungal Biofilms: Targets for the Development of Novel Strategies in Plant Disease Management. Front. Microbiol. 2017, 8, 654. [Google Scholar] [CrossRef]
- Glick, B.R. The Enhancement of Plant Growth by Free-Living Bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Mercier, J.; Wilson, C.L. Colonization of Apple Wounds by Naturally Occurring Microflora and Introduced Candida Oleophila and Their Effect on Infection by Botrytis Cinerea during Storage. Biol. Control. 1994, 4, 138–144. [Google Scholar] [CrossRef]
- Castoria, R.; De Curtis, F.; Lima, G.; Caputo, L.; Pacifico, S.; De Cicco, V. Aureobasidium Pullulans (LS-30) an Antagonist of Postharvest Pathogens of Fruits: Study on Its Modes of Action. Postharvest Biol. Technol. 2001, 22, 7–17. [Google Scholar] [CrossRef]
- Antoun, H. Plant-Growth-Promoting Rhizobacteria. In Brenner’s Encyclopedia of Genetics; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Rosier, A.; Medeiros, F.H.V.; Bais, H.P. Defining Plant Growth Promoting Rhizobacteria Molecular and Biochemical Networks in Beneficial Plant-Microbe Interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The Role of Plant Growth-Promoting Rhizobacteria (PGPR) in Mitigating Plant’s Environmental Stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of Plant Growth Promoting Rhizobacteria for Sustainable Development in Agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Xiong, H.; Kakei, Y.; Kobayashi, T.; Guo, X.; Nakazono, M.; Takahashi, H.; Nakanishi, H.; Shen, H.; Zhang, F.; Nishizawa, N.K.; et al. Molecular Evidence for Phytosiderophore-Induced Improvement of Iron Nutrition of Peanut Intercropped with Maize in Calcareous Soil. Plant Cell Environ. 2013, 36, 1888–1902. [Google Scholar] [CrossRef]
- Al Raish, S.M.; Saeed, E.E.; Alyafei, D.M.; El-Tarabily, K.A.; AbuQamar, S.F. Evaluation of Streptomycete Actinobacterial Isolates as Biocontrol Agents against Royal Poinciana Stem Canker Disease Caused by the Fungal Pathogen Neoscytalidium Dimidiatum. Biol. Control. 2021, 164, 104783. [Google Scholar] [CrossRef]
- Al Hamad, B.M.; Al Raish, S.M.; Ramadan, G.A.; Saeed, E.E.; Alameri, S.S.A.; Al Senaani, S.S.; AbuQamar, S.F.; El-Tarabily, K.A. Effectiveness of Augmentative Biological Control of Streptomyces Griseorubens UAE2 Depends on 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Activity against Neoscytalidium Dimidiatum. J. Fungi 2021, 7, 885. [Google Scholar] [CrossRef]
- Al Raish, S.M.; Saeed, E.E.; Sham, A.; Alblooshi, K.; El-Tarabily, K.A.; AbuQamar, S.F. Molecular Characterization and Disease Control of Stem Canker on Royal Poinciana (Delonix regia) Caused by Neoscytalidium dimidiatum in the United Arab Emirates. Int. J. Mol. Sci. 2020, 21, 1033. [Google Scholar] [CrossRef]
- Radzki, W.; Gutierrez Mañero, F.J.; Algar, E.; Lucas García, J.A.; García-Villaraco, A.; Ramos Solano, B. Bacterial Siderophores Efficiently Provide Iron to Iron-Starved Tomato Plants in Hydroponics Culture. Antonie Van Leeuwenhoek 2013, 104, 321–330. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Li, C.; Ma, Y. Antifungal and Plant Growth Promotion Activity of Volatile Organic Compounds Produced by Bacillus amyloliquefaciens. MicrobiologyOpen 2019, 8, e00813. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty Years of Postharvest Biocontrol Research: Is It Time for a New Paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Korsten, L. Biological Control of Postharvest Diseases of Fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and Plants—With Special Reference to Induced Systemic Resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Nazari, M.; Smith, D.L. A PGPR-Produced Bacteriocin for Sustainable Agriculture: A Review of Thuricin 17 Characteristics and Applications. Front. Plant Sci. 2020, 11, 1619–1630. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Dubey, S.; Sharma, S. “Next-Generation Bioformulations” for Plant Growth Promotion and Stress Mitigation: A Promising Approach for Sustainable Agriculture. J. Plant Growth Regul. 2023, 42, 6741–6759. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.-P. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. 2009, Volume 309. Available online: http://data.europa.eu/eli/reg/2009/1107/oj (accessed on 1 January 2025).
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance). 2019, Volume 170. Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 1 January 2025).
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; van Lenteren, J.C. The Status of Biological Control and Recommendations for Improving Uptake for the Future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, P.P.; Singh, S.K.; Verma, H. 5—Sustainable Agriculture and Benefits of Organic Farming to Special Emphasis on PGPR. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Kumar, A., Singh, A.K., Choudhary, K.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 75–87. ISBN 978-0-12-817004-5. [Google Scholar]
- Sun, X.; Wang, W.; Yi, S.; Zheng, F.; Zhang, Z.; Alharbi, S.A.; Filimonenko, E.; Wang, Z.; Kuzyakov, Y. Microbial Composition in Saline and Alkaline Soils Regulates Plant Growth with P-Solubilizing Bacteria. Appl. Soil Ecol. 2024, 203, 105653. [Google Scholar] [CrossRef]
- Sharma, N. Biological Controls for Preventing Food Deterioration: Strategies for Pre- and Postharvest Management; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 978-1-118-53306-2. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Lee, S.-K.; Lur, H.-S.; Lo, K.-J.; Cheng, K.-C.; Chuang, C.-C.; Tang, S.-J.; Yang, Z.-W.; Liu, C.-T. Evaluation of the Effects of Different Liquid Inoculant Formulations on the Survival and Plant-Growth-Promoting Efficiency of Rhodopseudomonas palustris Strain PS3. Appl. Microbiol. Biotechnol. 2016, 100, 7977–7987. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-44367-2. [Google Scholar]
- Fadiji, A.E.; Babalola, O.O. Exploring the Potentialities of Beneficial Endophytes for Improved Plant Growth. Saudi J. Biol. Sci. 2020, 27, 3622–3633. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef]
- Shukla, K.P.; Sharma, S.; Singh, N.K.; Singh, V.; Tiwari, K.; Singh, S. Nature and Role of Root Exudates: Efficacy in Bioremediation. Afr. J. Biotechnol. 2011, 10, 9717–9724. [Google Scholar] [CrossRef]
- Estrela, A.B.; Abraham, W.-R. Fungal Metabolites for the Control of Biofilm Infections. Agriculture 2016, 6, 37. [Google Scholar] [CrossRef]
- Lachance, M.-A.; Starmer, W.T. Chapter 4—Ecology and Yeasts. In The Yeasts, 4th ed.; Kurtzman, C.P., Fell, J.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 21–30. ISBN 978-0-444-81312-1. [Google Scholar]
- Starmer, W.T.; Lachance, M.-A. Chapter 6—Yeast Ecology. In The Yeasts, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: London, UK, 2011; pp. 65–83. ISBN 978-0-444-52149-1. [Google Scholar]
- Yurkov, A. Yeasts in Forest Soils. In Yeasts in Natural Ecosystems: Diversity; Buzzini, P., Lachance, M.-A., Yurkov, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 87–116. ISBN 978-3-319-62683-3. [Google Scholar]
- Ciancio, A.; Pieterse, C.M.J.; Mercado-Blanco, J. Editorial: Harnessing Useful Rhizosphere Microorganisms for Pathogen and Pest Biocontrol—Second Edition. Front. Microbiol. 2019, 10, 1935. [Google Scholar] [CrossRef]
- Mohanram, S.; Kumar, P. Rhizosphere Microbiome: Revisiting the Synergy of Plant-Microbe Interactions. Ann. Microbiol. 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Salomon, M.V.; Funes Pinter, I.; Piccoli, P.; Bottini, R. Use of Plant Growth-Promoting Rhizobacteria as Biocontrol Agents: Induced Systemic Resistance Against Biotic Stress in Plants. In Microbial Applications: Biomedicine, Agriculture and Industry; Kalia, V.C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 2, pp. 133–152. ISBN 978-3-319-52669-0. [Google Scholar]
- Raza, W.; Yousaf, S.; Rajer, F.U. Plant Growth Promoting Activity of Volatile Organic Compounds Produced by Biocontrol Strains. Sci. Lett. 2016, 4, 40–43. [Google Scholar]
- Pérez-de-Luque, A.; Tille, S.; Johnson, I.; Pascual-Pardo, D.; Ton, J.; Cameron, D.D. The Interactive Effects of Arbuscular Mycorrhiza and Plant Growth-Promoting Rhizobacteria Synergistically Enhance Host Plant Defences against Pathogens. Sci. Rep. 2017, 7, 16409. [Google Scholar] [CrossRef]
- Gupta, S.; Khan, I.M.; Kunal; Parihar, N.; Kumar, D. Introduction to the Rhizosphere World. In Rhizosphere Revolution; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-003-57029-5. [Google Scholar]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The Rhizosphere: A Playground and Battlefield for Soilborne Pathogens and Beneficial Microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Anandakumar, S.; Senthamilselvi, D.; Kalaiselvi, T. Microbial Consortium with Multifunctional Plant Growth-Promoting Traits and Its Significant Contribution in Sustainable Agriculture. In Progress in Soil Microbiome Research; Parray, J.A., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 53–75. ISBN 978-3-031-71487-0. [Google Scholar]
- Dzvene, A.R.; Chiduza, C. Application of Biofertilizers for Enhancing Beneficial Microbiomes in Push–Pull Cropping Systems: A Review. Bacteria 2024, 3, 271–286. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Field, D.T.; Stockman, A.; Kendall, T.J. Colonisation or Invasion: A Diagnostic Dilemma in a ‘Benign’ Gallbladder. Diagn. Histopathol. 2024, 30, 264–267. [Google Scholar] [CrossRef]
- Gegenbauer, C.; Bellaire, A.; Schintlmeister, A.; Schmid, M.C.; Kubicek, M.; Voglmayr, H.; Zotz, G.; Richter, A.; Mayer, V.E. Exo- and Endophytic Fungi Enable Rapid Transfer of Nutrients from Ant Waste to Orchid Tissue. New Phytol. 2023, 238, 2210–2223. [Google Scholar] [CrossRef]
- Sundari, S.K.; Prakash, A.; Yadav, P.; Kumari, A. Plant Growth-Promoting Microbes as Front-Runners for On-Site Remediation of Organophosphate Pesticide Residues in Agriculture Soils. In Phyto and Rhizo Remediation; Arora, N.K., Kumar, N., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2019; Volume 9, pp. 249–285. ISBN 978-981-329-663-3. [Google Scholar]
- Woolgar, J.A.; Triantafyllou, A. Pitfalls and Procedures in the Histopathological Diagnosis of Oral and Oropharyngeal Squamous Cell Carcinoma and a Review of the Role of Pathology in Prognosis. Oral Oncol. 2009, 45, 361–385. [Google Scholar] [CrossRef]
- Chaturvedi, H.; Prakash, A. Exploitation of Plant Tissue Invading Rhizospheric Microbes as Bio-Fertilizers. In Rhizosphere Microbes: Soil and Plant Functions; Sharma, S.K., Singh, U.B., Sahu, P.K., Singh, H.V., Sharma, P.K., Eds.; Springer: Singapore, 2020; pp. 315–329. ISBN 978-981-15-9154-9. [Google Scholar]
- Ishida, J.K.; Bini, A.P.; Creste, S.; Van Sluys, M.-A. Towards Defining the Core Saccharum Microbiome: Input from Five Genotypes. BMC Microbiol. 2022, 22, 193. [Google Scholar] [CrossRef]
- Al-Turki, A.; Murali, M.; Omar, A.F.; Rehan, M.; Sayyed, R.Z. Recent Advances in PGPR-Mediated Resilience toward Interactive Effects of Drought and Salt Stress in Plants. Front. Microbiol. 2023, 14, 1214845. [Google Scholar] [CrossRef]
- Hijri, M. Analysis of a Large Dataset of Mycorrhiza Inoculation Field Trials on Potato Shows Highly Significant Increases in Yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef]
- Smith, J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2009; Volume 73. [Google Scholar] [CrossRef]
- Baslam, M.; Esteban, R.; García-Plazaola, J.I.; Goicoechea, N. Effectiveness of Arbuscular Mycorrhizal Fungi (AMF) for Inducing the Accumulation of Major Carotenoids, Chlorophylls and Tocopherol in Green and Red Leaf Lettuces. Appl. Microbiol. Biotechnol. 2013, 97, 3119–3128. [Google Scholar] [CrossRef]
- Chaudhary, P.; Agri, U.; Chaudhary, A.; Kumar, A.; Kumar, G. Endophytes and Their Potential in Biotic Stress Management and Crop Production. Front. Microbiol. 2022, 13, 933017. [Google Scholar] [CrossRef]
- Selvasekaran, P.; Chidambaram, R. Agriculturally Important Fungi for Crop Protection. In Agriculturally Important Fungi for Sustainable Agriculture: Volume 2: Functional Annotation for Crop Protection; Yadav, A.N., Mishra, S., Kour, D., Yadav, N., Kumar, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–53. ISBN 978-3-030-48474-3. [Google Scholar]
- Joubert, P.M.; Doty, S.L. Endophytic Yeasts: Biology, Ecology and Applications. In Endophytes of Forest Trees: Biology and Applications; Pirttilä, A.M., Frank, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–14. ISBN 978-3-319-89833-9. [Google Scholar]
- Nutaratat, P.; Srisuk, N.; Arunrattiyakorn, P.; Limtong, S. Plant Growth-Promoting Traits of Epiphytic and Endophytic Yeasts Isolated from Rice and Sugar Cane Leaves in Thailand. Fungal Biol. 2014, 118, 683–694. [Google Scholar] [CrossRef]
- Vujanovic, V. Tremellomycetes Yeasts in Kernel Ecological Niche: Early Indicators of Enhanced Competitiveness of Endophytic and Mycoparasitic Symbionts against Wheat Pathobiota. Plants 2021, 10, 905. [Google Scholar] [CrossRef]
- Chiva, R.; Celador-Lera, L.; Uña, J.A.; Jiménez-López, A.; Espinosa-Alcantud, M.; Mateos-Horganero, E.; Vega, S.; Santos, M.Á.; Velázquez, E.; Tamame, M. Yeast Biodiversity in Fermented Doughs and Raw Cereal Matrices and the Study of Technological Traits of Selected Strains Isolated in Spain. Microorganisms 2021, 9, 47. [Google Scholar] [CrossRef]
- Cullen, N.P.; Fetters, A.M.; Ashman, T.-L. Integrating Microbes into Pollination. Curr. Opin. Insect Sci. 2021, 44, 48–54. [Google Scholar] [CrossRef]
- de Souza, R.S.C.; Okura, V.K.; Armanhi, J.S.L.; Jorrín, B.; Lozano, N.; da Silva, M.J.; González-Guerrero, M.; de Araújo, L.M.; Verza, N.C.; Bagheri, H.C.; et al. Unlocking the Bacterial and Fungal Communities Assemblages of Sugarcane Microbiome. Sci. Rep. 2016, 6, 28774. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Höfte, M. Chapter 6 Rhizobacteria-Induced Systemic Resistance. In Advances in Botanical Research; Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2009; Volume 51, pp. 223–281. [Google Scholar]
- Jetiyanon, K.; Kloepper, J.W. Mixtures of Plant Growth-Promoting Rhizobacteria for Induction of Systemic Resistance against Multiple Plant Diseases. Biol. Control 2002, 24, 285–291. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, S.P.; Cueto, L.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Singh, S.P.; Blázquez, M.A.; Sansinenea, E. Auxins of Microbial Origin and Their Use in Agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 8549–8565. [Google Scholar] [CrossRef]
- Sun, P.-F.; Fang, W.-T.; Shin, L.-Y.; Wei, J.-Y.; Fu, S.-F.; Chou, J.-Y. Indole-3-Acetic Acid-Producing Yeasts in the Phyllosphere of the Carnivorous Plant Drosera indica L. PLoS ONE 2014, 9, e114196. [Google Scholar] [CrossRef] [PubMed]
- Anand, G.; Gupta, R.; Marash, I.; Leibman-Markus, M.; Bar, M. Cytokinin Production and Sensing in Fungi. Microbiol. Res. 2022, 262, 127103. [Google Scholar] [CrossRef] [PubMed]
- Naili, M.B.; Alghazeer, R.O.; Saleh, N.A.; Al-Najjar, A.Y. Evaluation of Antibacterial and Antioxidant Activities of Artemisia campestris (Astraceae) and Ziziphus lotus (Rhamnacea). Arab. J. Chem. 2010, 3, 79–84. [Google Scholar] [CrossRef]
- Demain, A.L.; Martens, E. Production of Valuable Compounds by Molds and Yeasts. J. Antibiot. 2017, 70, 347–360. [Google Scholar] [CrossRef]
- Bennett, J.W.; Hung, R.; Lee, S.; Padhi, S. 18 Fungal and Bacterial Volatile Organic Compounds: An Overview and Their Role as Ecological Signaling Agents. In Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 373–393. ISBN 978-3-642-30826-0. [Google Scholar]
- Fincheira, P.; Quiroz, A. Microbial Volatiles as Plant Growth Inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef]
- Tabacchioni, S.; Passato, S.; Ambrosino, P.; Huang, L.; Caldara, M.; Cantale, C.; Hett, J.; Del Fiore, A.; Fiore, A.; Schlüter, A.; et al. Identification of Beneficial Microbial Consortia and Bioactive Compounds with Potential as Plant Biostimulants for a Sustainable Agriculture. Microorganisms 2021, 9, 426. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Uwaremwe, C.; Zhao, X.; Yue, L.; Zhou, Q.; Wang, Y.; Tran, L.-S.P.; Li, W.; Chen, G.; et al. Characterization of Three New Plant Growth-Promoting Microbes and Effects of the Interkingdom Interactions on Plant Growth and Disease Prevention. Plant Cell Rep. 2023, 42, 1757–1776. [Google Scholar] [CrossRef]
| Microorganism | Challenges |
|---|---|
| PGPR | Field performance variability, survival in diverse conditions, regulatory hurdles |
| Filamentous fungi | Environmental sensitivity, short shelf life, pathogen resistance, field application complexities |
| Yeasts | Transient soil presence, limited host range, scale-up challenges, safety concerns |
| Rhizobacteria | Competition with native microbes, host specificity, formulation stability |
| PGR | Function | Yeast Example |
|---|---|---|
| Auxins (IAA) | Promote root elongation and branching | Rhodotorula, Saccharomyces cerevisiae |
| Cytokinins | Stimulate cell division and shoot growth | Candida, Pichia |
| Gibberellins | Enhance stem elongation, seed germination, and flowering | Debaryomyces hansenii |
| ACC Deaminase | Reduces ethylene levels, alleviating stress-induced growth inhibition | Various yeast species |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Raish, S.M.; Sourani, O.M.; Abu-Elsaoud, A.M. Plant Growth-Promoting Microorganisms as Biocontrol Agents: Mechanisms, Challenges, and Future Prospects. Appl. Microbiol. 2025, 5, 44. https://doi.org/10.3390/applmicrobiol5020044
Al Raish SM, Sourani OM, Abu-Elsaoud AM. Plant Growth-Promoting Microorganisms as Biocontrol Agents: Mechanisms, Challenges, and Future Prospects. Applied Microbiology. 2025; 5(2):44. https://doi.org/10.3390/applmicrobiol5020044
Chicago/Turabian StyleAl Raish, Seham M., Osama M. Sourani, and Abdelghafar M. Abu-Elsaoud. 2025. "Plant Growth-Promoting Microorganisms as Biocontrol Agents: Mechanisms, Challenges, and Future Prospects" Applied Microbiology 5, no. 2: 44. https://doi.org/10.3390/applmicrobiol5020044
APA StyleAl Raish, S. M., Sourani, O. M., & Abu-Elsaoud, A. M. (2025). Plant Growth-Promoting Microorganisms as Biocontrol Agents: Mechanisms, Challenges, and Future Prospects. Applied Microbiology, 5(2), 44. https://doi.org/10.3390/applmicrobiol5020044






