Antifungal Potential of Cyanobacterium Nostoc sp. BCAC 1226 Suspension as a Biocontrol Agent Against Phytopathogenic Fungi and Oomycetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacterial Cultivation
2.2. Cultivation of Phytopathogens
2.3. Experimental Design
2.4. Data and Statistical Analysis
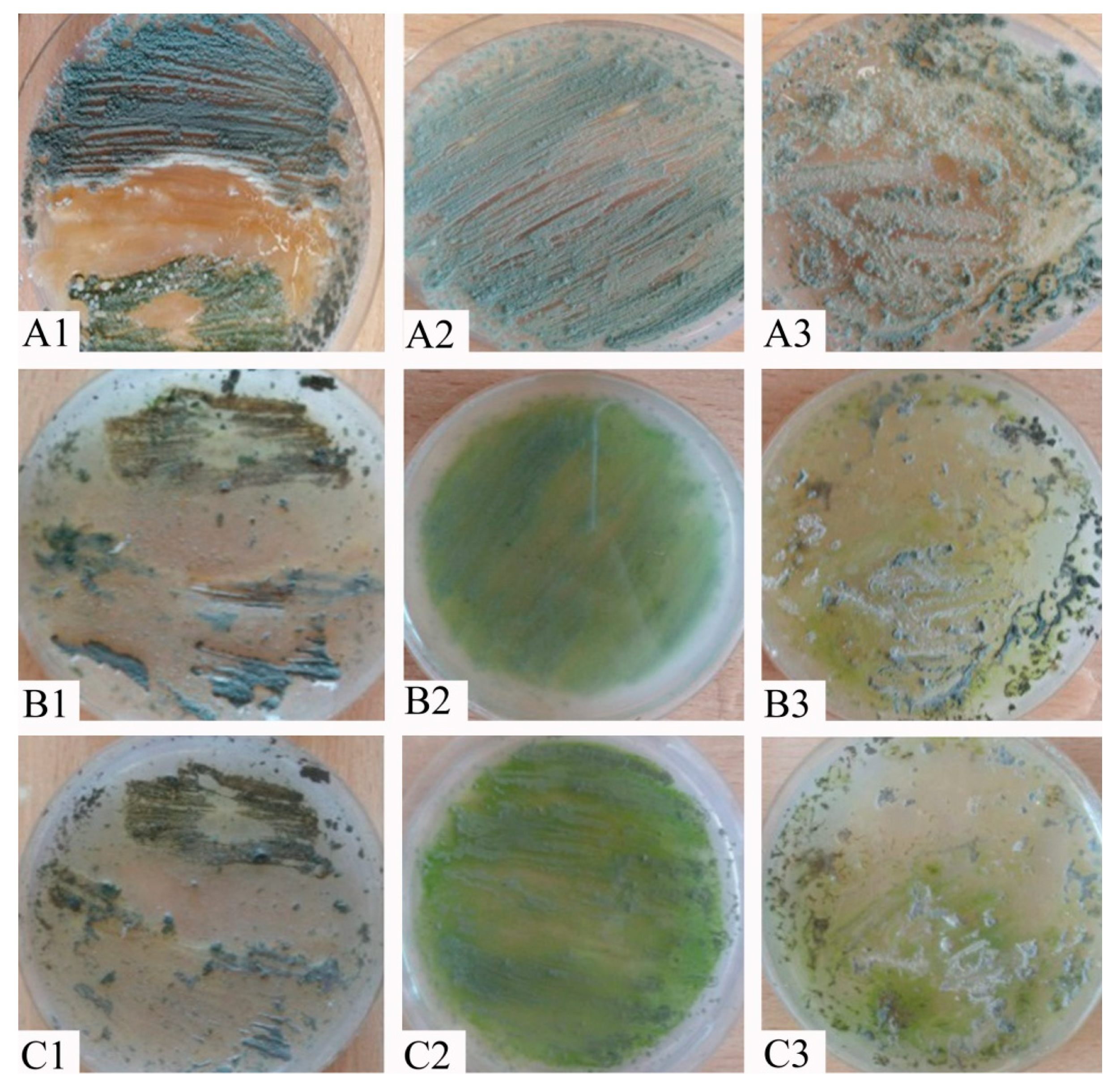
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marrez, D.A.; Sultan, Y.Y. Antifungal activity of the cyanobacterium Microcystis aeruginosa. J. Appl. Pharm. Sci. 2016, 6, 191–198. [Google Scholar] [CrossRef]
- Khakimov, A.A.; Omonlikov, A.U.; Utaganov, S.B.U. Current status and prospects of the use of biofungicides against plant diseases. GSC Biol. Pharm. Sci. 2020, 13, 119–126. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Pilarska, A.A.; Niewiadomska, A.; Piotrowska-Cyplik, A. Fungi of the Trichoderma Genus: Future Perspectives of Benefits in Sustainable Agriculture. Appl. Sci. 2023, 13, 6434. [Google Scholar] [CrossRef]
- Dutilloy, E.; Oni, F.E.; Esmaeel, Q.; Clément, C.; Barka, E.A. Plant Beneficial Bacteria as Bioprotectants against Wheat and Barley Diseases. J. Fungi 2022, 8, 632. [Google Scholar] [CrossRef] [PubMed]
- Dutilloy, E.; Arguelles Arias, A.; Richet, N.; Guise, J.-F.; Duban, M.; Leclère, V.; Selim, S.; Jacques, P.; Jacquard, C.; Clément, C.; et al. Bacillus velezensis BE2 controls wheat and barley diseases by direct antagonism and induced systemic resistance. Appl. Microbiol. Biotechnol. 2024, 108, 1–24. [Google Scholar] [CrossRef]
- Kenfaoui, J.; Dutiloy, E.; Benchlih, S.; Lahlali, R.; Ait-Barka, E.; Esmaeel, Q. Bacillus velezensis: A Versatile Ally in the Battle Against Phytopathogens—Insights and Prospects. Appl. Microbiol. Biotechnol. 2024, 108, 439. [Google Scholar] [CrossRef]
- Manathunga, K.K.; Gunasekara, N.W.; Meegahakumbura, M.K.; Ratnaweera, P.B.; Faraj, T.K.; Wanasinghe, D.N. Exploring Endophytic Fungi as Natural Antagonists against Fungal Pathogens of Food Crops. J. Fungi 2024, 10, 606. [Google Scholar] [CrossRef]
- Muhammad, M.; Basit, A.; Ali, K.; Ahmad, H.; Li, W.J.; Khan, A.; Mohamed, H.I. A review on endophytic fungi: A potent reservoir of bioactive metabolites with special emphasis on blight disease management. Arch. Microbiol. 2024, 206, 129. [Google Scholar] [CrossRef]
- De Castro, M.T.; de Lima Ferreira, A.D.C.; do Nascimento, I.N.; Rocha, G.T.; Celestino, M.F.; Freire, Í.A.; Moreira, I.C.F.; Gomes, G.C.; dos Reis Cunha, B.B.; Montalvão, S.C.L.; et al. Endophytic Bacillus spp. of coffee plants (Coffea arabica L.) and its potential in the biocontrol of phytopathogenic fungi and Lepidoptera larvae. Egypt. J. Biol. Pest. Control 2025, 35, 8. [Google Scholar] [CrossRef]
- Kulik, M.M. The potential for using cyanobacteria (blue-green algae) and algae in the biological control of plant pathogenic bacteria and fungi. Eur. J. Plant Pathol. 1995, 101, 585–599. [Google Scholar] [CrossRef]
- Castenholz, R.W. Phylum BX. Cyanobacteria. In Bergey′s Manual of Determinative Bacteriology; Boone, D.R., Castenholz, R.W., Garrity, G.M., Eds.; Springer: New York, NY, USA, 2001; pp. 473–599. [Google Scholar]
- Domracheva, L.I.; Kondakova, L.V.; Popov, L.B.; Zykova, Y.N. Bioremediation capabilities of soil cyanobacteria (review). Theor. Appl. Ecol. 2009, 1, 8–17. [Google Scholar] [CrossRef]
- Soltani, N.; Khavari-Nejad, R.A.; Tabatabaei, Y.M.; Shokravi, S.; Fernández-Valiente, E. Screening of soil cyanobacteria for antifungal and antibacterial activity. Pharm. Biol. 2005, 43, 455–459. [Google Scholar] [CrossRef]
- Al-Nedawe, R.A.D.; Yusof, Z.N.B. Cyanobacteria as a Source of Bioactive Compounds with Anticancer, Antibacterial, Antifungal, and Antiviral Activities: A Review. Microb. Bioact. 2023, 6, 1–16. [Google Scholar]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria—A profile source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Mo, S.; Krunic, A.; Chlipala, G.; Orjal, J. Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod. 2009, 72, 894–899. [Google Scholar] [CrossRef]
- Asthana, R.K.; Srivastava, A.; Singh, A.P.; Deepali; Singh, S.P.; Nath, G.; Srivastava, R.; Srivastava, B.S. Identification of an antimicrobial entity from Fischerella sp. colonizing neem tree bark. J. Appl. Phycol. 2006, 18, 33–39. [Google Scholar] [CrossRef]
- Rojas, V.; Rivas, L.; Cardenas, C.; Guzman, F. Cyanobacteria and Eukaryotic Microalgae as Emerging Sources of Antibacterial Peptides. Molecules 2020, 25, 5804. [Google Scholar] [CrossRef]
- Matei, E.; Basu, R.; Furey, W.; Shi, J.; Calnan, C.; Aiken, C.; Gronenborn, A.M. Structure and glycan binding of a new cyanovirin-N homolog. J. Biol. Chem. 2016, 291, 18967–18976. [Google Scholar] [CrossRef]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef]
- Vestola, J.; Shishido, T.K.; Jokela, J.; Fewer, D.P.; Aitio, O.; Permi, P.; Wahlsten, M.; Wang, H.; Rouhiainen, L.; Sivonen, K. Hassallidins, antifungal glycolipopeptides, are widespread among cyanobacteria and are the end-product of a nonribosomal pathway. Proc. Natl. Acad. Sci. USA 2014, 111, 1909–1917. [Google Scholar] [CrossRef]
- Shishido, T.K.; Humist, A.; Jokela, J.; Liu, L.; Wahlsten, M.; Tamrakar, A.; Fewer, D.P.; Permi, P.; Andreote, A.P.D.; Fiore, M.F.; et al. Antifungal compounds from cyanobacteria. Mar. Drugs 2015, 13, 2124–2140. [Google Scholar] [CrossRef] [PubMed]
- Humisto, A.; Jokela, J.; Teigen, K.; Wahlsten, M.; Permi, P.; Sivonen, K.; Herfindal, L. Characterization of the interaction of the antifungal and cytotoxic cyclic glycolipopeptide hassallidin with sterol-containing lipid membranes. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Erhonyota, C.; Edo, G.I.; Onoharigho, F.O. Comparison of poison plate and agar well diffusion method determining the antifungal activity of protein fractions. Acta Ecol. Sin. 2022, 43, 684–689. [Google Scholar] [CrossRef]
- Kumar, N.S.S.; Sivasubramanian, V.; Mukund, S. Antimicrobial and antifungal activity of extracts of Phormidium fragile Gomont. J. Algal Biomass Utln. 2013, 4, 66–71. [Google Scholar]
- Águila-Carricondo, P.; Román, R.; Marín-Guirao, J.I.; Cantón, Y. Native biocrust cyanobacteria strains showing antagonism against three soilborne pathogenic fungi. Pathogens 2024, 13, 579. [Google Scholar] [CrossRef]
- Abo-Shady, A.M.; Al-Ghaffar, B.A.; Rahhal, M.; Abd-El Monem, H. Biological control of faba bean pathogenic fungi by three cyanobacterial filtrates. Pak. J. Biol. Sci. 2007, 10, 3029–3038. [Google Scholar]
- Sand-Jensen, K.; Jespersen, T.S. Tolerance of the widespread cyanobacterium Nostoc commune to extreme temperature variations (−269 to 105 °C), pH and salt stress. Oecologia 2012, 169, 331–339. [Google Scholar] [CrossRef]
- Bataeva, Y.V.; Grigoryan, L.N. Ecological features and adaptive capabilities of cyanobacteria in desert ecosystems: A review. Eurasian Soil. Sci. 2024, 57, 430–445. [Google Scholar] [CrossRef]
- Dal-Ferro, L.S.; Schenider, A.; Missiaggia, D.G.; Silva, L.J.; Maciel-Silva, A.S.; Figueredo, C.C. Organizing a global list of cyanobacteria and algae from soil biocrusts evidenced great geographic and taxonomic gaps. FEMS Microbiol. Ecol. 2024, 100, 7. [Google Scholar] [CrossRef]
- El-Fayoumy, E.A.; Shanab, S.M.; Hassan, O.M.A.; Shalaby, E.A. Enhancement of active ingredients and biological activities of Nostoc linckia biomass cultivated under modified BG-110 medium composition. Biomass Convers. Biorefin. 2021, 13, 6049–6066. [Google Scholar] [CrossRef]
- Eladl, S.A.; Elnabawy, A.M.; Eltanahy, E.G. Recent biotechnological applications of value-added bioactive compounds from microalgae and seaweeds. Bot. Stud. 2024, 65, 28. [Google Scholar] [CrossRef] [PubMed]
- Iskusnykh, O.Y.; Iskusnykh, A.Y.; Iskusnykh, D.O. Cyanobacteria—Promising objects of biotechnology and medicine. Vestn. VGUIT 2021, 83, 70–77. [Google Scholar] [CrossRef]
- Todorova, A.K.; Juettner, F.; Linden, A.; Pluess, T.; von Philipsborn, W. Nostocyclamide: A new macrocyclic, thiazole-containing allelochemical from Nostoc sp. 31 (Cyanobacteria). J. Org. Chem. 1995, 60, 7891–7895. [Google Scholar] [CrossRef]
- Jüttner, F.; Todorova, A.K.; Walch, N.; von Philipsborn, W. Nostocyclamide M: A cyanobacterial cyclic peptide with allelopathic activity from Nostoc sp. 31. Phytochemistry 2001, 57, 613–619. [Google Scholar] [CrossRef]
- Todorova, A.; Jüttner, F. Ecotoxicological analysis of Nostocyclamide, a modified cyclic hexapeptide from Nostoc. Phycologia 1996, 35, 183–188. [Google Scholar] [CrossRef]
- Kajiyama, S.; Kanzaki, H.; Kawazu, K.; Kobayashi, A. Nostofungicidine, an antifungal lipopeptide from the field-grown terrestrial blue-green alga Nostoc commune. Tetrahedron Lett. 1998, 39, 3737–3740. [Google Scholar] [CrossRef]
- Heinilä, L.M.P.; Fewer, D.P.; Jokela, J.K.; Wahlsten, M.; Jortikka, A.; Sivonen, K. Shared PKS module in biosynthesis of synergistic laxaphycins. Front. Microbiol. 2020, 11, 578878. [Google Scholar] [CrossRef]
- Heinilä, L.M.P.; Fewer, D.P.; Jokela, J.K.; Wahlsten, M.; Ouyang, X.; Permi, P.; Jortikka, A.; Sivonen, K. The structure and biosynthesis of heinamides A1–A3 and B1–B5, antifungal members of the laxaphycin lipopeptide family. Org. Biomol. Chem. 2021, 19, 5577–5588. [Google Scholar] [CrossRef]
- Chaganty, S.; Golakoti, T.; Heltzel, C.; Moore, R.E.; Yoshida, W.Y. Isolation and structure determination of cryptophycins 38, 326, and 327 from the terrestrial cyanobacterium Nostoc sp. GSV 224. J. Nat. Prod. 2004, 67, 1403–1406. [Google Scholar] [CrossRef]
- Nowruzi, B.; Khavari-Nejad, R.-A.; Sivonen, K.; Kazemi, B.; Najafi, F.; Nejadsattari, T. Identification and toxigenic potential of a Nostoc sp. Algae 2012, 27, 303–313. [Google Scholar] [CrossRef]
- Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive peptides produced by cyanobacteria of the genus nostoc: A review. Mar. Drug. 2019, 17, 561. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of pigments from microalgae and cyanobacteria—A review on current methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- Cano, M.M.S.D.; De Mul, M.C.Z.; De Caire, G.Z.; De Halperin, D.R. Inhibition of Candida albicans and Staphylococcus aureus by phenolic compounds from the terrestrial cyanobacterium Nostoc muscorum. J. Appl. Phycol. 1990, 2, 79–81. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Osman, M.E.H.; Dyab, M.A.; Amer, M.S. Production and characterization of antimicrobial active substance from the cyanobacterium Nostoc muscorum. Environ. Toxicol. Pharmacol. 2006, 21, 42–50. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.I.I.; Abo-Elyousr, K.A.M.; Abdel-Rahim, I.R. Fungicidal activity of extracellular products of cyanobacteria against Alternaria porri. Eur. J. Phycol. 2015, 50, 239–245. [Google Scholar] [CrossRef]
- Locher, H.H.; Ritz, D.; Pfaff, P.; Gaertner, M.; Knezevic, A.; Sabato, D.; Schroeder, S.; Barbaras, D.; Gademann, K. Dimers of Nostocarboline with potent antibacterial activity. Chemotherapy 2010, 56, 318–324. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; Allori, E.; Sanz, J.M.; Gonda, M.; Alconada, T.; Cavello, I.; Dib, J.R.; Diaz, M.A.; Nally, C.; et al. Microbial Biopesticides: Diversity, Scope, and Mechanisms Involved in Plant Disease Control. Diversity 2023, 15, 457. [Google Scholar] [CrossRef]
- Christodoulou, M.; Jokela, J.; Wahlsten, M.; Saari, L.; Economou-Amilli, A.; de Fiore, M.F.; Sivonen, K. Description of Aliinostoc Alkaliphilum Sp. Nov. (Nostocales, Cyanobacteria), a New Bioactive Metabolite-Producing Strain from Salina Verde (Pantanal, Brazil) and Taxonomic Distribution of Bioactive Metabolites in Nostoc and Nostoc-like Genera. Water 2022, 14, 2470. [Google Scholar] [CrossRef]
- Nowruzi, B.; Nemati, F. Evaluation of hydrolytic enzymes and antifungal activity of extracellular bioactive compounds of Desmonostoc alborizicum and Neowestiellopsis persica against Plant Pathogenic Fungi. Acta Biol. Slov. 2023, 66, 4–12. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Yadav, A.; Sahoo, A.; Kumari, P.; Singh, L.A.; Swapnil, P.; Meena, M.; Kumar, S. Microalgal-based sustainable bio-fungicides: A promising solution to enhance crop yield. Discov. Sustain. 2025, 6, 39. [Google Scholar] [CrossRef]
- López-Arellanes, M.E.; López-Pacheco, L.D.; Elizondo-Luevano, J.H.; González-Meza, G.M. Algae and Cyanobacteria Fatty Acids and Bioactive Metabolites: Natural Antifungal Alternative Against Fusarium sp. Microorganisms 2025, 13, 439. [Google Scholar] [CrossRef] [PubMed]
- Abad, Z.G.; Burgess, T.I.; Bourret, T.; Bensch, K.; Cacciola, S.O.; Scanu, B.; Mathew, B.; Kasiborski, B.; Srivastava, S.; Kageyama, K.; et al. Phytophthora: Taxonomic and phylogenetic revision of the genus. Stud. Mycol. 2023, 106, 259–348. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, J.H.C.; Hanse, B.; van Leeuwen, G.C.M.; Groenewald, J.Z.; Crous, P.W. Stemphylium revisited. Stud. Mycol. 2017, 87, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Kroon, L.P.N.M.; Brouwer, H.; De Cock, A.W.A.M.; Govers, F. The Genus Phytophthora anno 2012. Phytopathology 2012, 102, 348–364. [Google Scholar] [CrossRef]
- Scott, P.; Bader, M.K.-F.; Burgess, T.; Hardy, G.; Williams, N. Global biogeography and invasion risk of the plant pathogen genus Phytophthora. Environ. Sci. Policy 2019, 101, 175–182. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Richardson, D.M.; Leroux, J.J.; Strasberg, D.; Edwards, J.; Roets, F.; Hubka, V.; Taylor, P.W.; Heykoop, M.; et al. Fungal Planet description sheets: 400–468. Persoonia 2016, 36, 316–458. [Google Scholar] [CrossRef]
- Brahamanage, R.S.; Hyde, K.D.; Li, X.H.; Jayawardena, R.S.; McKenzie, E.H.C.; Yan, J.Y. Are pathogenic isolates of Stemphylium host-specific and cosmopolitan? Plant Pathol. Quar. 2018, 8, 153–164. [Google Scholar] [CrossRef]
- Sánchez-Torres, P.; Vilanova, L.; Ballester, A.R.; López-Pérez, M.; Teixidó, N.; Viñas, I.; Usall, J.; González-Candelas, L.; Torres, R. Unravelling the contribution of the Penicillium expansum PeSte12 transcription factor to virulence during apple fruit infection. Food Microbiol. 2018, 69, 123–135. [Google Scholar] [CrossRef]
- Yan, H.; Li, Y.; Lv, Y.; Zhao, X.; Man, Z.; Chen, Z.; Guo, L.; Huo, J.; Sang, M.; Li, C.C.; et al. Postharvest fruit rot on blue honeysuckle (Lonicera caerulea L.) caused by Penicillium oxalicum newly reported in China. Crop Prot. 2025, 193, 107195. [Google Scholar] [CrossRef]
- Gálvez, L.; Gil-Serna, J.; García, M.; Iglesias, C.; Palmero, D. Stemphylium leaf blight of garlic (Allium sativum) in Spain: Taxonomy and in vitro fungicide response. Plant Pathol. J. 2016, 32, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.A.; Engle, C.A.; Meyer, F.W.; Watkins, C.B. Penicillium expansum invades apples through stems during controlled atmosphere storage. Plant Health Prog. 2006, 7, 1. [Google Scholar] [CrossRef]
- Vico, I.; Duduk, N.; Vasic, M.; Nikolic, M. Identification of Penicillium expansum causing postharvest blue mold decay of apple fruit. Pestic. Phytomed. 2014, 29, 257–266. [Google Scholar] [CrossRef]
- Carmichael, W.W. Isolation, Culture, and Toxicity Testing of Toxic Freshwater Cyanobacteria (Blue-Green Algae). In Fundamental Research in Homogenous Catalysis 3; Shilov, V., Ed.; Gordon & Breach: New York, NY, USA, 1986; pp. 1249–1262. [Google Scholar]
- Kawachi, M.; Noël, M.H. Sterilization and Sterile Technique. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 65–81. [Google Scholar]
- Atanasova-Pancevska, N.; Kungulovski, D.; Angelovska, D.; Mirkovik, L.; Stojanoska, M.; Boskovski, O.; Kostandinovska, S. In vitro evaluation of soil bacillus strains isolated from the Bucim copper mine for biocontrol against grapevine downy mildew. J. Agric. Plant Sci. 2024, 22, 2. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Alsenani, F.; Tupally, K.R.; Chua, E.T.; Eltanahy, E.; Alsufyani, H.; Parekh, H.S.; Schenk, P.M. Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm. J. 2020, 28, 1834–1841. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Maniam, G.P.; Ramaraj, R.; Govindan, N. Exploration of bioactive compounds and antibacterial activity of marine blue-green microalgae (Oscillatoria sp.) isolated from the coastal region of West Malaysia. SN Appl. Sci. 2020, 2, 1906. [Google Scholar] [CrossRef]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Geometric Morphometrics for Biologists: A Primer; Elsevier Academic Press: New York, NY, USA; London, UK, 2004. [Google Scholar]
- Edjabou, M.E.; Martín-Fernández, J.A.; Scheutz, C.; Astrup, T.F. Statistical analysis of solid waste composition data: Arithmetic mean, standard deviation, and correlation coefficients. Waste Manag. 2017, 69, 13–23. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of Student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef]
- Yadav, S.; Agrawal, M.; Raipuria, N.; Agrawal, M.K. Antimicrobial activity of Nostoc calcicola (Cyanobacteria) isolated from central India against human pathogens. Asian J. Pharm. 2016, 10, S554–S559. [Google Scholar]
- Osman, M.; El-Sheekh, M.; Metwally, M.; Ismail, A.; Ismail, M. Antagonistic activity of some fungi and cyanobacteria species against Rhizoctonia solani. Int. J. Plant Pathol. 2011, 2, 101–114. [Google Scholar] [CrossRef][Green Version]
- Poveda, J. Cyanobacteria in plant health: Biological strategy against abiotic and biotic stresses. Crop Prot. 2021, 141, 105450. [Google Scholar] [CrossRef]
- Behzadnia, A.; Moosavi-Nasab, M.; Oliyaei, N. Anti-biofilm activity of marine algae-derived bioactive compounds. Front. Microbiol. 2024, 12, 1270174. [Google Scholar] [CrossRef] [PubMed]
- Tomek, P.; Hrouzek, P.; Kuzma, M.; Sýkora, R.; Fišer, R.; Cěrný, J.; Novák, P.; Bártová, S.; Šimek, P.; Hof, M.; et al. Cytotoxic lipopeptide muscotoxin A, isolated from soil cyanobacterium DesmoNostoc muscorum, permeabilizes phospholipid membranes by reducing their fluidity. Chem. Res. Toxicol. 2016, 28, 216–224. [Google Scholar] [CrossRef]
- Cheel, J.; Hájek, J.; Kuzma, M.; Saurav, K.; Smýkalová, I.; Ondráčková, E.; Urajová, P.; Vu, D.L.; Faure, K.; Kopecký, J.; et al. Application of HPCCC combined with polymeric resins and HPLC for the separation of cyclic lipopeptides muscotoxins A-C and their antimicrobial activity. Molecules 2018, 23, 2653. [Google Scholar] [CrossRef]
- Fidor, A.; Grabski, M.; Gawor, J.; Gromadka, R.; Węgrzyn, G.; Mazur-Marzec, H. Nostoc edaphicum CCNP1411 from the Baltic Sea—A new producer of Nostocyclopeptides. Mar. Drugs 2020, 18, 442. [Google Scholar] [CrossRef]
- Bishoyi, A.K.; Sahoo, C.R.; Padhy, R.N. Recent progression of cyanobacteria and their pharmaceutical utility: An update. J. Biomol. Struct. Dyn. 2022, 41, 4219–4252. [Google Scholar] [CrossRef]
- Horváth, Á.N.; Németh, L.; Vörös, L.; Stirk, W.A.; van Staden, J.; Ördög, V. Cataloguing microalgae and cyanobacteria strains from the Mosonmagyaróvár algal culture collection with in vitro antagonistic activity against phytopathogenic fungi and oomycetes. Phytoparasitica 2023, 51, 747–762. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; El-Beltagi, H.S.; Shanab, S.M.M.; El-fayoumy, E.A.; Shalaby, E.A.; Bendary, E.S.A. Potential Antioxidant and Anticancer Activities of Secondary Metabolites of Nostoc linckia Cultivated under Zn and Cu Stress Conditions. Processes 2021, 9, 1972. [Google Scholar] [CrossRef]
- Domracheva, L.I.; Shirokikh, I.G.; Fokina, A.I. Cyanobacteria and actinomycetes influence against Fusarium species in soil and rhizosphere. Mycol. Phytopathol. 2009, 43, 157–165. [Google Scholar]
- Alwathnani, H.A.; Perveen, K. Biological control of Fusarium wilt of tomato by antagonist fungi and cyanobacteria. Afr. J. Biotechnol. 2012, 11, 1100–1105. [Google Scholar] [CrossRef]
- Nowruzi, B.; Salehi, M.; Talebi, A. Study of the effects of bioactive compounds of cyanobacterium Desmonostoc alborizicum on pathogenic fungi of wheat. Acta Biol. Slov. 2024, 67, 3. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, J.; Zhang, D.; Li, Y.; Li, H.; He, H. Effect of heterocystous nitrogen-fixing cyanobacteria against rice sheath blight and the underlying mechanism. Appl. Soil. Ecol. 2020, 153, 1–9. [Google Scholar] [CrossRef]
- Pandey, R.N.; Jaisani, P.; Singh, H.B. Trichoderma: Agricultural Applications and Beyond. In Biopesticides; Woodhead Publishing: Sawston, UK, 2022; pp. 353–381. [Google Scholar]
- Morsy, F.M.; Nafady, N.A.; Abd-Alla, M.H.; Elhady, D.A. Green synthesis of silver nanoparticles by water-soluble fraction of the extracellular polysaccharides/matrix of the cyanobacterium Nostoc commune and its application as a potent fungal surface sterilizing agent of seed crops. Univ. J. Microbiol. Res. 2014, 2, 36–43. [Google Scholar] [CrossRef]
- Volk, R.-B.; Furkert, F.H. Antialgal, antibacterial, and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol. Res. 2006, 161, 180–186. [Google Scholar] [CrossRef]
- Yu, H.; Jia, S.; Dai, Y. Accumulation of exopolysaccharides in liquid suspension culture of Nostoc flagelliforme cells. Appl. Biochem. Biotechnol. 2010, 160, 552–560. [Google Scholar] [CrossRef]
- Domracheva, L.I.; Trefilova, L.V.; Tretyakova, A.N.; Grebneva, O.I.; Dudoladova, G.M. Biological Protection of Seedlings from Diseases in Nurseries. In Forests of the Kirov Region; Vidyakina, A.I., Ashikhmina, T.Y., Novoselova, S.D., Eds.; Kirov Regional Printing House: Kirov, Russia, 2008; pp. 292–299. [Google Scholar]
- Kovina, A.L.; Popov, L.B.; Domracheva, L.I.; Elkina, T.S.; Kovin, D.A. The Use of Biological Products when Growing Asters in an Urban Environment. In Proceedings of the VIth All-Russian Scientific and Practical Conference with International Participation “Problems of Regional Ecology in the Context of Sustainable Development”; VINITI: Kirov, Russia, 2008; pp. 279–281. [Google Scholar]
- Renganathan, P.; Puente, E.O.; Sukhanova, N.V.; Gaysina, L.A. Hydroponics with Microalgae and Cyanobacteria: Emerging Trends and Opportunities in Modern Agriculture. BioTech 2024, 13, 27. [Google Scholar] [CrossRef]
- Surapuram, V.; Setzer, W.N.; McFeeters, R.L.; McFeeters, H. Antifungal activity of plant extracts against Aspergillus niger and Rhizopus stolonifer. Nat. Prod. Commun. 2014, 9, 1934578X1400901118. [Google Scholar] [CrossRef]
- Kim, T.Y.; Hwang, S.H.; Noh, J.S.; Cho, J.Y.; Maung, C.E. Antifungal potential of Bacillus velezensis CE 100 for the control of different Colletotrichum species through isolation of active dipeptide, cyclo-(d-phenylalanyl-d-prolyl). Int. J. Mol. Sci. 2022, 23, 7786. [Google Scholar] [CrossRef]

| Variant of Experiment | Xmin, cm2 | Xmax, cm2 | X ± S, cm2 | σ | Me | CV (%) | t | |
|---|---|---|---|---|---|---|---|---|
| 7th Day Post-Inoculation | ||||||||
| Phytophthora sp. | Control | 11.35 | 29.29 | 18.30 ± 1.68 | 5.31 | 16.78 | 29.03 | - |
| Experiment | 5.32 | 13.28 | 8.55 ± 0.74 | 2.35 | 8.14 | 27.44 | 5.31 * | |
| Stemphylium sp. | Control | 11.94 | 24.17 | 17.76 ± 1.28 | 4.04 | 17.54 | 22.72 | - |
| Experiment | 10.43 | 16.74 | 13.50 ± 0.73 | 2.31 | 13.24 | 17.08 | 2.89 * | |
| Penicillium sp. | Control | 11.36 | 25.38 | 17.64 ± 1.46 | 4.63 | 17.47 | 26.24 | - |
| Experiment | 6.62 | 10.93 | 8.90 ± 0.36 | 1.13 | 8.95 | 12.72 | 5.80 * | |
| 14th Day Post-Inoculation | ||||||||
| Phytophthora sp. | Control | 11.13 | 29.30 | 18.03 ± 1.73 | 5.46 | 16.04 | 30.26 | - |
| Experiment | 3.17 | 7.16 | 4.96 ± 0.41 | 1.29 | 4.70 | 26.03 | 7.37 * | |
| Stemphylium sp. | Control | 11.78 | 24.03 | 17.60 ± 1.28 | 4.04 | 17.20 | 22.95 | - |
| Experiment | 6.97 | 9.97 | 8.71 ± 0.31 | 0.97 | 8.83 | 11.09 | 6.77 * | |
| Penicillium sp. | Control | 11.20 | 24.37 | 17.50 ± 1.43 | 4.53 | 18.06 | 25.88 | - |
| Experiment | 3.91 | 7.33 | 5.54 ± 0.32 | 1.03 | 5.31 | 18.52 | 8.15 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusupova, A.; Kartabayeva, B.; Sushchenko, R.; Gaysina, K.; Renganathan, P.; Gaysina, L.A. Antifungal Potential of Cyanobacterium Nostoc sp. BCAC 1226 Suspension as a Biocontrol Agent Against Phytopathogenic Fungi and Oomycetes. Appl. Microbiol. 2025, 5, 46. https://doi.org/10.3390/applmicrobiol5020046
Yusupova A, Kartabayeva B, Sushchenko R, Gaysina K, Renganathan P, Gaysina LA. Antifungal Potential of Cyanobacterium Nostoc sp. BCAC 1226 Suspension as a Biocontrol Agent Against Phytopathogenic Fungi and Oomycetes. Applied Microbiology. 2025; 5(2):46. https://doi.org/10.3390/applmicrobiol5020046
Chicago/Turabian StyleYusupova, Adele, Bakhyt Kartabayeva, Rezeda Sushchenko, Kamilla Gaysina, Prabhaharan Renganathan, and Lira A. Gaysina. 2025. "Antifungal Potential of Cyanobacterium Nostoc sp. BCAC 1226 Suspension as a Biocontrol Agent Against Phytopathogenic Fungi and Oomycetes" Applied Microbiology 5, no. 2: 46. https://doi.org/10.3390/applmicrobiol5020046
APA StyleYusupova, A., Kartabayeva, B., Sushchenko, R., Gaysina, K., Renganathan, P., & Gaysina, L. A. (2025). Antifungal Potential of Cyanobacterium Nostoc sp. BCAC 1226 Suspension as a Biocontrol Agent Against Phytopathogenic Fungi and Oomycetes. Applied Microbiology, 5(2), 46. https://doi.org/10.3390/applmicrobiol5020046









