Abstract
In response to the escalating demand for sustainable agricultural methodologies, the utilization of microbial volatile organic compounds (VOCs) as antagonists against phytopathogens has emerged as a viable eco-friendly alternative. Microbial volatiles exhibit rapid diffusion rates, facilitating prompt chemical interactions. Moreover, microorganisms possess the capacity to emit volatiles constitutively, as well as in response to biological interactions and environmental stimuli. In addition to volatile compounds, these bacteria demonstrate the ability to produce soluble metabolites with antifungal properties, such as APE Vf, pyoverdin, and fragin. In this study, we identified two Pseudomonas strains (BJa3 and MCal1) capable of inhibiting the in vitro mycelial growth of the phytopathogenic fungus Aspergillus flavus, which serves as the causal agent of diseases in sugarcane and maize. Utilizing GC/MS analysis, we detected 47 distinct VOCs which were produced by these bacterial strains. Notably, certain volatile compounds, including 1-heptoxydecane and tridecan-2-one, emerged as primary candidates for inhibiting fungal growth. These compounds belong to essential chemical classes previously documented for their antifungal activity, while others represent novel molecules. Furthermore, examination via confocal microscopy unveiled significant morphological alterations, particularly in the cell wall, of mycelia exposed to VOCs emitted by both Pseudomonas species. These findings underscore the potential of the identified BJa3 and MCal1 Pseudomonas strains as promising agents for fungal biocontrol in agricultural crops.
Keywords:
bacteria volatile compounds; Pseudomonas; biological control; phytopathogenic fungus; microbial antagonistic activity Key Contribution:
New Pseudomonas species can inhibit the growth of the fungus Aspergillus flavus.
1. Introduction
Fungi belonging to the genus Aspergillus have historically been utilized as biological agents for controlling the gray sugarcane mealybug Pseudococcus sacchari, a significant pest affecting sugarcane crops worldwide [1]. However, this approach was developed previously to the discovery of aflatoxins, hepatocarcinogenic secondary metabolites produced by fungi, in the early 1960s [2,3] (Coomes and Sanders, 1963; Hartley et al., 1963. Aflatoxins represent pervasive contaminants in food and feed globally, primarily affecting consumers through the ingestion of contaminated seeds and edible plant parts. Aspergillus flavus, known for its production of aflatoxin types B and G, has been identified in maize and traditional herbal products such as yerba mate [4,5]. Moreover, it stands as the predominant phytopathogenic species affecting sugarcane and its by-products in Brazil [6]. Fungal proliferation in crop plants not only incurs substantial economic losses but also poses significant health risks [7,8,9]. Consequently, researchers have endeavored to develop effective strategies for mitigating fungal growth, both pre- and post-harvest [8,9]. Biological control methods have emerged as a particularly prominent avenue due to their cost-effectiveness and environmental compatibility [8]. Estimates suggest that fungal diseases contribute to approximately 30% of global crop losses [1]. Various microorganisms offer potential for suppressing plant diseases by enhancing host immunity or directly inhibiting pathogens [3]. Accordingly, there is a pressing need to explore plant growth-promoting bacteria (PGPB), also known as multifunctional bacteria, which serve as antagonists against phytopathogens, offering an eco-friendly alternative to conventional agrochemicals. PGPBs employ diverse mechanisms to promote plant health and growth, among which the production of volatile organic compounds (VOCs) holds particular significance. VOCs, derived from various biochemical pathways, possess low boiling points and high vapor pressures [10], rendering them potent signaling molecules with potential applications in disease management and plant stimulation.
Volatile organic compounds (VOCs) comprise a diverse array of metabolites that exhibit volatility and are synthesized by various microorganisms. This class of compounds includes alcohols, ketones, thioesters, phenols, and benzene derivatives [11]. Notably, VOCs offer significant advantages as they are biodegradable and can be employed as gaseous treatments, thereby mitigating the accumulation of toxic residues on fruits [12,13,14]. Recent years have seen the growth of interest in bacterial VOCs due to their essential role as signaling molecules in the biocontrol of phytopathogens and the stimulation of plant growth. The gaseous nature of VOCs facilitates their efficient dispersion through porous soil, enabling them to traverse considerable distances. Consequently, VOCs emerge as promising candidates for biocontrol strategies, bolstering the efficacy of antagonistic mechanisms against specific microorganisms [15,16].
Among VOC-producing microorganisms, the bacterial genus Pseudomonas has emerged as a promising biofumigation agent for combatting various fungal diseases. Studies have demonstrated its efficacy in controlling gray mold caused by Botrytis cinerea in Medicago truncatula plants [17,18] and apples [19], as well as tomato wilt induced by Ralstonia solanacearum [20], and blue mold affecting apple and citrus fruits caused by Penicillium expansum and Penicillium italicum [21]. These findings underscore the potential of Pseudomonas-derived VOCs as an appealing alternative for postharvest disease management strategies.
In addition to volatile organic compounds (VOCs), attention should also be directed towards diffusible and soluble compounds, as they cover a vast array of potentially biologically active substances capable of inhibiting the growth of phytopathogens [22,23,24,25]. Among these compounds are cyclic lipopeptides belonging to the iturin, surfactin, and fengicin families, which are renowned for their potent activity against a broad spectrum of phytopathogens, including bacteria, fungi, oomycetes, and viruses [26,27,28].
In the present study, we identified two soil Pseudomonas species (BJa3 and MCal1) that emit relevant volatile signals that were able to inhibit the mycelial growth of A. flavus. Our findings reveal that these bacterial isolates produce volatile organic compounds (VOCs) which exhibit potent antagonistic activity when co-cultivated with the fungus. To characterize the VOCs emitted by our Pseudomonas isolates during co-cultivation with A. flavus and in isolation, we employed solid-phase microextraction coupled with gas chromatography–mass spectrometry (SPME/GC–MS) analysis. Furthermore, confocal laser scanning microscopy (CLSM) analysis was conducted to ascertain the damage inflicted by the bacterial VOCs on the hyphae, mycelia, and conidia of A. flavus, thereby impeding its development. Additionally, genomic and experimental analyses indicated that the bacterial isolates produce soluble and diffusible compounds capable of inhibiting the mycelial growth of A. flavus. Overall, our in vitro and in silico results underscore the multifaceted antimicrobial activity exhibited by strains BJa3 and MCal1 at both the diffusible and volatile levels, thus underscoring their potential utility as valuable fungicidal agents.
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
The phytopathogenic fungus A. flavus (URM 7262) was kindly provided by the Collection of cultures—Micoteca URM, from the Biological Sciences Center of the Federal University of Pernambuco. Fungus was cultivated on potato dextrose agar (PDA; agar, 15 g/L; dextrose, 20 g/L; potato extract, 4 g/L) (Sigma-Aldrich, Burlington, MA, USA) for VOC assays and on Vogel Medium (Na3C6H5O7. 2H2O, 125 g/L; KH2PO4, 250 g/L; NH4NO3, 100 g/L; MgSO4. 7H2O, 10 g/L; CaCl2. 2H2O, 5 g/L; Trace element solution, 5 mL/L; biotin solution, 2.5 mL/L; sucrose, 15 g/L) for diffusion assay. The 10 evaluated bacteria (all of which belong to the Pseudomonas genus) were isolated from soil and sugarcane juice samples in the region of Ribeirão Preto, Brazil (21°10′40″ S; 47°48′36″ W). Bacteria were isolated using abundant and inexpensive carbon sources such as sugarcane juice (MCal1, M3 and MGal98 strains) and sodium benzoate (BJa3 and Bagro211 strains). After, all bacteria were routinely grown on Luria–Bertani (LB; NaCl, 10 g/L; Tryptone, 10 g/L; Yeast Extract, 5 g/L) (Sigma-Aldrich, Burlington, MA, USA) medium at 30 °C.
2.2. Phylogenetic Analysis of BJa3 and MCal1 Isolates
Total DNA of Pseudomonas spp. selected strains was extracted using the GeneJET Genomic Purification Kit (Thermo Fischer Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The concentration of DNA was determined fluorometrically using the Qubit® 3.0 (Qubit® dsDNA Broad Range Assay Kit, Life Technologies, Carlsbad, CA, USA). DNA library was prepared using the Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA, USA), assessed for quality using the 2100 Bioanalyzer (Agilent Genomics, Santa Clara, CA, USA), and subsequently submitted to sequencing using the Illumina HiSeq (2 × 150 bp) platform (Illumina, San Diego, CA, USA). The phylogenetic analysis of the evaluated isolates was performed using the whole genome. To assemble the genomes, the sequencing quality was first analyzed using the FastQC tool (v. 0.11.9) followed by trimming with the Trimmomatic program (version 0.38.0) [29] and the removal of possible human contamination reads with the bowtie2 tool (v. 2.3.5.1) [30]. The SPAdes assembler (v. 3.12) [31] was chosen for genome assembly and the MEGAHIT tool (v. 1.2.9) [32] was used as a secondary assembler. Finally, the FGAP (v. 7.17) [33], which consists of a tool to fill in the undefined bases of assemblies, was used with the extended paired-end bases. Additionally, the annotation of the genes was carried out with the PROKKA tool (v. 1.12) [34]. The phylogenetic identification of the isolates was performed using the TYGS platform [35], and the classification was based on the Digital DNA-DNA hybridization (dDDH) value [36].
2.3. Detection of VOCs Antagonistic Activity In Vitro
The impact of VOCs produced by the 10 bacteria from various genera was assessed for the initial screening using 2-compartment Petri dishes. A. flavus was raised in a Petri dish filled with PDA medium and incubated for five days in a growth chamber at 28 °C. One compartment of the plate containing LB medium was spread with 100 μL of a fresh bacterial suspension (about 5 × 108 CFU), and the other compartment containing PDA was dripped with a spore solution containing 106 spores mL−1. The plates were covered with Parafilm® and kept at 28 °C for two days of incubation. Plates without bacterial inoculum (negative control) or containing bacteria E. coli DH5α and P. putida KT 2440 were used as experiment controls.
The 5 bacteria that had the more significant visual effects on the growth of A. flavus from an initial screening were then tested in triplicate. Then, the top 2 isolates in terms of fungal inhibition were selected for ensuing examinations.
For VOCs synergy tests on fungal growth, the 2 most promising isolates were placed on the same plate and cultivated jointly. Each bacterial inoculum was spread out over 50 μL (about 5 × 108 CFU) in one of the divided plate compartments. A solution containing roughly 106 A. flavus spores was inoculated on the opposing side. Plates without bacterial inoculum (negative control) or containing bacteria E. coli DH5α and P. putida KT 2440 were used as experiment controls.
2.4. Detection of Soluble Antagonist Compounds
To detect the presence of antifungal compounds in bacterial cultures cultivated in liquid medium, strains BJa3, MCal1, and DH5α were cultivated in 50 mL of LB medium at 30 °C and 200 rpm for 16 h. A. flavus fungus was cultivated in 50 mL of Vogel medium at 28 °C and 150 rpm during 48 h. Then, inocula were collected and mixed at a ratio of 1/10 (100 μL of supernatant of A. flavus to 900 μL of supernatant of bacterial isolates) and incubated again in LB medium at 30 °C and 200 rpm for 16 h. Inocula containing only bacteria were also made. After this time, the inocula were collected and centrifuged at 10,000 rpm for 10 min, and supernatants were filtered through a 0.22 mm filter. For the negative control, Vogel, Vogel plus LB, and LB media were used instead of cell-free supernatant. One mL of the supernatant was mixed with agar–LB and poured into a Petri dish. A 5 μL solution of A. flavus was inoculated in the center of each plate and incubated for 2 days at 28 °C. Later, mycelial growth was measured. Three independent assays were performed. In addition, the online tool antiSMASH (https://antismash.secondarymetabolites.org/#!/start) (accessed on 24 May 2023) [12] was used to determine which soluble compounds were produced by the BJa3 and MCal1 bacteria, using the assembled genomes of the respective bacteria as input.
2.5. Statistical Data Analysis
In order to determine if there were significant differences between the 2 groups, one-way ANOVA test was used. Tukey’s test was used to compare means and standard deviations using GraphPad Prism v.8.0.2 software (San Diego, CA, USA). p values less than 0.05 were considered statistically significant.
2.6. Morphological Characterization of A. flavus after VOC Exposure of BJa3, MCal1, and DH5α by Confocal Laser Scanning Microscopy (CLSM)
Changes in the external morphology of A. flavus after exposure to the selected isolates, BJa3, MCal1, and DH5α, at a final concentration of 5 × 108 CFU of bacterial solution were examined using CLSM. DH5α strain as well as microcultures not exposed to bacterial cultures were used as negative controls. Fungus were cultivated, with minor modifications, according to the protocol by Li et al. (2010) [37]. A. flavus fungus was collected after 2 days of growth using an inoculation loop to preserve the integrity of the mycelia and placed at the four corners of a PDA-medium rectangle. This structure was placed on a microscope slide and inserted into a 50 mL conical flask. Previously, cultures of the selected bacteria in 5 mL of LB medium were cultivated inside the flask. This system was incubated for 2 days at 28 °C. Later, the samples were washed in Phosphate-Buffered Saline (PBS) and stained with 50 μL of calcofluor white (Sigma-Aldrich, USA) for 10 min. Images were acquired in Confocal Microscopy Multiuser Laboratory (LMMC—USP) with a Leica SP5 microscope (Leica Microsystems, Wetzlar, Germany) using plan apochromatic 40× (NA 1.25, oil) and 63× (NA 1.4, oil). Calcofluor-stained samples were recorded in blue channel (395 nm) and the emission wavelength was 440 nm.
2.7. Bacterial VOCs Identification by Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry (SPME/GC–MS)
Volatilome analysis of the antagonists was performed at the Organic Micromolecule Mass Spectrometry Center (CEMMO) located at FCFRP/USP (Ribeirão Preto, SP, Brazil). Fifty microliters of bacterial suspension (~108 CFU) of the selected isolates (BJa3, MCal1 and DH5α) and of the A. flavus fungus were inoculated in 10 mL flasks with screw caps and PTFE/Silicone septa (Supelco, Bellefonte, PA, USA), and 1 mL of the respective culture medium was deposited on each side of the flask (LB for bacteria and PDA for fungus). Microorganisms were incubated for 48 h at 28 °C in quadruplicates. For control samples, a flask containing only culture medium without bacterial inoculum was used as well as E. coli DH5α.
VOCs produced were collected by using the solid-phase microextraction (SPME) technique [38] with a StableFlex fiber 2 cm of 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (Supelco, Bellefonte, PA, USA). The SPME fiber was inserted into the 10 mL glass vials equipped with screw caps and PTFE/Silicone septa and extraction was performed in headspace mode at 55 °C for 30 min. Then, the fiber was desorbed into the GC port at 240 °C for 3 min in split mode and separated in a GC-MS QP2010 Ultra (Shimadzu, Kyoto, Japan) equipped with a ZB-5MS column (30 m × 0.25 mm inner diameter × 0.25 µm film thickness). The temperature program was from 40 °C (held for 3 min) to 220 °C at 7 °C/min and then to 260 °C at 12 °C/min (held for 2 min). The mass spectrometer was operated in the electron ionization mode at 70 eV. The ion source and transfer line temperature were set, respectively, at 250 °C and 260 °C. Mass spectra were scanned in the range m/z 35–400 amu. For VOCs identification, mass spectra were compared with those of the Global Natural Products Social Molecular Networking (GNPS) mass spectrometry library [39].
2.8. Bacterial Volatilome Analysis
The Shimadzu GGD files were converted to CDF format with Openchrom software (v. 15.0) (https://lablicate.com/platform/openchrom) (accessed on 7 July 2023). The converted spectra were uploaded to the GNPS platform [15] and pre-processing was performed with MSHUB-GC workflow (https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=9532209f010b442a9efe95a7897efd66) (accessed on 7 July 2023). The deconvoluted fragmentation spectra obtained from pre-processing were submitted to the MOLECULAR-LIBRARY SEARCH-GC workflow (https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=b6d863f7266f4bb7a0918e576abe64fb) (accessed on 7 July 2023) to perform spectral networking and automated spectral matching, which was where the compound annotations were obtained from. Principal Component Analysis was performed after spectra total peak area normalization and auto-scaling. Heatmaps were plotted using the mean of the spectra peak area relative to the abundance of each group (A. flavus alone; A. flavus + bacteria; bacteria alone). We searched for statistically significant differences in spectra peak area in each group using the Kruskal–Wallis test [40] (α = 0.05).
3. Results and Discussion
3.1. BJa3 and MCal1 Produce Volatile Compounds with Antifungal Activity
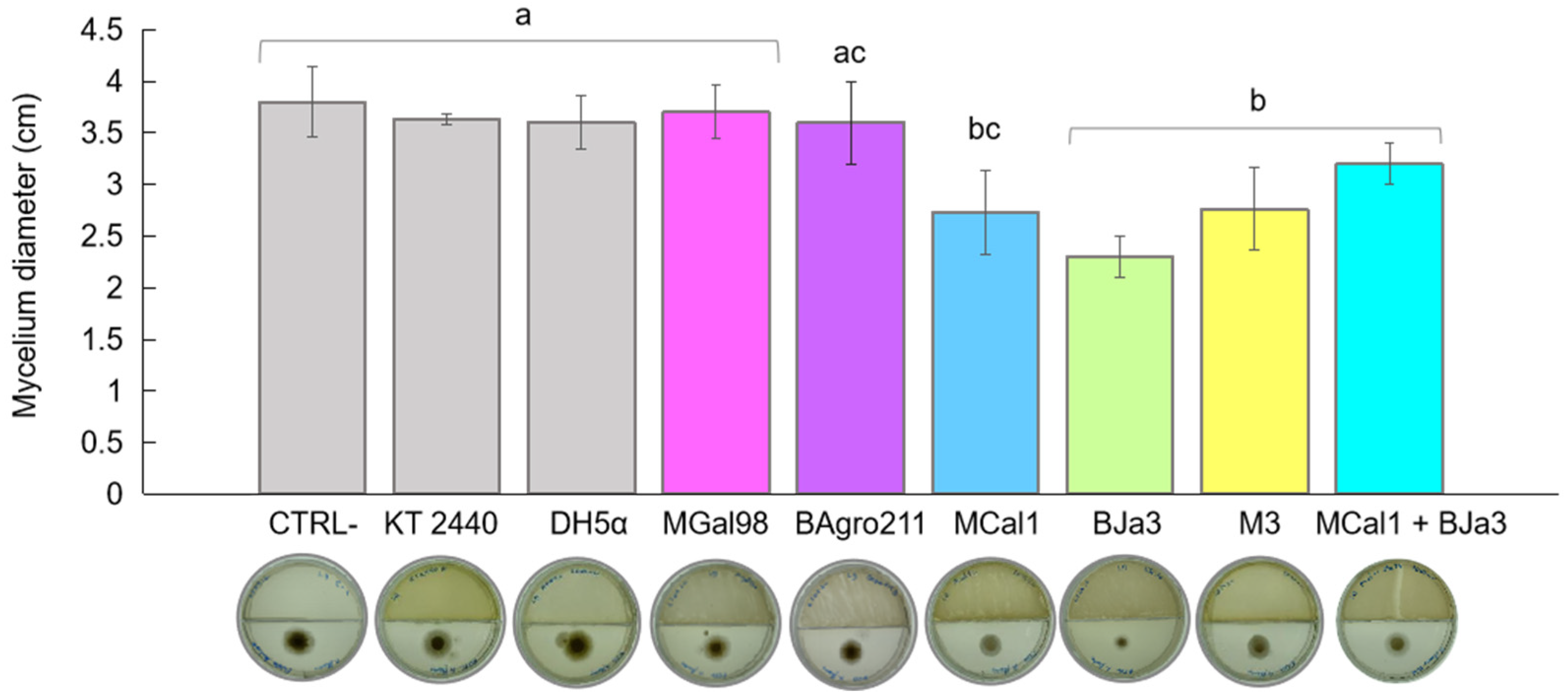
Numerous species within the genus Pseudomonas are known inhabitants of soil and rhizosphere environments, and concurrently display antagonistic behaviors against phytopathogenic fungi [41,42,43,44,45,46]. Motivated by these findings, we investigated Pseudomonas spp. isolates sourced from soil and sugarcane juice as potential candidates for biocontrol agents. Initially, we conducted a screening of 10 bacterial isolates to identify VOC-producing strains with inhibitory effects against the growth of A. flavus. Based on quantitative assessments of mycelium diameter (Figure 1), we identified the top five most promising bacteria. Escherichia coli DH5α and the soil bacterium Pseudomonas putida KT 2440 were employed as controls. As depicted in Figure 1, the strains BJa3 and MCal1 exhibited approximately 50% inhibition of A. flavus growth, followed by M3 at 40%, relative to the control (without bacterial inoculum). Notably, cultivation of A. flavus in the presence of strain MCal1 resulted in conspicuous alterations to the mycelium’s appearance, characterized by discernible differences in color and structure compared to the negative control (Figure S1). In contrast, E. coli DH5α and P. putida KT 2440 did not exhibit an inhibition of fungal growth. Consistent with our observations, E. coli DH5α previously demonstrated a lack of antifungal activity in the literature [20]. Subsequently, the isolates which demonstrated the most potent antifungal activity (BJa3 and MCal1) were selected for further experimentation. The measurements of the halos were carried out after two days of incubation, because after this period, the supply of nutrients for the bacteria becomes sparser, favoring a drop in their growth and, consequently, a lower production of VOCs.
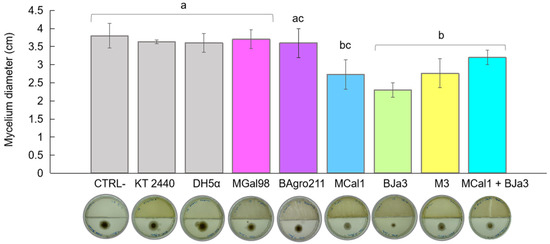
Figure 1.
Effect of bacterial VOCs on A. flavus growth inhibition after 2 days of co-culture. Inhibitory effect of bacterial VOCs from monocultures and mixtures of BJa3 with MCal1. Controls correspond to A. flavus without the bacterial inoculum, E. coli DH5α and Pseudomonas KT 2440. Different letters indicate significant differences between treatments. Standard errors are indicated by vertical lines (Tukey’s test, p-value < 0.05).
A variety of factors affect the composition of the volatile metabolome subset, also known as the volatilome. These factors include elements such as culture media, temperature, pH, interactions with other organisms, and growth stage [45,47]. Remarkably, prior investigations have revealed that nutrient-rich media, such as Luria–Bertani (LB), not only promote bacterial growth and fungal inhibition but also enhance the production of volatile organic compounds (VOCs) by bacteria [48]. Moreover, distinct volatile compositions have been observed between monocultures and microbial mixtures [48]. To evaluate the potential synergistic antagonistic effect of VOC emissions against A. flavus, we conducted co-cultivation experiments involving the two most promising bacteria, BJa3 and MCal1, in conjunction with the fungus. Contrary to our expectations, the co-cultivation of the BJa3 and MCal1 strains did not result in a significant inhibition of fungal growth (Figure 1). Recent investigations have elucidated that interactions among different bacterial species can either induce or suppress the emission of volatile chemicals that modulate microbial growth dynamics [49,50]. These findings highlighted a predisposition for greater antifungal activity when antagonistic bacteria are cultivated in nutrient-rich media and separately.
Despite the demonstrated efficacy of VOCs in defending against phytopathogens in controlled laboratory assays, their effectiveness in enhancing plant defenses demands a range of additional tests that include various factors that must be considered. Methodologies for the direct application of VOCs in agricultural fields and greenhouses are rarely explored and require further investigation. Only a few studies have tested the effect of VOCs on plant growth promotion and disease suppression under field conditions through soil-drench methods, spray applications, and seed treatments [51,52,53,54].
Several factors may cause differences between in vitro and in vivo tests. One of these is the fact that laboratory experiments demonstrating the efficacy of VOCs typically involve much higher bacterial concentrations than those achievable in open fields. Additionally, while the high biodegradability of VOCs may minimize their negative environmental impact, the reactivity of VOCs limits the distance they travel in fields and barns, thereby limiting their persistence and activity [55,56,57]. Moreover, the very essence of VOCs (volatility) means that these compounds are highly influenced by meteorological factors such as wind speed and direction, humidity, rainfall, and temperature.
Another factor that should be considered is aeration conditions. Under aerobic conditions, bacteria utilize any carbon source for cell growth, with only a small fraction being used for VOC production. Under microaerophilic and anaerobic conditions, bacteria initiate fermentation by utilizing carbon sources, and these are involved in the biosynthesis and emission of various VOCs [58,59].
Despite all the challenges in field conditions, VOCs can still be effectively utilized, as the necessary concentration of VOCs can be maintained in enclosed environments [59]. However, to fully assess their effects, multiple analyses and investigations are required to examine the efficacy of VOCs produced under natural conditions during plant–microorganism interactions.
3.2. The Strain MCal1 Represents a Novel Species within the Pseudomonas Genus, while BJa3 Is Categorized within the Pseudomonas Soli Species
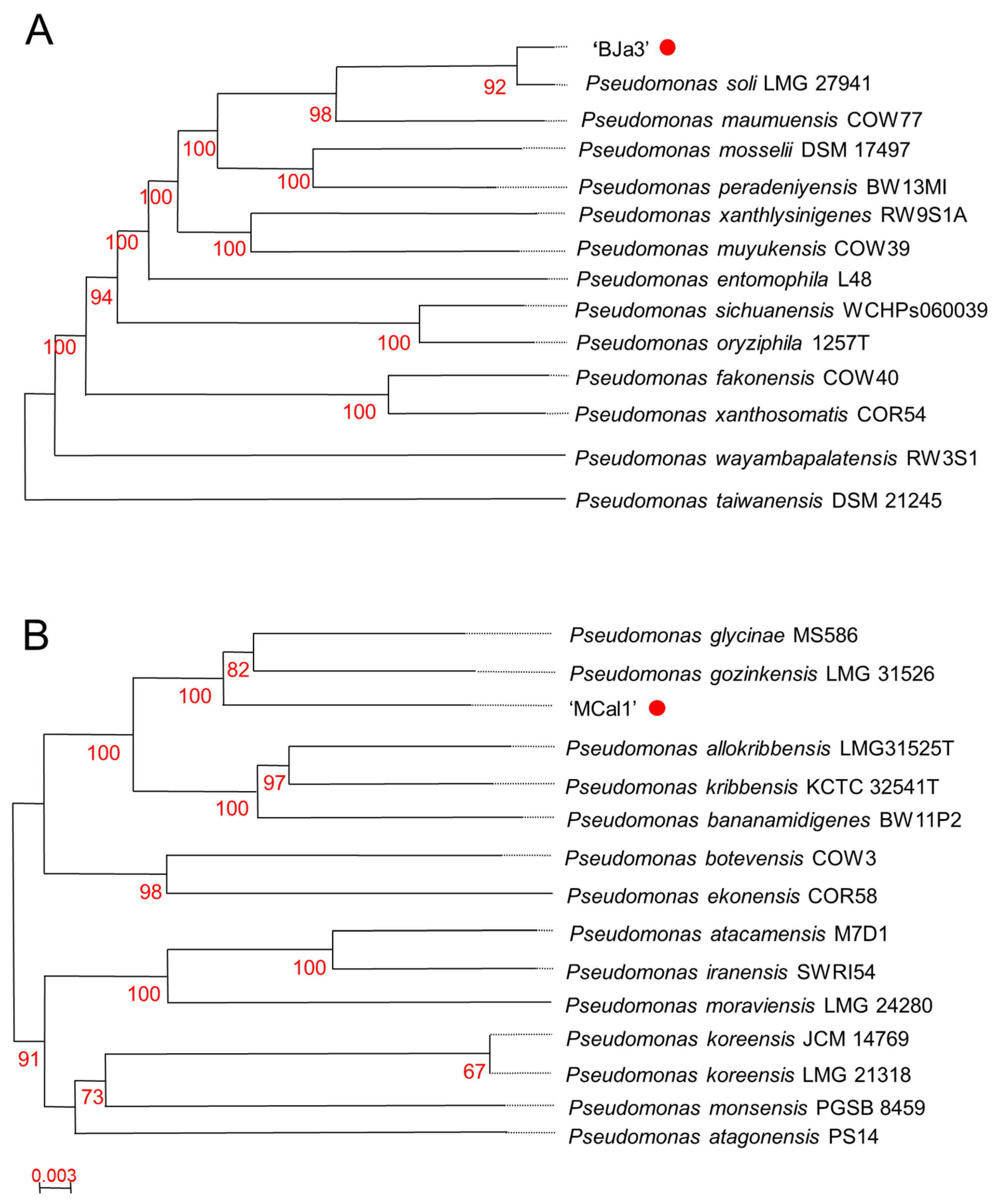
Based on the antifungal activity screening results, we elected to take a deeper look into the genomic characteristics of the strains MCal1 and BJa3. These strains, isolated from soil (BJa3) and sugarcane juice (MCal1), underwent genome sequencing. Digital DNA-DNA hybridization (dDDH) analysis revealed their affiliation with the genus Pseudomonas (Figure 2). Specifically, the BJa3 strain exhibited a close similarity to Pseudomonas soli species, with a dDDH4 value exceeding 90%, indicating that they very possibly belong to the same species (Table S1 and Figure 2A) [36]. Conversely, the Pseudomonas sp. MCal1 strain clustered within the same monophyletic group as Pseudomonas glycinae and Pseudomonas gozinkensis (refer to Figure 2B). However, upon comparing the MCal1 genome with 14 closely related Pseudomonas species genomes available on GenBank, the dDDH4 value surpassed 50% only with the two most similar strains (Table S2). This result suggests that the Pseudomonas sp. MCal1 may be a new Pseudomonas species.
The genome size of BJA3 was estimated to be 5.9 Mb with 5272 coding sequences and the GC content was estimated to be 64.05% (Table S3). The MCal1 strain has a genome size of 6.2 Mb, 5650 coding sequences and a GC percentage of 60.42%. Both DNA GC content and genome size can be considered as important taxonomic markers. It was observed that, in Pseudomonas, the GC content varies from 48.3% to 68.3% [60,61]. Genome size differences vary within the genus, as does the corresponding gene content, and, in this case, genome size is directly related to the number of coding sequences. In Pseudomonas, coding genes ranged from 2803 (genome size 3.03 Mb) to 6895 (genome size 7.38 Mb) [62]. Therefore, both the genome size and coding sequences and the GC content of the sequenced genomes are within the expected ranges of bacteria belonging to the Pseudomonas genus.
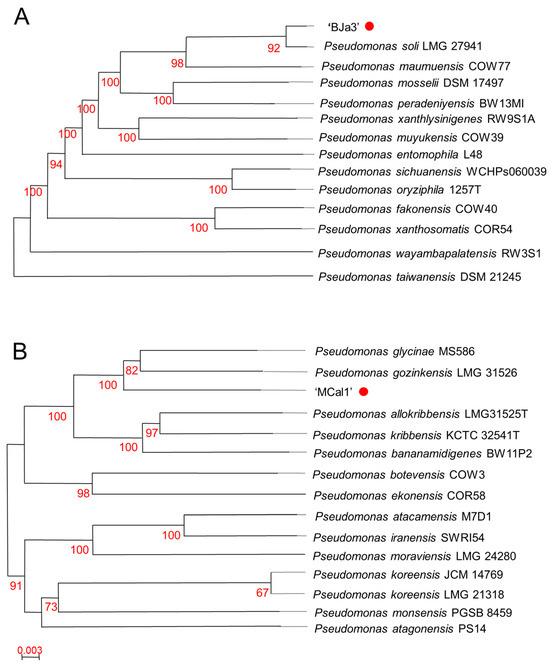
Figure 2.
Taxonomic affiliation of Pseudomonas sp. strains BJa3 and MCal1. Tree of BJa3 (A) and MCal1 (B) inferred with FastME [63] from Genome BLAST Distance Phylogeny method (GBDP) distances calculated from genome sequences. Branch lengths are scaled in terms of GBDP distance formula d5; numbers above branches are GBDP pseudo-bootstrap support values from 100 replications. The trees were constructed using the TYGS tool.
Figure 2.
Taxonomic affiliation of Pseudomonas sp. strains BJa3 and MCal1. Tree of BJa3 (A) and MCal1 (B) inferred with FastME [63] from Genome BLAST Distance Phylogeny method (GBDP) distances calculated from genome sequences. Branch lengths are scaled in terms of GBDP distance formula d5; numbers above branches are GBDP pseudo-bootstrap support values from 100 replications. The trees were constructed using the TYGS tool.
3.3. MCal1 and BJa3 Also Exhibit Antimicrobial Effects on A. flavus through Diffusible Compounds Production
Considering that the strains BJa3 and MCal1 were able to hamper fungal growth in two-compartment Petri dishes with VOCs, we wondered if antagonistic activity was also produced by agar-diffusible compounds. It has been shown that some compounds that are soluble in liquid media are capable of inhibiting fungal development and altering some characteristics of the mycelium [64]. To check whether our bacterial strains produced soluble compounds of interest, we first carried out a search for secondary metabolites using the whole genomes of these bacteria as inputs to the antiSMASH tool (v. 7.1.0) (Table S4).
The genomic results showed shared antifungal secondary metabolites between strains BJa3 and MCal1, notably APE Vf, pyoverdin, and fragin. These compounds exhibit distinct mechanisms of action. The arylpolyene gene cluster (APE Vf) identified in the genome of strain BJa3 represents a compound with multifaceted benefits, contributing to plant protection against oxidative stress and exhibiting various biological functions. This includes effective biocontrol against the pathogenic fungus Fusarium oxysporum, thereby enhancing plant survival [65,66].
Another crucial identified gene cluster involves the siderophore pyoverdin, which plays a significant role in impeding biofilm formation on the phytopathogenic fungus Aspergillus fumigatus [67,68]. Additionally, fragin, categorized as a metallophore, demonstrates noteworthy antifungal activity primarily based on its ability to chelate metals, thereby disrupting fungal growth [69].
Several noteworthy secondary metabolites were exclusively identified within the BJa3 strain. One such compound, putisolvin, stands out as a lipopeptide, functioning as a biosurfactant. Its significance lies in facilitating swarming motility and biofilm formation while exhibiting zoosporicidal and antifungal activities [70]. Another compelling cluster identified at the genome level was pseudopyronine A/pseudopyronine B, demonstrating potent activity against plant pathogenic fungi [71]. Moreover, the genomic cluster responsible for the lipopeptide viscosin production was also identified. Similar to pyoverdin, this compound displays remarkable biocontrol properties against Aspergillus fumigatus [72].
The exclusive soluble compounds detected solely within the MCal1 strain encompass bacillomycin D and fengycin. Within the category of lipopeptides, bacillomycin D, classified among the iturin family, stands out as an antifungal secondary metabolite. This compound operates by impairing the cell wall and membrane of fungal hyphae and spores, causing disruptions within the cytoplasm and organelles, resulting in exudation and empty cavities within the cell structure [73]. Similarly, fengycin, another potent antifungal compound potentially produced by MCal1, constitutes a lipopeptide complex specifically targeting filamentous fungi. Notably, it exhibits an ineffectiveness against yeasts and bacteria [74].
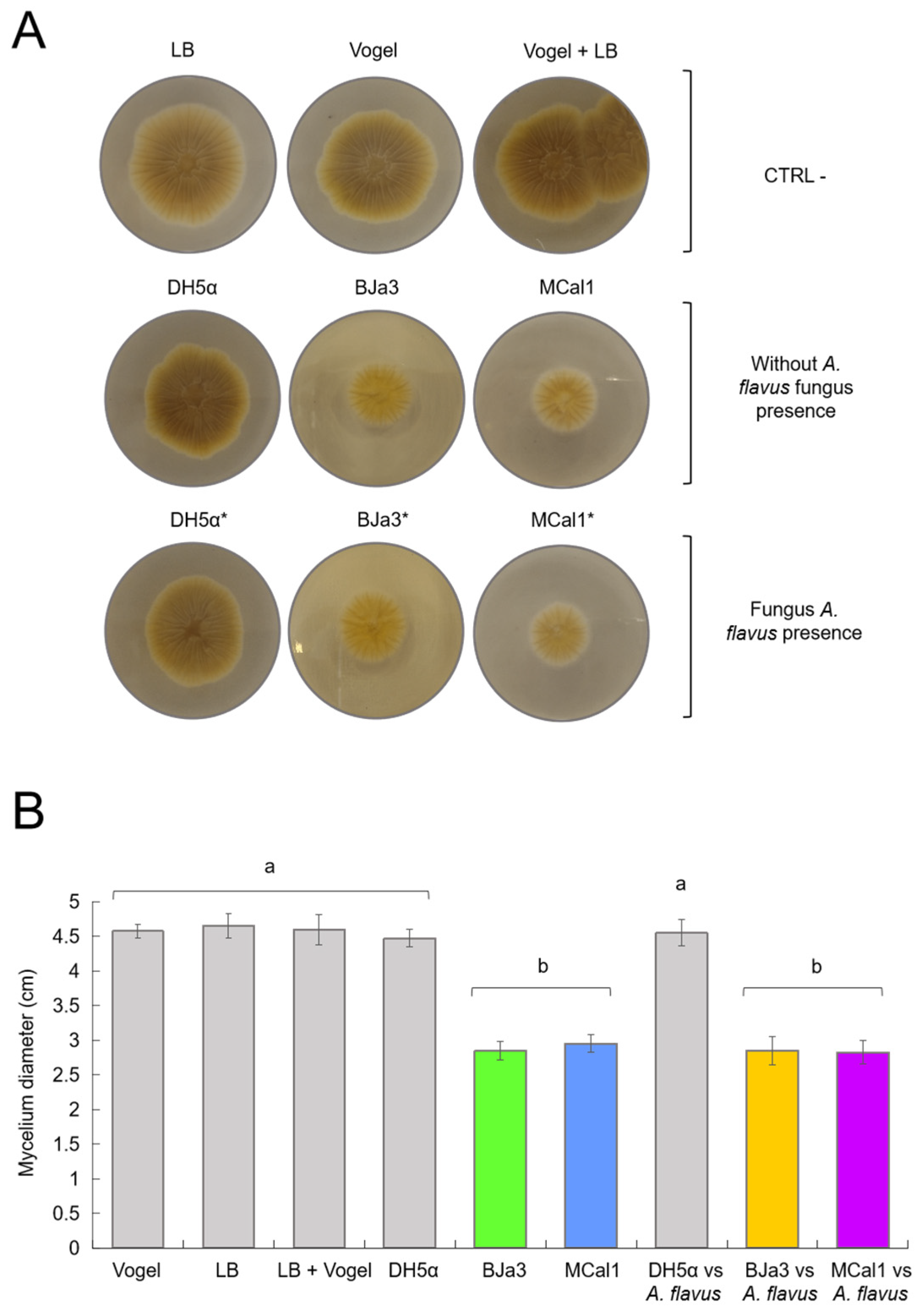
In order to check that the strains were indeed also able to inhibit fungal growth through soluble compounds, we carried out experiments in LB agar and Vogel agar culture media supplemented with cell-free supernatants of the BJa3 and MCal1 strains. The results shown in Figure 3 indicate that the strains BJa3 and MCal1’s supernatants contained diffusible compounds that led to the inhibition of the growth of A. flavus when the bacteria were cultivated alone in LB broth compared to the negative control (A. flavus cultivated alone) (Figure 3).
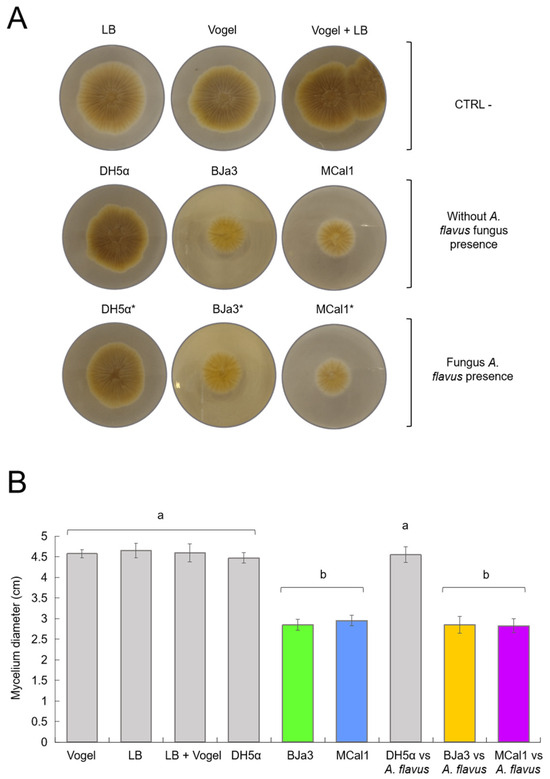
Figure 3.
Effect of bacterial-diffusible compounds on A. flavus growth inhibition after 2 days of co-culture. (A) Controls (CTRL-) corresponding to A. flavus without the bacterial inoculum, using Vogel, LB, and Vogel + LB medium, respectively. Effects of diffusible compounds in the absence of fungi with the bacteria E. coli DH5α and Pseudomonas BJa3 and MCal1, respectively, on the mycelial growth of the fungus A. flavus are shown in the second line. Effects of diffusible compounds on the mycelial growth of the fungus A. flavus from the bacteria E. coli DH5α and Pseudomonas BJa3 and MCal1, respectively, when cultivated in the presence of the fungus in liquid medium are represented in the third line. (B) Inhibitory effect of soluble bacterial compounds from the 2 selected isolates on the growth of A. flavus mycelium. The asterisk (*) indicates the condition of co-culture of bacteria and fungus in the inoculum. Different letters indicate significant statistical differences between treatments. Standard errors are indicated by vertical lines (Tukey’s test, p-value < 0.05).
As the two strains proved to be capable of producing diffusible compounds with antimicrobial activity when cultivated alone, we wondered if they were also able to produce them when cultivated in the presence of the fungus in liquid medium, a condition that should induce a metabolic response in bacterial metabolism. As shown in Figure 3, the growth of A. flavus was similarly inhibited both in cell-free supernatants of BJa3 and MCal1 cultivated alone or with A. flavus after 24 h of incubation. Thus, in both conditions (bacteria cultivated with or without the fungus), fungal growth decreased by approximately 60% compared to the negative control (Figure 3).
Also, we used supernatants of E. coli DH5α as control bacteria, in both the conditions of cultivation alone and with A. flavus, with no apparent influence on fungal growth, similar to the negative control. Moreover, the impact of LB and Vogel culture media and their combination on bacteria cultivation was also verified. Thus, as observed in Figure 3, none of the controls (E. coli DH5α or culture media) hampered the fungus’ growth or morphology.
3.4. VOCs Produced by BJa3 and MCal1 Strains Affect the Morphology of A. flavus
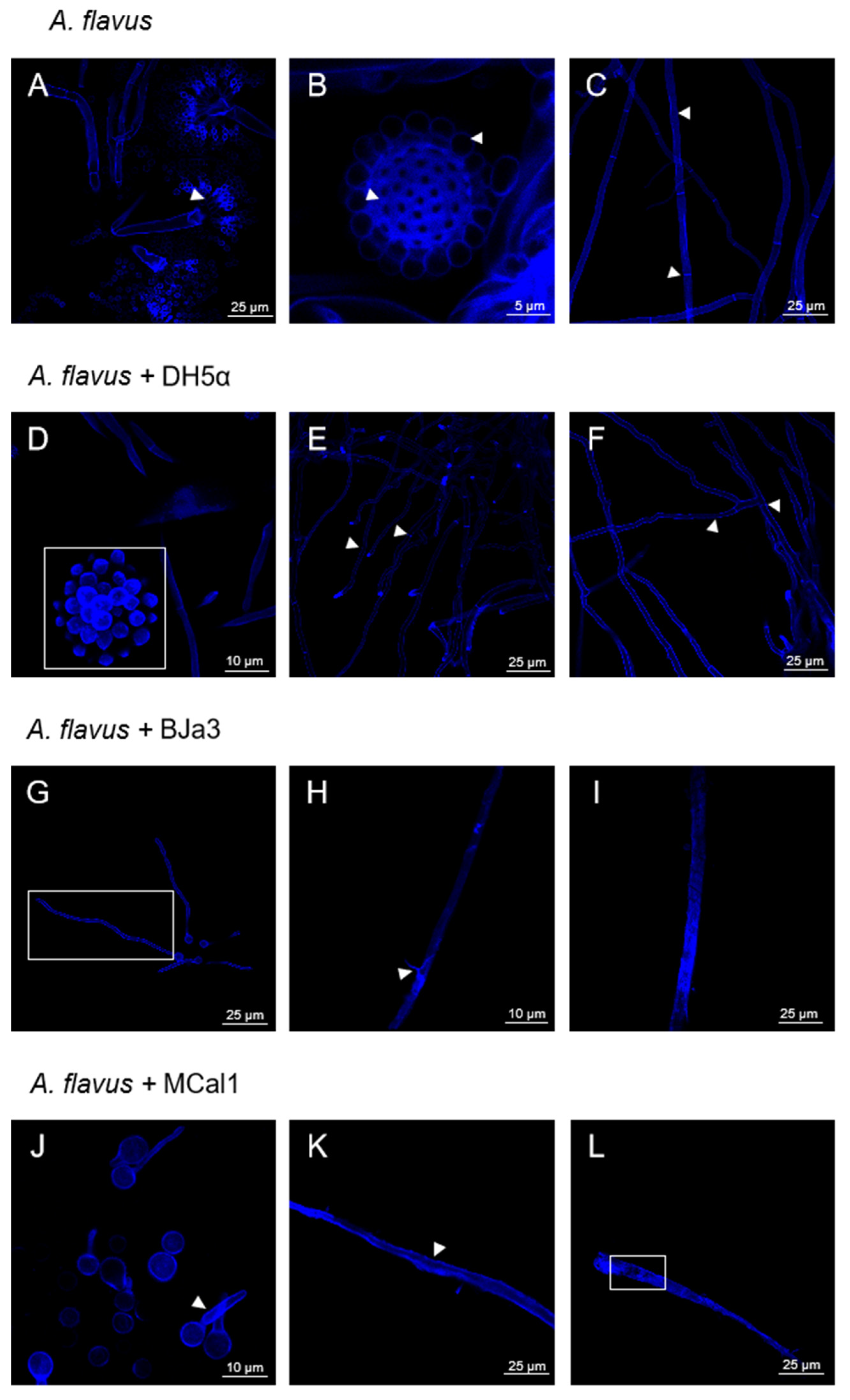
Once we had demonstrated the inhibition of the growth of A. flavus by the new Pseudomonas strains, we looked into the structural and morphological alterations in A. flavus when it was exposed to the volatiles produced by BJa3, MCal1, and DH5α. To achieve this goal, microcultures of A. flavus were created and incubated for 48 h before CLSM analysis. As shown in Figure 4, it was possible to observe that all fungal structures were preserved when A. flavus was cultivated without bacteria in the microculture (negative control). The biseriate conidial head, with the primary separation into metulae and phialides, which is very characteristic of the A. flavus species [44,75], is in early formation and is well characterized and preserved in the control condition (Figure 4A, arrow head). It is also possible to observe that the vesicle and conidia are being formed and their morphology is intact (Figure 4B, arrow head). Conidia of A. flavus have relatively thin walls which are finely to moderately roughened. Their shape can vary from spherical to elliptical [75]. Furthermore, the walls of the hyphae are intact, with well-demarcated septa (Figure 4C, arrow head).
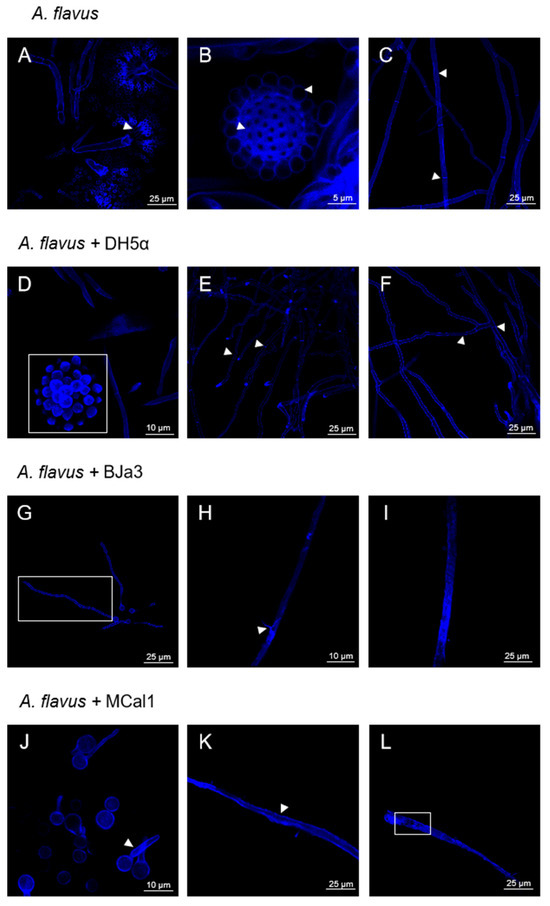
Figure 4.
Representative confocal laser scanning microscopy of the mycelium of A. flavus. (A) Conidial head of A. flavus, with phialides highlighted by the arrow head. (B) Vesicle and conidia structures are intact and developed, as highlighted by the arrow heads. (C) Developed hyphae with demarcated septa, shown by the arrow heads. Images (A–C) are fungal structures preserved in the absence of bacteria. (D) Conidial head with developed conidia, as highlighted by the white square. (D,F) Preserved and intact hyphae, with septa and intact hyphae structure, as indicated by the arrowheads. Images (D–F) are fungal structures preserved in the presence of volatiles from E. coli DH5α. (G) Fungus exhibits signs of late germination, such as thinner and less developed hyphae, delimited by white rectangle. (H,I) Hyphae with a scaly appearance, as highlighted by the arrowhead and without septa. Images (G–I) are fungal structures damaged in the presence of volatiles from strain BJa3. (J) Late germination of hyphae. (K) Hyphae with internal cell darkening, as highlighted by the arrow head. (L) Hyphae with internal disorganization and absence of septa, as demarcated by the white rectangle. Images (J–L) are fungal structures damaged in the presence of volatiles from strain MCal1.
Similarly, when A. flavus was cultivated in the presence of E. coli DH5α, the resulting fungal structures were similar to the negative control, with the formation of the conidial head and conidia (Figure 4D, white square). The hyphae were also intact and had well-defined septa (Figure 4E,F, arrow head). Thus, according to these results, E. coli does not have an impact on the morphology of A. flavus or interfere with the development of its vegetative and reproductive structures.
On the other hand, exposing the fungus to the Pseudomonas volatiles caused changes in the cell wall and hyphal germination of A. flavus (Figure 4G–L). Accordingly, after 48 h of exposure to the BJa3 isolate, the fungus exhibited signs of late germination, including thinner and less developed hyphae (Figure 4G, white rectangle). Additionally, the hyphae had a scaly, vacuolated appearance without septa (Figure 4H, arrow head and Figure 4I). Similar alterations in the structures of the cell wall and germination were found in A. flavus in the presence of the MCal1 strain. In addition to delayed germination (Figure 4J, arrow head), disorganization and destruction of the hyphal structure, as well as internal cell darkening, were also seen, resulting in abnormal fungal cell growth (Figure 4K, arrow head and Figure 4L, white rectangle). Comparable alterations in fungal morphology due to the presence of volatile compounds produced by bacteria were found in the literature [20,76,77,78,79]. For example, some VOCs have been reported to cause structural changes to the cell envelope or even damage the cell wall in the Thielaviopsis ethacetica fungus [80]. In this study, the authors proposed that the overexpression of ABC transporter proteins may be related to their role in osmo-adaptation and the maintenance of cell integrity and survival in response to undesirable changes occurring to the cell [20,76,78].
Similarly, actinomycete volatiles may alter the morphology of conidiophores and hyphae in a number of fungi [77] and prevent Cladosporium spp. conidiophores from germinating [79], as observed in our work (Figure 4G,J). According to Hernandez-León et al. (2015), some Pseudomonas fluorescens strains produced VOCs that prevented the growth of the phytopathogenic fungus Botrytis cinerea [17]. Analogous to this, Agrobacterium tumefaciens and Agrobacterium vitis were both inhibited by the VOCs produced by the P. fluorescens strain B-4117 [81]. Furthermore, it is essential to note that many current commercially used fungicides also have the membrane and cell wall as their primary targets [82,83].
3.5. Strains BJa3, MCal1, and DH5α Produce VOCs That Are Species-Specific and Pseudomonas Strains Produce Compounds with Antifungal Potential
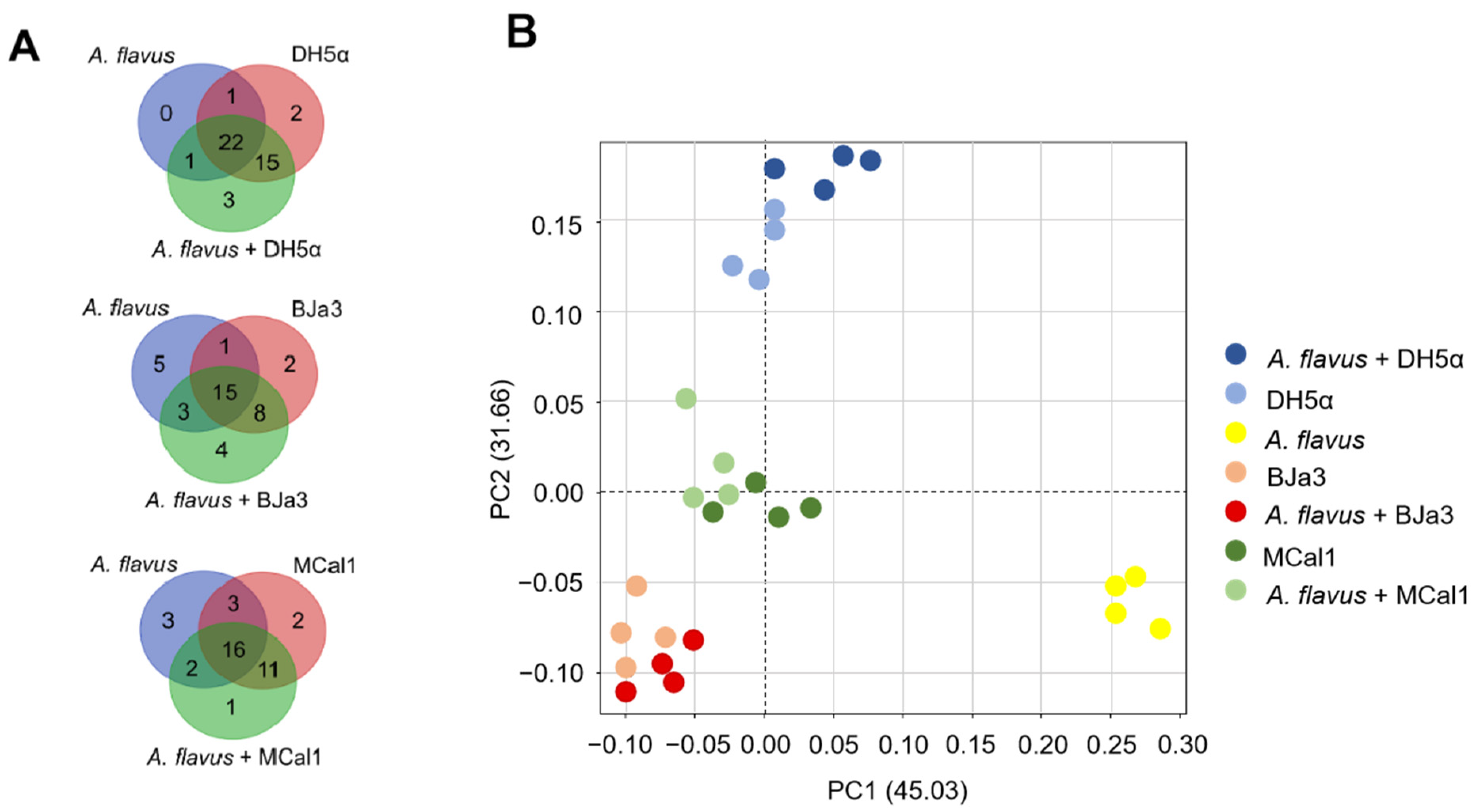
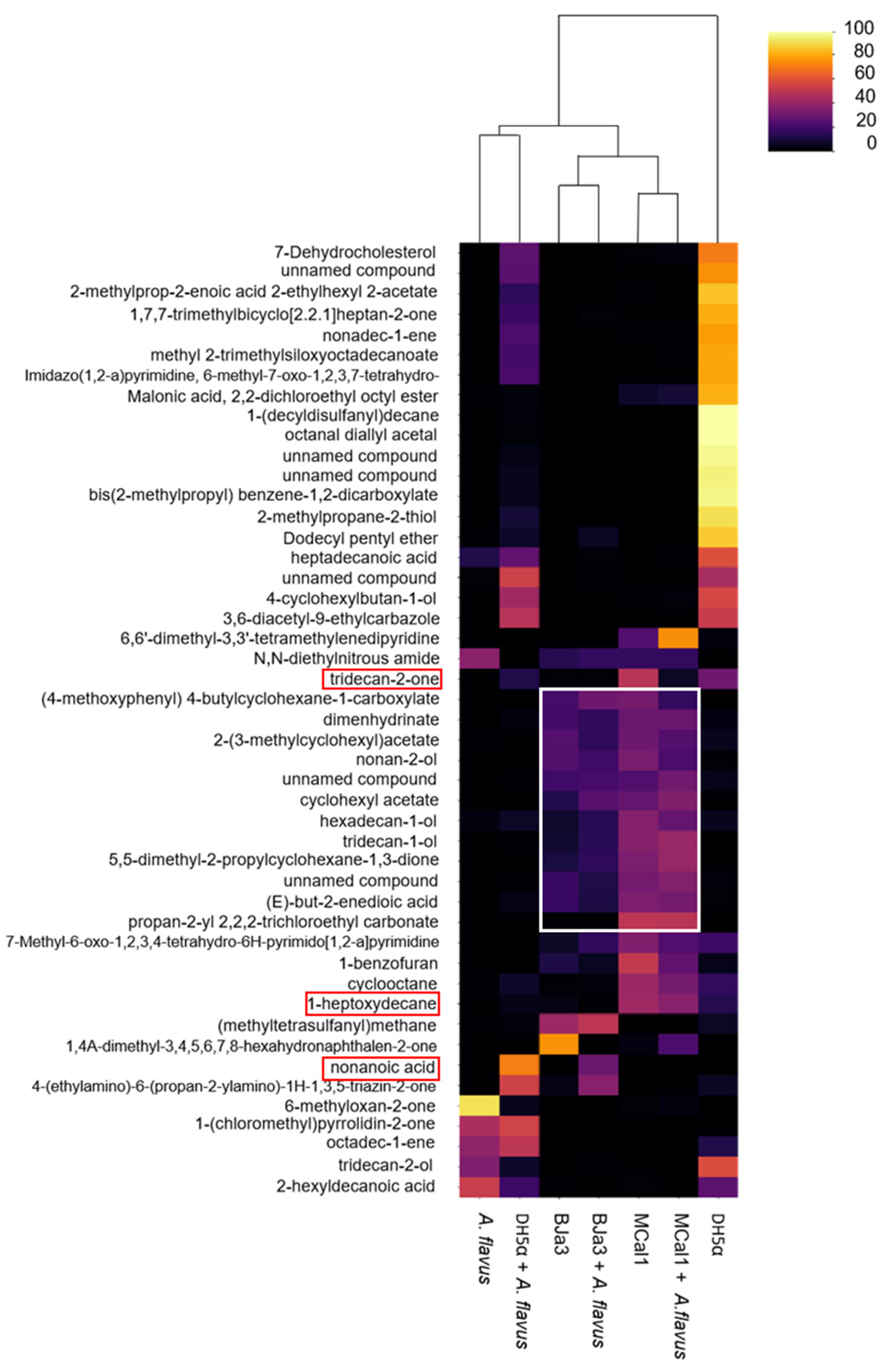
In order to identify which volatiles were produced by microorganisms, the volatilome profile of the Pseudomonas strains, E. coli DH5α, and A. flavus were analyzed by SPME/GC–MS. Although we had a total of 606 compounds, we considered the 47 VOCs that were annotated by GNPS and had statistically significant differences in the spectra peak area in at least one group according to the Kruskal–Wallis test (a = 0.05) (Table S5). From them, fourteen, fourteen, and twenty VOCs were detected in the volatilomes of BJa3, MCal1, and DH5α, respectively. On the other hand, four, one, and three VOCs were detected exclusively in the volatilomes of the bacteria cultivated in the presence of the fungus (i.e., BJa3 + A. flavus, Mcal1 + A. flavus and DH5α + A. flavus, respectively) (Figure 5A). As can be observed in the Venn diagram (Figure 5A), some compounds were shared by the three isolates. These compounds comprise several chemical classes, including alcohols, ketones, benzenoids, alkenes, and others with more complex composition (Table S4). This approach allowed us to identify a greater variety of compounds produced by our isolates in comparison to the literature, which reports the production of nine to thirty VOCs by Pseudomonas strains in different studies [17,64,80,84,85,86].
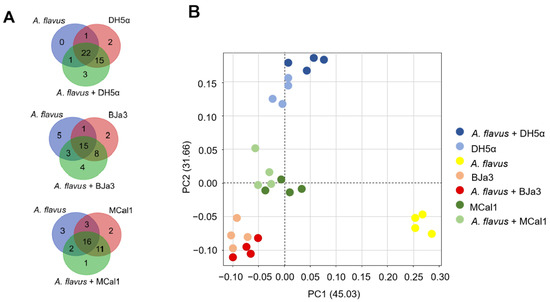
Figure 5.
Analysis of the volatilomes produced by the bacteria BJa3, MCal1, E. coli DH5α and fungus A. flavus. (A) Venn diagram considering only the 47 VOCs that have statistically significant differences in spectra peak area in each group. (B) PCA of the compounds annotated in the volatilome of the bacteria and fungus.
To elucidate the discriminatory patterns within the volatilomes of the bacterial isolates under investigation, principal component analysis (PCA) was conducted (Figure 5B). As anticipated, bacterial strains cultivated independently exhibited clustering proximal to those cultivated concurrently with the fungus. Notably, the Pseudomonas isolates BJa3 and MCal1 demonstrated a closer spatial arrangement in the PCA plot compared to their juxtaposition with E. coli DH5α (Figure 5B). Intriguingly, the volatile profile of the fungus cultivated in isolation manifested the most distinctive characteristics among the samples examined.
A heatmap (Figure 6) was generated to show the variations in the relative abundance of selected annotated VOCs produced by antagonist bacteria under different culture conditions. As shown in Figure 6, a clear difference in VOC profiles was observed in this analysis. For example, it was possible to observe that there was a clustering between BJa3 and Bja3 + A. flavus and also between Mcal1 and Mcal1 + A. flavus, as expected. In these cases, it is possible to see that the production of VOCs was similar, although the amount and presence of some compounds is altered. An exception is for E. coli DH5α, in which the VOCs produced by the bacteria cultivated separately did not cluster with the VOCs produced by E. coli + A. flavus. Although several reasons may have contributed to this result, we suggest that E. coli, while sensing the presence of A. flavus, is not capable of producing VOCs that alter the growth and production of fungal secondary metabolites when fungus grows together with the bacteria. Thus, it is possible to observe in Figure 6 that the VOCs produced by E. coli DH5α co-cultivated with A. flavus were grouped with the A. flavus volatilome. It is interesting to note that this finding corroborates previous results, in which E. coli DH5α does not interfere with fungal growth [20]. Also, it is worth noting that our study showed that co-cultures of bacteria and fungi have emerging volatile metabolomic properties. Therefore, the metabolome of the co-culture cannot be predicted from the sum of its constituents.
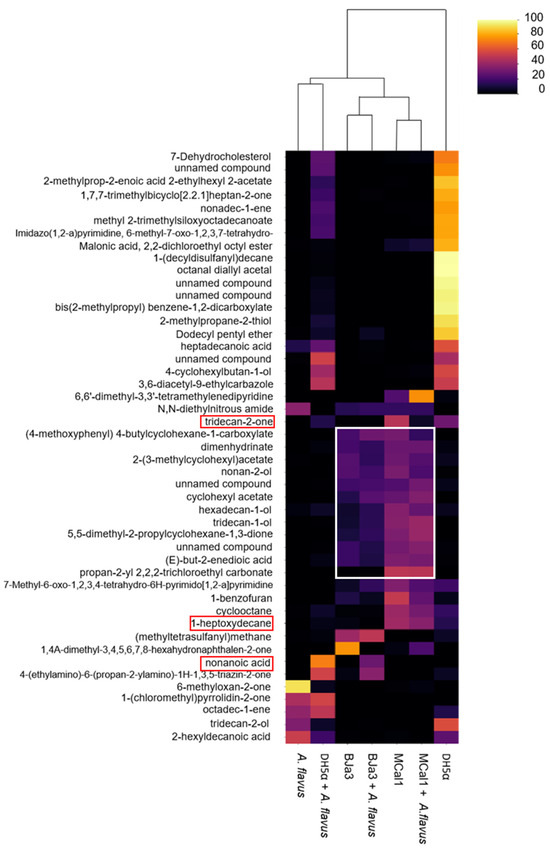
Figure 6.
Heatmap of the compounds annotated in the volatilome of the bacteria and fungus. Columns represent each sample condition. Rows represent the different VOCs evaluated. The color code indicates the abundance of each compound (scale from black, low abundance, to yellow, high abundance). The white rectangle indicates the set of volatile compounds produced by the BJa3 and MCal1 bacteria of interest and through the interaction between the bacteria and the A. flavus fungus. The red rectangles show some volatile compounds whose antifungal action has already been described in the literature. The columns were clustered based on Euclidean distance from the peak area average of each sample.
Considering the chemical nature of the compounds annotated in this study, it is possible to notice that some classes of compounds are common among Pseudomonas isolates (Figure 6). Moreover, some of these classes have already been validated in the literature for their biocontrol activity, such as alcohols [80,87,88,89], the unsaturated aliphatic hydrocarbons (i.e., 1-heptoxydecane) that were shown to inhibit the growth of several pathogenic fungus [80,90,91], and ketones (i.e., tridecan-2-one) that were confirmed as a bioactive compound against Fusarium oxysporum [92]. Furthermore, the same volatile compound may have opposite effects on different target organisms. For example, volatile sulfur compounds such as dimethyl disulfide, dimethyl trisulfide, and dimethyl tetrasulfide can strongly inhibit fungus (Rhizoctonia solani and Fusarium culmorum) and oomycete (Pythium ultimum) growth while promoting the development of some bacteria (Pseudomonas sp.) [93,94,95] and some plants [96].
The main mechanism underlying the antifungal properties of VOCs involves the disruption of fungal cell wall and membrane structures, resulting in intracellular lysate leakage and the induction of oxidative stress [97]. This phenomenon is facilitated by the ability of VOCs to permeate fungal cells via hydrogen bonding interactions. Consequently, the forces generated during this interaction perturb the aqueous environment of cell membranes, thereby interfering with their cellular physiology and functionality [97]. Thus, B. cinerea treated with VOCs derived from Streptomyces globisporus shows excessive vesiculation, thickened walls and retracted membranes [98]. In addition, Trichoderma sp., Phoma sp., and Colletotrichum sp. Exposed to VOCs from Chromobacterium vaccinii show extensive morphological abnormalities, such as swollen hyphal cells, vacuolar deposits, and cell wall alterations [99]. This action corroborates our findings in the microscopy of fungi exposed to VOCs.
Several VOCs exhibit a direct targeting mechanism towards fungal cell membranes, thereby increasing membrane permeability and inducing cell leakage. Notably, certain VOCs such as nonanoic acid, synthesized by Pseudomonas sp., have been identified as enhancing cell membrane fluidity. This phenomenon is attributed to the alteration of membrane protein conformation, consequently leading to the leakage of intracellular contents [100]. Furthermore, Bergsson et al. (2001) [101] have elucidated the destructive effects of decanoic acid on Candida albicans cell membranes, culminating in the release of cytoplasmic contents.
Zhang et al. (2020) [102] found that, in addition to morphological changes in the hyphae and the destruction of the cell membrane, there was a significant accumulation of ROS in Ceratocystis fimbriata cells after exposure to VOCs from Pseudomonas chlororaphis. Thus, oxidative stress-induced mitochondrial dysfunction and decreased ATP levels inhibited the growth of C. fimbriata. In addition, organic acids such as decanoic acid produced by S. cerevisiae significantly decrease intracellular ATP levels and inhibit the growth of B. cinerea, possibly through mechanisms related to energy metabolism [103]. In general, VOC-induced ROS accumulation and oxidative stress lead to the inhibition of the growth of pathogenic fungi.
4. Conclusions
In this study, we explored the efficacy of soil Pseudomonas isolates BJa3 and MCal1 as biocontrol agents against the phytopathogenic fungus A. flavus. Our findings revealed the significant antifungal activity of both the BJa3 and MCal1 Pseudomonas strains, effectively inhibiting the mycelial growth of A. flavus. In summary, our work underscores the potential of Pseudomonas species to be used as biocontrol agents against phytopathogenic fungi through the production of both diffusible and volatile compounds. Future research endeavors should delve into elucidating the specific mechanisms underlying the antifungal activity of these compounds and exploring their applications in sustainable crop protection practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biotech13020008/s1, Figure S1. Antibacterial activity of VOCs of Mcal1 strain on LB medium in 2-compartment Petri dishes. Table S1. Digital DNA-DNA hybridization (dDDH) values between the BJa3 genome and the selected typestrain genomes. Table S2. Digital DNA-DNA hybridization (dDDH) values between the MCal1 genome and the selected types of strain genomes. Table S3. Genome statistics for Pseudomonas sp. BJa3 and MCal1. Table S4. Secondary metabolite gene clusters potentially present in BJa3 and MCal1. Table S5. VOCs produced by the bacterial antagonists.
Author Contributions
Conceptualization, F.R., R.C.A., M.d.L.T.d.M.P. and M.-E.G.; methodology, F.R. and R.C.A.; validation, F.R., T.C.B. and R.C.A.; formal analysis, F.R. and T.C.B.; investigation, F.R.; resources, M.-E.G.; data curation, T.C.B., R.R.d.S. and R.S.-R.; writing—original draft preparation, F.R. and M.-E.G.; writing—review and editing, F.R. and M.-E.G.; supervision, M.-E.G.; project administration, M.-E.G.; funding acquisition, M.-E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the São Paulo State Foundation (FAPESP, award # 2021/01748-5). FRO was the beneficiary of a CAPES scholarship. MEG was supported by CNPq Research Productivity Scholarship (award # 304089/2023-0). RS was supported by FAPESP award numbers 2017/18922-2, 2019/05026-4 and 2020/02207-5. TCB was supported by FAPESP award number 21/08235-3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
BJa3 and MCal1 genome sequence data were deposited in the NCBI GenBank database under accession numbers JAOXMC000000000 and JAKUMP000000000, respectively (Table S1).
Acknowledgments
The authors thank their laboratory colleagues for their insightful comments and suggestions throughout this study. The authors also would like to thank the lab technician Thalita Riul Prado for her assistance in the course of this work. Additionally, authors thank Izabel Cristina Casanova Turatti for her support on SPME/GC–MS experiments and Elisabete Rosa Milani for her assistance in microscopy experiments.
Conflicts of Interest
Authors are involved in the development of a patent related to the research topic investigated in this paper. RS-R is employed by ByMyCell Inova Simples.
References
- Abdallah, M.F.; Audenaert, K.; Saeger, S.D.; Houbraken, J. Revisiting an Aspergillus Flavus Strain Isolated from an Egyptian Sugarcane Field in 1930. Microorganisms 2020, 8, 1633. [Google Scholar] [CrossRef]
- Coomes, T.J.; Sanders, J.C. The Detection and Estimation of Aflatoxin in Groundnuts and Groundnut Materials. Part I. Paper-Chromatographic Procedure. Analyst 1963, 88, 209. [Google Scholar] [CrossRef]
- Hartley, R.D.; Nesbitt, B.F.; O’Kelly, J. Toxic Metabolites of Aspergillus Flavus. Nature 1963, 198, 1056–1058. [Google Scholar] [CrossRef]
- Viaro, H.P.; da Silva, J.J.; de Souza Ferranti, L.; Bordini, J.G.; Massi, F.P.; Fungaro, M.H.P. The First Report of A. Novoparasiticus, A. Arachidicola and A. Pseudocaelatus in Brazilian Corn Kernels. Int. J. Food Microbiol. 2017, 243, 46–51. [Google Scholar] [CrossRef]
- Silva, J.J.; Puel, O.; Lorber, S.; Ferranti, L.S.; Ortiz, L.F.; Taniwaki, M.H.; Iamanaka, B.T.; Fungaro, M.H.P. Occurrence and Diversity of Aspergillus in Commercial Yerba Mate Elaborated for the Brazilian Beverage ‘Chimarrão’. Food Res. Int. 2019, 121, 940–946. [Google Scholar] [CrossRef]
- Iamanaka, B.T.; de Souza Lopes, A.; Martins, L.M.; Frisvad, J.C.; Medina, A.; Magan, N.; Sartori, D.; Massi, F.P.; Fungaro, M.H.P.; Taniwaki, M.H. Aspergillus Section Flavi Diversity and the Role of A. novoparasiticus in Aflatoxin Contamination in the Sugarcane Production Chain. Int. J. Food Microbiol. 2019, 293, 17–23. [Google Scholar] [CrossRef]
- Ismaiel, A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential Economic Losses to the US Corn Industry from Aflatoxin Contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Umesha, S.; Manukumar, H.M.G.; Chandrasekhar, B.; Shivakumara, P.; Shiva Kumar, J.; Raghava, S.; Avinash, P.; Shirin, M.; Bharathi, T.R.; Rajini, S.B.; et al. A Flatoxins and Food Pathogens: Impact of Biologically Active Aflatoxins and Their Control Strategies: Aflatoxins and Food Pathogens. J. Sci. Food Agric. 2017, 97, 1698–1707. [Google Scholar] [CrossRef]
- Lemfack, M.-C.; Bahl, H.; Piechulla, B.; Magnus, N. The Domain of Bacteria and Their Volatile Metabolic Potential. In Bacterial Volatile Compounds as Mediators of Airborne Interactions; Ryu, C.-M., Weisskopf, L., Piechulla, B., Eds.; Springer: Singapore, 2020; pp. 1–38. ISBN 9789811572920. [Google Scholar]
- Tilocca, B.; Cao, A.; Migheli, Q. Scent of a Killer: Microbial Volatilome and Its Role in the Biological Control of Plant Pathogens. Front. Microbiol. 2020, 11, 41. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay Control in the Postharvest System: Role of Microbial and Plant Volatile Organic Compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Qin, X.; Xiao, H.; Cheng, X.; Zhou, H.; Si, L. Hanseniaspora Uvarum Prolongs Shelf Life of Strawberry via Volatile Production. Food Microbiol. 2017, 63, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Toffano, L.; Fialho, M.B.; Pascholati, S.F. Potential of Fumigation of Orange Fruits with Volatile Organic Compounds Produced by Saccharomyces Cerevisiae to Control Citrus Black Spot Disease at Postharvest. Biol. Control 2017, 108, 77–82. [Google Scholar] [CrossRef]
- Peñuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic Volatile Emissions from the Soil: Biogenic Volatile Emissions from the Soil. Plant Cell Environ. 2014, 37, 1866–1891. [Google Scholar] [CrossRef]
- Schenkel, D.; Deveau, A.; Niimi, J.; Mariotte, P.; Vitra, A.; Meisser, M.; Buttler, A.; Splivallo, R. Linking Soil’s Volatilome to Microbes and Plant Roots Highlights the Importance of Microbes as Emitters of Belowground Volatile Signals. Environ. Microbiol. 2019, 21, 3313–3327. [Google Scholar] [CrossRef]
- Hernández-León, R.; Rojas-Solís, D.; Contreras-Pérez, M.; del Carmen Orozco-Mosqueda, M.; Macías-Rodríguez, L.I.; Reyes-de la Cruz, H.; Valencia-Cantero, E.; Santoyo, G. Characterization of the Antifungal and Plant Growth-Promoting Effects of Diffusible and Volatile Organic Compounds Produced by Pseudomonas Fluorescens Strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Liang, S.; Zhou, Z.; Zhang, C.; Li, K.; Peng, X.; Qin, S.; Xing, K. Antifungal Activity of Volatile Compounds from Bacillus tequilensis XK29 against Botrytis Cinerea Causing Gray Mold on Cherry Tomatoes. Postharvest Biol. Technol. 2023, 198, 112239. [Google Scholar] [CrossRef]
- Wallace, R.L.; Hirkala, D.L.; Nelson, L.M. Mechanisms of Action of Three Isolates of Pseudomonas Fluorescens Active against Postharvest Grey Mold Decay of Apple during Commercial Storage. Biological Control 2018, 117, 13–20. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Liu, D.; Wei, Z.; Huang, Q.; Shen, Q. Volatile Organic Compounds Produced by Pseudomonas Fluorescens WR-1 Restrict the Growth and Virulence Traits of Ralstonia Solanacearum. Microbiol. Res. 2016, 192, 103–113. [Google Scholar] [CrossRef]
- Gandía, M.; Kakar, A.; Giner-Llorca, M.; Holzknecht, J.; Martínez-Culebras, P.; Galgóczy, L.; Marx, F.; Marcos, J.F.; Manzanares, P. Potential of Antifungal Proteins (AFPs) to Control Penicillium Postharvest Fruit Decay. JoF 2021, 7, 449. [Google Scholar] [CrossRef]
- Ling, L.; Jiang, K.; Cheng, W.; Wang, Y.; Pang, M.; Luo, H.; Lu, L.; Gao, K.; Tu, Y. Biocontrol of Volatile Organic Compounds Obtained from Bacillus subtilis CL2 against Aspergillus Flavus in Peanuts during Storage. Biol. Control 2022, 176, 105094. [Google Scholar] [CrossRef]
- Natarajan, S.; Balachandar, D.; Senthil, N.; Velazhahan, R.; Paranidharan, V. Volatiles of Antagonistic Soil Yeasts Inhibit Growth and Aflatoxin Production of Aspergillus Flavus. Microbiol. Res. 2022, 263, 127150. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ishihara, A.; Nakajima, H. Isolation of Anteiso-C 17, Iso-C 17, Iso-C 16, and Iso-C 15 Bacillomycin D from Bacillus amyloliquefaciens SD-32 and Their Antifungal Activities against Plant Pathogens. J. Agric. Food Chem. 2014, 62, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Li, B.; Zhang, N.; Waseem, R.; Shen, Q.; Huang, Q. Production of Bacillomycin- and Macrolactin-Type Antibiotics by Bacillus amyloliquefaciens NJN-6 for Suppressing Soilborne Plant Pathogens. J. Agric. Food Chem. 2012, 60, 2976–2981. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Li, N.; Li, B.; Wang, S.; Wang, Y.; Meng, X.; Li, B.; Cao, K.; Hu, T. An Endophytic Strain Bacillus velezensis JZ51 Controlled Pink Mold Rot of Postharvest Apple Fruit via Antagonistic Action and Disease Resistance Induction. Postharvest Biol. Technol. 2024, 210, 112793. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Vollenbroich, D.; Özel, M.; Vater, J.; Kamp, R.M.; Pauli, G. Mechanism of Inactivation of Enveloped Viruses by the Biosurfactant Surfactin from Bacillus subtilis. Biologicals 1997, 25, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An Ultra-Fast Single-Node Solution for Large and Complex Metagenomics Assembly via Succinct de Bruijn Graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Piro, V.C.; Faoro, H.; Weiss, V.A.; Steffens, M.B.; Pedrosa, F.O.; Souza, E.M.; Raittz, R.T. FGAP: An Automated Gap Closing Tool. BMC Res. Notes 2014, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Göker, M. TYGS Is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Zhao, C.-A.; Liu, C.-J.; Xu, X.-F. Endophytic Fungi Diversity of Aquatic/Riparian Plants and Their Antifungal Activity in Vitro. J. Microbiol. 2010, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of Volatile Organic Compounds by Aureobasidium Pullulans as a Potential Mechanism of Action against Postharvest Fruit Pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Nachar, N. The Mann-Whitney U: A Test for Assessing Whether Two Independent Samples Come from the Same Distribution. Tutor. Quant. Methods Psychol. 2008, 4, 13–20. [Google Scholar] [CrossRef]
- Chiniquy, D.; Barnes, E.M.; Zhou, J.; Hartman, K.; Li, X.; Sheflin, A.; Pella, A.; Marsh, E.; Prenni, J.; Deutschbauer, A.M.; et al. Microbial Community Field Surveys Reveal Abundant Pseudomonas Population in Sorghum Rhizosphere Composed of Many Closely Related Phylotypes. Front. Microbiol. 2021, 12, 598180. [Google Scholar] [CrossRef]
- Castaldi, S.; Masi, M.; Sautua, F.; Cimmino, A.; Isticato, R.; Carmona, M.; Tuzi, A.; Evidente, A. Pseudomonas Fluorescens Showing Antifungal Activity against Macrophomina Phaseolina, a Severe Pathogenic Fungus of Soybean, Produces Phenazine as the Main Active Metabolite. Biomolecules 2021, 11, 1728. [Google Scholar] [CrossRef]
- Castro Tapia, M.P.; Madariaga Burrows, R.P.; Ruiz Sepúlveda, B.; Vargas Concha, M.; Vera Palma, C.; Moya-Elizondo, E.A. Antagonistic Activity of Chilean Strains of Pseudomonas Protegens Against Fungi Causing Crown and Root Rot of Wheat (Triticum aestivum L.). Front. Plant Sci. 2020, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gao, J.; Zhang, M.; Xue, J.; Zhang, X. Pseudomonas Aeruginosa Ld-08 Isolated from Lilium Davidii Exhibits Antifungal and Growth-Promoting Properties. PLoS ONE 2022, 17, e0269640. [Google Scholar] [CrossRef] [PubMed]
- Melo, I.; Souza, W.; Silva, L.; Santos, S.; Assalin, M.; Zucchi, T.; Queiroz, S. Antifungal Activity of Pseudomonas Frederiksbergensis CMAA 1323 Isolated from the Antarctic Hair Grass Deschampsia Antarctica. Br. Microbiol. Res. J. 2016, 14, 1–11. [Google Scholar] [CrossRef]
- Müller, T.; Ruppel, S.; Behrendt, U.; Lentzsch, P.; Müller, M.E.H. Antagonistic Potential of Fluorescent Pseudomonads Colonizing Wheat Heads Against Mycotoxin Producing Alternaria and Fusaria. Front. Microbiol. 2018, 9, 2124. [Google Scholar] [CrossRef]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial Volatiles and Their Action Potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; Zweers, H.; de Boer, W.; Garbeva, P. Volatiles in Inter-Specific Bacterial Interactions. Front. Microbiol. 2015, 6, 166507. [Google Scholar] [CrossRef]
- Abis, L.; Loubet, B.; Ciuraru, R.; Lafouge, F.; Houot, S.; Nowak, V.; Tripied, J.; Dequiedt, S.; Maron, P.A.; Sadet-Bourgeteau, S. Reduced Microbial Diversity Induces Larger Volatile Organic Compound Emissions from Soils. Sci. Rep. 2020, 10, 6104. [Google Scholar] [CrossRef] [PubMed]
- van Agtmaal, M.; van Os, G.J.; Hol, W.H.G.; Hundscheid, M.P.J.; Runia, W.T.; Hordijk, C.A.; de Boer, W. Legacy Effects of Anaerobic Soil Disinfestation on Soil Bacterial Community Composition and Production of Pathogen-Suppressing Volatiles. Front. Microbiol. 2015, 6, 701. [Google Scholar] [CrossRef]
- Song, G.; Ryu, C.-M. Two Volatile Organic Compounds Trigger Plant Self-Defense against a Bacterial Pathogen and a Sucking Insect in Cucumber under Open Field Conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef]
- Goelen, T.; Vuts, J.; Sobhy, I.S.; Wäckers, F.; Caulfield, J.C.; Birkett, M.A.; Rediers, H.; Jacquemyn, H.; Lievens, B. Identification and Application of Bacterial Volatiles to Attract a Generalist Aphid Parasitoid: From Laboratory to Greenhouse Assays. Pest Manag. Sci. 2021, 77, 930–938. [Google Scholar] [CrossRef]
- Chen, W.-H.; You, F. Smart Greenhouse Control under Harsh Climate Conditions Based on Data-Driven Robust Model Predictive Control with Principal Component Analysis and Kernel Density Estimation. J. Process Control 2021, 107, 103–113. [Google Scholar] [CrossRef]
- Gan, Z.; Zhou, Q.; Zheng, C.; Wang, J. Challenges and Applications of Volatile Organic Compounds Monitoring Technology in Plant Disease Diagnosis. Biosens. Bioelectron. 2023, 237, 115540. [Google Scholar] [CrossRef]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have Biopesticides Come of Age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- McFrederick, Q.S.; Fuentes, J.D.; Roulston, T.; Kathilankal, J.C.; Lerdau, M. Effects of Air Pollution on Biogenic Volatiles and Ecological Interactions. Oecologia 2009, 160, 411–420. [Google Scholar] [CrossRef]
- Blande, J.D.; Holopainen, J.K.; Niinemets, Ü. Plant Volatiles in Polluted Atmospheres: Stress Responses and Signal Degradation. Plant Cell Environ. 2014, 37, 1892–1904. [Google Scholar] [CrossRef]
- Insam, H.; Seewald, M.S.A. Volatile Organic Compounds (VOCs) in Soils. Biol. Fertil. Soils 2010, 46, 199–213. [Google Scholar] [CrossRef]
- Rani, A.; Rana, A.; Dhaka, R.K.; Singh, A.P.; Chahar, M.; Singh, S.; Nain, L.; Singh, K.P.; Minz, D. Bacterial Volatile Organic Compounds as Biopesticides, Growth Promoters and Plant-Defense Elicitors: Current Understanding and Future Scope. Biotechnol. Adv. 2023, 63, 108078. [Google Scholar] [CrossRef]
- Girard, L.; Lood, C.; Höfte, M.; Vandamme, P.; Rokni-Zadeh, H.; van Noort, V.; Lavigne, R.; De Mot, R. The Ever-Expanding Pseudomonas Genus: Description of 43 New Species and Partition of the Pseudomonas Putida Group. Microorganisms 2021, 9, 1766. [Google Scholar] [CrossRef] [PubMed]
- Saati-Santamaría, Z.; Peral-Aranega, E.; Velázquez, E.; Rivas, R.; García-Fraile, P. Phylogenomic Analyses of the Genus Pseudomonas Lead to the Rearrangement of Several Species and the Definition of New Genera. Biology 2021, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Lalucat, J.; Gomila, M.; Mulet, M.; Zaruma, A.; García-Valdés, E. Past, Present and Future of the Boundaries of the Pseudomonas Genus: Proposal of Stutzerimonas Gen. Nov. Syst. Appl. Microbiol. 2022, 45, 126289. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Klenk, H.-P.; Göker, M. Taxonomic Use of DNA G+C Content and DNA–DNA Hybridization in the Genomic Age. Int. J. Syst. Evol. Microbiol. 2014, 64, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Corallo, B.; Amarelle, V.; Stewart, S.; Pan, D.; Tiscornia, S.; Fabiano, E. Paenibacillus sp. Strain UY79, Isolated from a Root Nodule of Arachis villosa, Displays a Broad Spectrum of Antifungal Activity. Appl. Environ. Microbiol. 2022, 88, e01645-21. [Google Scholar] [CrossRef] [PubMed]
- Remali, J.; Sarmin, N.; Izzah, M.; Ng, C.L.; Tiong, J.J.L.; Aizat, W.M.; Keong, L.K.; Zin, N.M. Genomic Characterization of a New Endophytic Streptomyces Kebangsaanensis Identifies Biosynthetic Pathway Gene Clusters for Novel Phenazine Antibiotic Production. PeerJ 2017, 5, e3738. [Google Scholar] [CrossRef] [PubMed]
- Siupka, P.; Piński, A.; Babicka, D.; Piotrowska-Seget, Z. Genome Mining Revealed a High Biosynthetic Potential for Antifungal streptomyces sp. S-2 Isolated from Black Soot. Int. J. Mol. Sci. 2020, 21, 2558. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Nazik, H.; Penner, J.; Shah, H.; Ansari, S.R.; Clemons, K.V.; Groleau, M.-C.; Dietl, A.-M.; Visca, P.; Haas, H.; et al. Studies of Pseudomonas Aeruginosa Mutants Indicate Pyoverdine as the Central Factor in Inhibition of Aspergillus Fumigatus Biofilm. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Sharma, D.; Kumar, P.; Chandra Dubey, R. Biocontrol Potential of Bacillus spp. for Resilient and Sustainable Agricultural Systems. Physiol. Mol. Plant Pathol. 2023, 128, 102173. [Google Scholar] [CrossRef]
- Jenul, C.; Sieber, S.; Daeppen, C.; Mathew, A.; Lardi, M.; Pessi, G.; Hoepfner, D.; Neuburger, M.; Linden, A.; Gademann, K.; et al. Biosynthesis of Fragin Is Controlled by a Novel Quorum Sensing Signal. Nat. Commun. 2018, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Kruijt, M.; Tran, H.; Raaijmakers, J.M. Functional, Genetic and Chemical Characterization of Biosurfactants Produced by Plant Growth-Promoting Pseudomonas Putida 267. J. Appl. Microbiol. 2009, 107, 546–556. [Google Scholar] [CrossRef]
- Nishanth Kumar, S.; Aravind, S.R.; Jacob, J.; Gopinath, G.; Lankalapalli, R.S.; Sreelekha, T.T.; Dileep Kumar, B.S. Pseudopyronine B: A Potent Antimicrobial and Anticancer Molecule Isolated from a Pseudomonas Mosselii. Front. Microbiol. 2016, 7, 1307. [Google Scholar] [CrossRef]
- Martin, H.C.; Ibáñez, R.; Nothias, L.-F.; Boya, P.C.A.; Reinert, L.K.; Rollins-Smith, L.A.; Dorrestein, P.C.; Gutiérrez, M. Viscosin-like Lipopeptides from Frog Skin Bacteria Inhibit Aspergillus Fumigatus and Batrachochytrium Dendrobatidis Detected by Imaging Mass Spectrometry and Molecular Networking. Sci. Rep. 2019, 9, 3019. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, H.; Tan, Z.; Xuan, Z.; Dahar, G.Y.; Li, Q.X.; Miao, W.; Liu, W. Antifungal Mechanism of Bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum Gloeosporioides Penz. Pestic. Biochem. Physiol. 2020, 163, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Vanittanakom, N.; Loeffler, W.; Koch, U.; Jung, G. Fengycin—A Novel Antifungal Lipopeptide Antibiotic Produced by Bacillus subtilis F-29-3. J. Antibiot. 1986, 39, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Soares, C.; Kozakiewicz, Z.; Paterson, R.R.M.; Lima, N.; Venâncio, A. Identification and Characterization of Aspergillus Flavus and Aflatoxins. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 527–534. [Google Scholar]
- Boukaew, S.; Plubrukam, A.; Prasertsan, P. Effect of Volatile Substances from Streptomyces Philanthi RM-1-138 on Growth of Rhizoctonia Solani on Rice Leaf. BioControl 2013, 58, 471–482. [Google Scholar] [CrossRef]
- Moore-Landecker, E.; Stotzky, G. Morphological Abnormalities of Fungi Induced by Volatile Microbial Metabolites. Mycologia 1973, 65, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K.; Fujita, K.; Takashima, Y.; Ardin, A.C.; Ooshima, T.; Matsumoto-Nakano, M. Role of ABC Transporter Proteins in Stress Responses of Streptococcus Mutans. Oral Health Dent. Manag. 2014, 13, 359–365. [Google Scholar] [PubMed]
- Rolim, J.M.; Walker, C.; Mezzomo, R.; Muniz, M.F. Antagonism and Effect of Volatile Metabolites of Trichoderma spp. on Cladosporium spp. Floresta Ambiente 2019, 26, e20170594. [Google Scholar] [CrossRef]
- Freitas, C.S.A.; Maciel, L.F.; Corrêa dos Santos, R.A.; Costa, O.M.M.M.; Maia, F.C.B.; Rabelo, R.S.; Franco, H.C.J.; Alves, E.; Consonni, S.R.; Freitas, R.O.; et al. Bacterial Volatile Organic Compounds Induce Adverse Ultrastructural Changes and DNA Damage to the Sugarcane Pathogenic Fungus Thielaviopsis Ethacetica. Environ. Microbiol. 2022, 24, 1430–1453. [Google Scholar] [CrossRef]
- Dandurishvili, N.; Toklikishvili, N.; Ovadis, M.; Eliashvili, P.; Giorgobiani, N.; Keshelava, R.; Tediashvili, M.; Vainstein, A.; Khmel, I.; Szegedi, E.; et al. Broad-Range Antagonistic Rhizobacteria Pseudomonas Fluorescens and Serratia Plymuthica Suppress Agrobacterium Crown Gall Tumours on Tomato Plants: Biocontrol of Agrobacterium by Bacterial Antagonists. J. Appl. Microbiol. 2011, 110, 341–352. [Google Scholar] [CrossRef]
- Jampilek, J. Potential of Agricultural Fungicides for Antifungal Drug Discovery. Expert Opin. Drug Discov. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Mazu, T.K.; Bricker, B.A.; Flores-Rozas, H.; Ablordeppey, S.Y. The Mechanistic Targets of Antifungal Agents: An Overview. Mini Rev. Med. Chem. 2016, 16, 555–578. [Google Scholar] [CrossRef]
- Farag, M.A.; Ryu, C.-M.; Sumner, L.W.; Paré, P.W. GC–MS SPME Profiling of Rhizobacterial Volatiles Reveals Prospective Inducers of Growth Promotion and Induced Systemic Resistance in Plants. Phytochemistry 2006, 67, 2262–2268. [Google Scholar] [CrossRef]
- Park, Y.-S.; Dutta, S.; Ann, M.; Raaijmakers, J.M.; Park, K. Promotion of Plant Growth by Pseudomonas Fluorescens Strain SS101 via Novel Volatile Organic Compounds. Biochem. Biophys. Res. Commun. 2015, 461, 361–365. [Google Scholar] [CrossRef]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile Organic Compounds Produced by Pseudomonas pseudoalcaligenes Alleviated drought Stress by Modulating Defense System in Maize (Zea mays L.). Physiol. Plant. 2021, 172, 896–911. [Google Scholar] [CrossRef]
- Che, J.; Liu, B.; Liu, G.; Chen, Q.; Lan, J. Volatile Organic Compounds Produced by Lysinibacillus sp. FJAT-4748 Possess Antifungal Activity against Colletotrichum Acutatum. Biocontrol Sci. Technol. 2017, 27, 1349–1362. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and Use of Potential Bacterial Organic Antifungal Volatiles in Biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.; Xiao, Y.; Wass, T.J.; Schenk, P.M. Identification of Soil Bacterial Isolates Suppressing Different Phytophthora spp. and Promoting Plant Growth. Front. Plant Sci. 2018, 9, 1502. [Google Scholar] [CrossRef]
- Hunziker, L.; Bönisch, D.; Groenhagen, U.; Bailly, A.; Schulz, S.; Weisskopf, L. Pseudomonas Strains Naturally Associated with Potato Plants Produce Volatiles with High Potential for Inhibition of Phytophthora Infestans. Appl. Environ. Microbiol. 2015, 81, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Lo Cantore, P.; Giorgio, A.; Iacobellis, N.S. Bioactivity of Volatile Organic Compounds Produced by Pseudomonas Tolaasii. Front. Microbiol. 2015, 6, 156562. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, J.; Li, C.; Ma, Y. Antifungal and Plant Growth Promotion Activity of Volatile Organic Compounds Produced by Bacillus Amyloliquefaciens. MicrobiologyOpen 2019, 8, e00813. [Google Scholar] [CrossRef]
- Garbeva, P.; Hordijk, C.; Gerards, S.; de Boer, W. Volatiles Produced by the Mycophagous Soil Bacterium Collimonas. FEMS Microbiol. Ecol. 2014, 87, 639–649. [Google Scholar] [CrossRef]
- Garbeva, P.; Hordijk, C.; Gerards, S.; de Boer, W. Volatile-Mediated Interactions between Phylogenetically Different Soil Bacteria. Front. Microbiol. 2014, 5, 91242. [Google Scholar] [CrossRef]
- Ali, Q.; Khan, A.R.; Tao, S.; Rajer, F.U.; Ayaz, M.; Abro, M.A.; Gu, Q.; Wu, H.; Kuptsov, V.; Kolomiets, E.; et al. Broad-spectrum Antagonistic Potential of Bacillus spp. Volatiles against Rhizoctonia solani and Xanthomonas oryzae Pv. Oryzae. Physiol. Plant. 2023, 175, e14087. [Google Scholar] [CrossRef]
- Meldau, D.G.; Meldau, S.; Hoang, L.H.; Underberg, S.; Wunsche, H.; Baldwin, I.T. Dimethyl Disulfide Produced by the Naturally Associated Bacterium Bacillus sp. B55 Promotes Nicotiana attenuata Growth by Enhancing Sulfur Nutrition. Plant Cell 2013, 25, 2731–2747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial Volatile Organic Compounds: Antifungal Mechanisms, Applications, and Challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Hsiang, T. Effects of Volatile Substances of Streptomyces Globisporus JK-1 on Control of Botrytis Cinerea on Tomato Fruit. Biol. Control 2012, 61, 113–120. [Google Scholar] [CrossRef]
- Ebadzadsahrai, G.; Higgins Keppler, E.A.; Soby, S.D.; Bean, H.D. Inhibition of Fungal Growth and Induction of a Novel Volatilome in Response to Chromobacterium Vaccinii Volatile Organic Compounds. Front. Microbiol. 2020, 11, 1035. [Google Scholar] [CrossRef]
- Avis, T.J.; Bélanger, R.R. Specificity and Mode of Action of the Antifungal Fatty Acid Cis-9-Heptadecenoic Acid Produced by Pseudozyma Flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, Ó.; Thormar, H. In Vitro Killing of Candida albicans by Fatty Acids and Monoglycerides. Antimicrob Agents Chemother 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Zhang, H.; Godana, E.A.; Sui, Y.; Yang, Q.; Zhang, X.; Zhao, L. Biological Control as an Alternative to Synthetic Fungicides for the Management of Grey and Blue Mould Diseases of Table Grapes: A Review. Crit. Rev. Microbiol. 2020, 46, 450–462. [Google Scholar] [CrossRef]
- Stevens, S.; Hofmeyr, J.-H. Effects of Ethanol, Octanoic and Decanoic Acids on Fermentation and the Passive Influx of Protons through the Plasma Membrane of Saccharomyces Cerevisiae. Appl. Microbiol. Biotechnol. 1993, 38, 656–663. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).