Metal Pollution as a Risk Factor for HIV Infection
Abstract
1. Introduction
2. The Hypothesis
3. Preliminary Data and Hypothesis Evaluation
3.1. Toxicogenomic Data Supports the Hypothesis
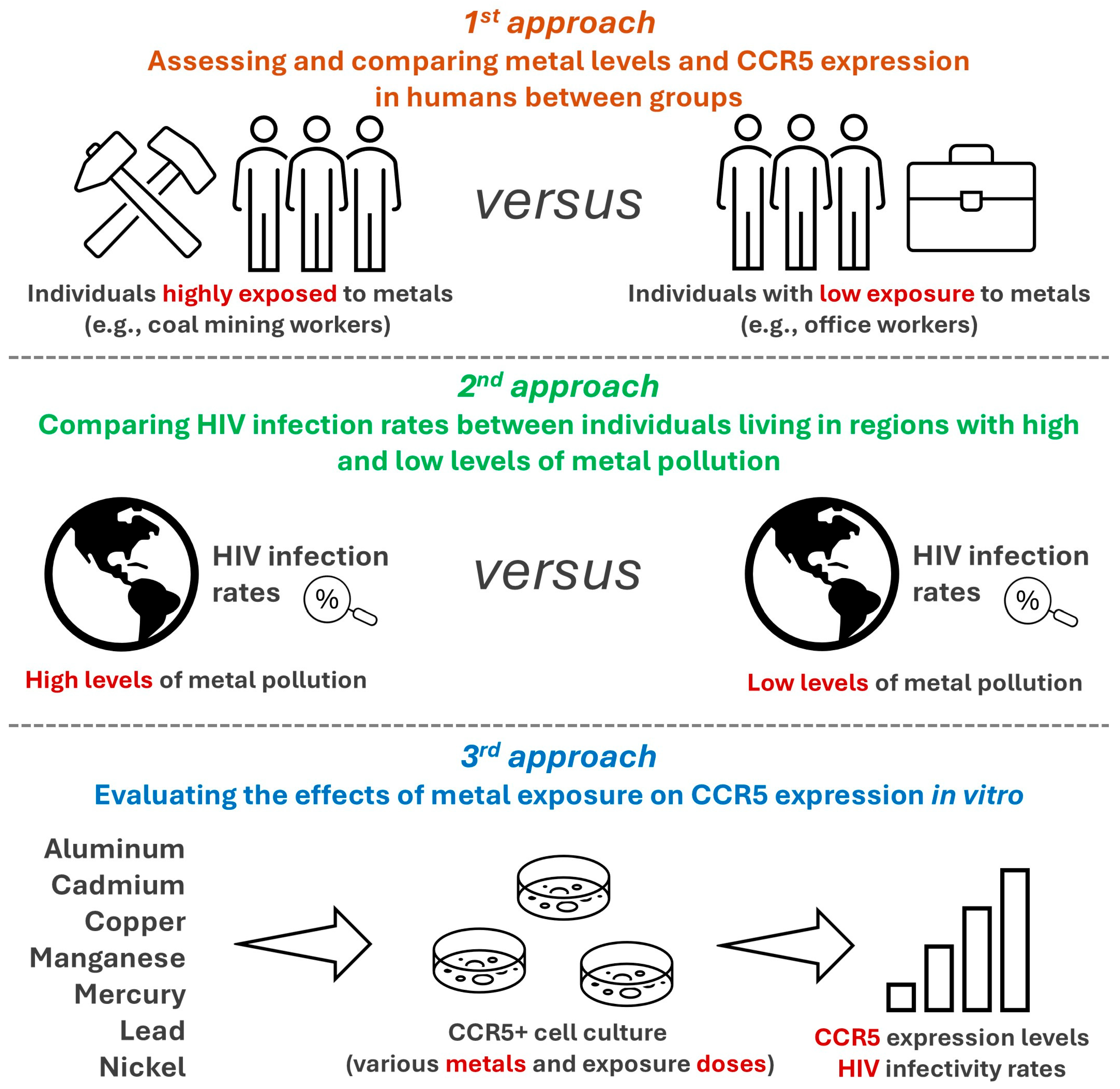
3.2. Other Approaches to Evaluate the Hypothesis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef]
- Gupta, G.R.; Parkhurst, J.O.; Ogden, J.A.; Aggleton, P.; Mahal, A. Structural approaches to HIV prevention. Lancet 2008, 372, 764–775. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Veazey, R.S. Mucosal immunology of HIV infection. Immunol. Rev. 2013, 254, 10–33. [Google Scholar] [CrossRef]
- McLaren, P.J.; Fellay, J. HIV-1 and human genetic variation. Nat. Rev. Genet. 2021, 22, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Hunter, E. HIV transmission. Cold Spring Harb. Perspect. Med. 2012, 2, a006965. [Google Scholar] [CrossRef] [PubMed]
- Pebody, R. Aidsmap: Estimated HIV Risk per Exposure. 2024. Available online: https://www.aidsmap.com/about-hiv/estimated-hiv-risk-exposure (accessed on 17 January 2025).
- Behbahani, H.; Popek, E.; Garcia, P.; Andersson, J.; Spetz, A.L.; Landay, A.; Flener, Z.; Patterson, B.K. Up-regulation of CCR5 expression in the placenta is associated with human immunodeficiency virus-1 vertical transmission. Am. J. Pathol. 2000, 157, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Kulmann-Leal, B.; Kaminski, V.L.; Rodrigues, A.G.; Bragatte, M.A.S.; Chies, J.A.B. Beyond HIV infection: Neglected and varied impacts of CCR5 and CCR5Δ32 on viral diseases. Virus Res. 2020, 286, 198040. [Google Scholar] [CrossRef]
- Tuttle, D.L.; Harrison, J.K.; Anders, C.; Sleasman, J.W.; Goodenow, M.M. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J. Virol. 1998, 72, 4962–4969. [Google Scholar] [CrossRef]
- Gornalusse, G.G.; Mummidi, S.; Gaitan, A.A.; Jimenez, F.; Ramsuran, V.; Picton, A.; Rogers, K.; Manoharan, M.S.; Avadhanam, N.; Murthy, K.K.; et al. Epigenetic mechanisms, T-cell activation, and CCR5 genetics interact to regulate T-cell expression of CCR5, the major HIV-1 coreceptor. Proc. Natl. Acad. Sci. USA 2015, 112, E4762–E4771. [Google Scholar] [CrossRef]
- Brelot, A.; Chakrabarti, L.A. CCR5 Revisited: How Mechanisms of HIV Entry Govern AIDS Pathogenesis. J. Mol. Biol. 2018, 430, 2557–2589. [Google Scholar] [CrossRef]
- Naif, H.M.; Li, S.; Alali, M.; Sloane, A.; Wu, L.; Kelly, M.; Lynch, G.; Lloyd, A.; Cunningham, A.L. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J. Virol. 1998, 72, 830–836. [Google Scholar] [CrossRef]
- Ostrowski, M.A.; Justement, S.J.; Catanzaro, A.; Hallahan, C.A.; Ehler, L.A.; Mizell, S.B.; Kumar, P.N.; Mican, J.A.; Chun, T.W.; Fauci, A.S. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J. Immunol. 1998, 161, 3195–3201. [Google Scholar] [CrossRef] [PubMed]
- Meditz, A.L.; Moreau, K.L.; MaWhinney, S.; Gozansky, W.S.; Melander, K.; Kohrt, W.M.; Wierman, M.E.; Connick, E. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J. Acquir. Immune Defic. Syndr. 2012, 59, 221–228. [Google Scholar] [CrossRef]
- Wu, L.; Paxton, W.A.; Kassam, N.; Ruffing, N.; Rottman, J.B.; Sullivan, N.; Choe, H.; Sodroski, J.; Newman, W.; Koup, R.A.; et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J. Exp. Med. 1997, 185, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef]
- Hossein-Khannazer, N.; Azizi, G.; Eslami, S.; Alhassan Mohammed, H.; Fayyaz, F.; Hosseinzadeh, R.; Usman, A.B.; Kamali, A.N.; Mohammadi, H.; Jadidi-Niaragh, F.; et al. The effects of cadmium exposure in the induction of inflammation. Immunopharmacol. Immunotoxicol. 2020, 42, 1–8. [Google Scholar] [CrossRef]
- Xu, X.; Hu, H.; Hong, Y.A. Body burden of heavy metals among HIV high risk population in USA. Environ. Pollut. 2017, 220, 1121–1126. [Google Scholar] [CrossRef]
- Xu, X.; Hu, H.; Dailey, A.B.; Kearney, G.; Talbott, E.O.; Cook, R.L. Potential health impacts of heavy metals on HIV-infected population in USA. PLoS ONE 2013, 8, e74288. [Google Scholar] [CrossRef]
- Folorunso, O.M.; Frazzoli, C.; Chijioke-Nwauche, I.; Bocca, B.; Orisakwe, O.E. Toxic Metals and Non-Communicable Diseases in HIV Population: A Systematic Review. Medicina 2021, 57, 492. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Chies, J.A.B. Toxicogenomics of the C-C chemokine receptor type 5 (CCR5): Exploring the potential impacts of chemical-CCR5 interactions on inflammation and human health. Food Chem. Toxicol. 2024, 186, 114511. [Google Scholar] [CrossRef]
- Wei, S.; Xu, T.; Sang, N.; Yue, H.; Chen, Y.; Jiang, T.; Jiang, T.; Yin, D. Mixed Metal Components in PM2.5 Contribute to Chemokine Receptor CCR5-Mediated Neuroinflammation and Neuropathological Changes in the Mouse Olfactory Bulb. Environ. Sci. Technol. 2024, 58, 4914–4925. [Google Scholar] [CrossRef]
- CTD—The Comparative Toxicogenomics Database. Available online: https://ctdbase.org/ (accessed on 3 January 2025).
- Davis, A.P.; Wiegers, T.C.; Sciaky, D.; Barkalow, F.; Strong, M.; Wyatt, B.; Wiegers, J.; McMorran, R.; Abrar, S.; Mattingly, C.J. Comparative Toxicogenomics Database’s 20th anniversary: Update 2025. Nucleic Acids Res. 2025, 53, D1328–D1334. [Google Scholar] [CrossRef]
- Harthill, M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011, 143, 1325–1336. [Google Scholar] [CrossRef]
- Rentschler, J.; Leonova, N. Global air pollution exposure and poverty. Nat. Commun. 2023, 14, 4432. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Lopez, M.E.; Lopes-Araújo, A.; Basta, P.C.; Soares-Silva, I.; de Souza, C.B.A.; Leal-Nazaré, C.G.; Santos-Sacramento, L.; Barthelemy, J.L.; Arrifano, G.P.; Augusto-Oliveira, M. Environmental pollution challenges public health surveillance: The case of mercury exposure and intoxication in Brazil. Lancet Reg. Health Am. 2024, 39, 100880. [Google Scholar] [CrossRef]
- Mody, A.; Sohn, A.H.; Iwuji, C.; Tan, R.K.J.; Venter, F.; Geng, E.H. HIV epidemiology, prevention, treatment, and implementation strategies for public health. Lancet 2024, 403, 471–492. [Google Scholar] [CrossRef]
- Padhi, B.K.; Khatib, M.N.; Ballal, S.; Bansal, P.; Bhopte, K.; Gaidhane, A.M.; Tomar, B.S.; Ashraf, A.; Kumar, M.R.; Chauhan, A.S.; et al. Association of exposure to air pollutants and risk of mortality among people living with HIV: A systematic review. BMC Public Health 2024, 24, 3251. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Liu, X.; Cheng, Q.Y.; Xiao, S.; Xia, L.X.; Yuan, B.F.; Feng, Y.Q. Heavy Metals Induce Decline of Derivatives of 5-Methycytosine in Both DNA and RNA of Stem Cells. ACS Chem. Biol. 2017, 12, 1636–1643. [Google Scholar] [CrossRef]
- Elkin, E.R.; Higgins, C.; Aung, M.T.; Bakulski, K.M. Metals Exposures and DNA Methylation: Current Evidence and Future Directions. Curr. Environ. Health Rep. 2022, 9, 673–696. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Vahter, M.; Concha, G.; Broberg, K. Low-level environmental cadmium exposure is associated with DNA hypomethylation in Argentinean women. Environ. Health Perspect. 2012, 120, 879–884. [Google Scholar] [CrossRef]
- Tarantini, L.; Bonzini, M.; Tripodi, A.; Angelici, L.; Nordio, F.; Cantone, L.; Apostoli, P.; Bertazzi, P.A.; Baccarelli, A.A. Blood hypomethylation of inflammatory genes mediates the effects of metal-rich airborne pollutants on blood coagulation. Occup. Environ. Med. 2013, 70, 418–425. [Google Scholar] [CrossRef]
- Chung, C.J.; Chang, C.H.; Liou, S.H.; Liu, C.S.; Liu, H.J.; Hsu, L.C.; Chen, J.S.; Lee, H.L. Relationships among DNA hypomethylation, Cd, and Pb exposure and risk of cigarette smoking-related urothelial carcinoma. Toxicol. Appl. Pharmacol. 2017, 316, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Stepanyan, A.; Petrackova, A.; Hakobyan, S.; Savara, J.; Davitavyan, S.; Kriegova, E.; Arakelyan, A. Long-term environmental metal exposure is associated with hypomethylation of CpG sites in NFKB1 and other genes related to oncogenesis. Clin. Epigenetics 2023, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde e Ambiente, Departamento de HIV, Aids, Tuberculose, Hepatites Virais e Infecções Sexualmente Transmissíveis. Boletim Epidemiológico, Número Especial Dezembro de 2024: HIV e Aids 2024; Ministério da Saúde: Brasília, DF, Brazil, 2024. [Google Scholar]
- Benzaken, A.S.; Sabidó, M.; Brito, I.; Bermúdez, X.P.D.; Benzaken, N.S.; Galbán, E.; Peeling, R.W.; Mabey, D. HIV and syphilis in the context of community vulnerability among indigenous people in the Brazilian Amazon. Int. J. Equity. Health 2017, 16, 92. [Google Scholar] [CrossRef]
- Ziliotto, M.; Chies, J.A.B.; Ellwanger, J.H. Toxicogenomics of persistent organic pollutants: Potential impacts on biodiversity and infectious diseases. Anthropocene 2024, 48, 100450. [Google Scholar] [CrossRef]
- Kriebel, D.; Tickner, J.; Epstein, P.; Lemons, J.; Levins, R.; Loechler, E.L.; Quinn, M.; Rudel, R.; Schettler, T.; Stoto, M. The precautionary principle in environmental science. Environ. Health Perspect. 2001, 109, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Smart—Servier Medical Art. Available online: https://smart.servier.com/ (accessed on 15 January 2025).
- Creative Commons. CC By 4.0, Attribution 4.0 International Deed. Available online: https://creativecommons.org/licenses/by/4.0/ (accessed on 1 July 2025).

| Interactions between air pollutants (particulate matter and particulate matter-like pollutants) with CCR5 (mRNA or protein) | |||||
| Chemical name | Chemical ID | CAS RN | Interaction | Interaction actions * | Number (n) of works supporting the interactions in CTD ** |
| Dust | D004391 | - | Dust results in increased expression of CCR5 mRNA | Increases ^ expression | n = 1 |
| Particulate matter | D052638 | - | Particulate matter results in decreased expression of CCR5 mRNA | Decreases ^ expression | n = 1 |
| Particulate matter results in increased expression of CCR5 mRNA | Increases ^ expression | n = 2 | |||
| Soot | D053260 | - | Soot results in increased expression of CCR5 mRNA | Increases ^ expression | n = 1 |
| Tobacco smoke pollution | D014028 | - | Tobacco smoke pollution affects the expression of CCR5 mRNA | Affects ^ expression | n = 1 |
| Tobacco smoke pollution results in decreased expression of CCR5 mRNA | Decreases ^ expression | n = 1 | |||
| Vehicle emissions | D001335 | - | Vehicle emissions affect the methylation of CCR5 gene | Affects ^ methylation | n = 1 |
| Vehicle emissions results in increased expression of CCR5 mRNA | Increases ^ expression | n = 2 | |||
| Interactions between metals and related compounds with CCR5 (mRNA or protein) | |||||
| Chemical name | Chemical ID | CAS RN | Interaction | Interaction actions | Number (n) of works supporting the interactions in CTD * |
| Arsenic | D001151 | 7440-38-2 | [Sodium arsenite results in increased abundance of arsenic] which results in increased expression of CCR5 mRNA | Increases ^ abundance|increases ^ expression | n = 1 |
| Cadmium | D002104 | 7440-43-9 | [Cadmium chloride results in increased abundance of cadmium] which results in increased expression of CCR5 mRNA | Increases ^ abundance|increases ^ expression | n = 1 |
| Cadmium chloride | D019256 | 10108-64-2 | [Cadmium chloride results in increased abundance of cadmium] which results in increased expression of CCR5 mRNA | Increases ^ abundance|increases ^ expression | n = 1 |
| Mercuric chloride | D008627 | 7487-94-7 | CCR5 protein results in increased susceptibility to mercuric chloride | Increases ^ response to substance | n = 1 |
| Mercuric chloride results in increased expression of CCR5 mRNA | Increases ^ expression | n = 1 | |||
| Mercuric chloride results in increased expression of CCR5 protein | Increases ^ expression | n = 1 | |||
| Nickel | D009532 | 7440-02-0 | Nickel affects the expression of CCR5 mRNA | Affects ^ expression | n = 1 |
| Nickel results in increased expression of CCR5 mRNA | Increases ^ expression | n = 1 | |||
| Trichostatin A inhibits the reaction [Nickel affects the expression of CCR5 mRNA] | Affects ^ expression|decreases ^ reaction | n = 1 | |||
| Nickel monoxide | C028007 | 1313-99-1 | Nickel monoxide results in increased expression of CCR5 mRNA | Increases ^ expression | n = 1 |
| Sodium arsenite | C017947 | 13768-07-5 | [Sodium arsenite results in increased abundance of arsenic] which results in increased expression of CCR5 mRNA | Increases ^ abundance|increases ^ expression | n = 1 |
| Titanium dioxide | C009495 | 13463-67-7 | [Titanium dioxide co-treated with azoxymethane co-treated with dextran sulfate] results in decreased expression of CCR5 mRNA | Affects ^ cotreatment|decreases ^ expression | n = 1 |
| Titanium dioxide results in increased expression of CCR5 mRNA | Increases ^ expression | n = 3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellwanger, J.H.; Valverde-Villegas, J.M.; Ziliotto, M.; Bogo Chies, J.A. Metal Pollution as a Risk Factor for HIV Infection. Immuno 2025, 5, 34. https://doi.org/10.3390/immuno5030034
Ellwanger JH, Valverde-Villegas JM, Ziliotto M, Bogo Chies JA. Metal Pollution as a Risk Factor for HIV Infection. Immuno. 2025; 5(3):34. https://doi.org/10.3390/immuno5030034
Chicago/Turabian StyleEllwanger, Joel Henrique, Jacqueline María Valverde-Villegas, Marina Ziliotto, and José Artur Bogo Chies. 2025. "Metal Pollution as a Risk Factor for HIV Infection" Immuno 5, no. 3: 34. https://doi.org/10.3390/immuno5030034
APA StyleEllwanger, J. H., Valverde-Villegas, J. M., Ziliotto, M., & Bogo Chies, J. A. (2025). Metal Pollution as a Risk Factor for HIV Infection. Immuno, 5(3), 34. https://doi.org/10.3390/immuno5030034








