Abstract
Synthetic dyes of various classes are widely applied in food production. Reliable and simple methods of dye determination are in demand for food quality control. Novel, sensitive, and selective voltammetric sensors based on glassy carbon electrodes modified with mixtures of metal oxide nanoparticles (NPs) dispersed in water or surfactant media have been developed for the first time for Sunset Yellow FCF, Brilliant Blue FCF, and Quinoline Yellow. Mixtures of CeO2 and SnO2 NPs dispersed in surfactants or CeO2 and Fe2O3 NPs are the best sensing layers for the determining of Sunset Yellow FCF and Quinoline Yellow or Brilliant Blue FCF.
1. Introduction
Synthetic dyes of various classes are widely applied in food production due to bright and reproducible colors, high stability, and improvement in the foodstuff appearance [1,2]. The dye content in food is strictly regulated due to a wide range of possible negative health effects [3]. Therefore, simple and reliable methods are in demand for food quality control. The presence of electroactive fragments in the structure of synthetic dyes makes possible the development of voltammetric sensors for their quantification.
Voltammetric sensors are a promising tool for the fast screening of synthetic dyes of various classes due to high sensitivity and selectivity, reliability, low costs, and possibilities of application on-site [4,5,6]. The sensing layer consisted of various types of nanomaterials, and their composites were used to provide sufficient analytical characteristics of dyes. Metal oxide nanomaterials are among the effective electrode surface modifiers. Nanostructured cerium(IV), tin(IV), iron(III), neodymium(III), zirconium(IV), and molybdenum(IV) oxides have been successfully applied as a sensing layer of voltammetric sensors to synthetic dyes [5,7,8,9,10,11]. The combination of several metal oxides providing a synergetic effect of each component is the further development in this field and shows improvement in the analytical parameters of the target dye analysis [12,13].
The current study is focused on the development of novel voltammetric sensors for Sunset Yellow FCF, Brilliant Blue FCF, and Quinoline Yellow based on a combination of metal oxide nanoparticles (NPs). Mixtures of cerium and tin dioxide NPs dispersed in cationic hexadecylpyridium bromide (HDPB) or non-ionic Brij® 35 surfactants have been shown to be the best sensing layers for the determination of Sunset Yellow FCF and Quinoline Yellow, respectively. The voltammetric sensor based on the mixture of cerium dioxide and iron(III) oxide NPs dispersed in water allows the determination of Brilliant Blue FCF. Sensors are characterized by scanning electron microscopy (SEM), electrochemical impedance spectroscopy, and voltammetry.
2. Materials and Methods
Sunset Yellow FCF (98% purity reagent), 85% Brilliant Blue FCF from Aldrich (Steinheim, Germany), and Quinoline Yellow from TCI (Tokyo, Japan) were used. Their stock solutions (1.0 × 10−3 M for Sunset Yellow FCF and Brilliant Blue FCF, 5060 mg L−1 for Quinoline Yellow) were prepared in distilled water.
CeO2 and SnO2 NPs, as well as CeO2·Fe2O3 NPs, were used as electrode surface modifiers. The mixture of CeO2 and SnO2 NPs (1 mg mL−1) was prepared from commercial reagents (10% CeO2 NPs water dispersion from Sigma-Aldrich (St. Louis, MO, USA) and SnO2 NP powder from Aldrich (Steinheim, Germany)) using surfactant dispersive media. The standard 1.0 mM solutions of surfactants in water were prepared from sodium lauryl sulfate (SLS) (Ph. Eur. Grade, Panreac (Barcelona, Spain), Brij® 35 (98%, Acros Organics (Geel, Belgium)), Tween® 80 (Merck, Steinheim, Germany), Triton X-100, and 98% HDPB from Aldrich (Steinheim, Germany). Dispersions of CeO2·Fe2O3 NPs (0.25–1.0 mg mL−1) were prepared by exact dilution of 20% aqueous dispersion of CeO2·Fe2O3 NPs (50:50 wt.%) from Alfa Aesar (Haverhill, MA, USA) and Cerion (Rochester, NY, USA). Sonication for 10 min in an ultrasonic bath (WiseClean WUC-A03H) (DAIHAN Scientific Co., Ltd., Wonju-si, Republic of Korea) was applied for the NPs’ dispersion preparation. Then, electrode surface modification was performed using the drop-casting method of 3–5 μL of NPs’ dispersion.
Electrochemical measurements were carried out at the potentiostat/galvanostat PGSTAT 302N with FRA 32M module (Metrohm B.V., Utrecht, The Netherlands) or PGSTAT 12 (Eco Chemie B.V., Utrecht, The Netherlands) with NOVA 1.10.1.9 software. GCE (3 mm diameter) from CH Instruments, Inc. (Bee Cave, TX, USA) or modified electrode, reference Ag/AgCl electrode, and auxiliary electrode (platinum wire) were placed in the electrochemical glass cell containing Britton–Robinson buffer (BRB) and cyclic or differential pulse voltammograms were recorded.
A MerlinTM high-resolution field emission scanning electron microscope (Carl Zeiss, Oberkochen, Germany), operated at 5 kV accelerating voltage and a 300 pA emission current, was applied for the electrode surface morphology characterization.
3. Results and Discussion
3.1. Voltammetric Sensors’ Characteristics
The voltammetric response of target dyes strongly depends on the sensing layer characteristics. The effect of modifier constituents (NPS and surfactants) and their concentrations were tested. The mixture of CeO2 and SnO2 NPs in various surfactants as dispersive media was studied. The nature of surfactants (anionic SLS, non-ionic Brij® 35, Tween® 80, and Triton X-100, cationic HDPB) and their concentration in the range of 0.050–1.0 mM were tested. Anionic SLS cannot be used as a dispersive medium as it promotes aggregation of NPs due to electrostatic effects. Non-ionic and cationic surfactants provide stable dispersions of CeO2 and SnO2 NPs. In the case of water dispersion of CeO2·Fe2O3 NPs, the effect of NPs concentration on the dye response was evaluated. The electrode parameters that provide the best response of the target dyes are summarized in Table 1.

Table 1.
Parameters of voltammetric sensors based on the mixed metal oxide NPs giving the best response to target synthetic dyes.
The electroactive surface area of the sensors was evaluated using the electro-oxidation of ferrocyanide ions in 0.1 M KCl. The data obtained (Table 1) indicate a significantly increased electroactive surface area vs. bare GCE (8.9 ± 0.3 mm2). The electron transfer rate constant calculated on the basis of the charge transfer resistance obtained by electrochemical impedance spectroscopy confirms an 8.5–38-fold increase for the sensors based on the mixed metal oxide NPs compared to bare GCE.
The surface morphology of the created sensors was studied by SEM (Figure 1). Spherical NPs were evenly distributed at the electrode surface forming porous coverage. Particles of average size of 28–90 nm with individual rhomboid inclusions were observed for NPs CeO2–SnO2–Brij® 35/GCE (Figure 1b). The NPs CeO2–SnO2–HDPB/GCE consisted of spherical NPs of 12–40 nm and of single aggregates up to 150 nm (Figure 1c). A fairly uniform porous coating of spherical particles with a diameter of 27-80 nm was obtained for NPs CeO2·Fe2O3/GCE (Figure 1d).

Figure 1.
SEM images of the sensor surface: (a) bare GCE; (b) NPs CeO2–SnO2–Brij® 35/GCE; (c) NPs CeO2–SnO2–HDPB/GCE; (d) NPs CeO2·Fe2O3/GCE.
3.2. Synthetic Dyes’ Quantification
BRB was used as a supporting electrolyte in order to cover a wider range of pH. The changes in the voltammetric characteristics of dyes were studied. The highest oxidation peak currents were obtained at pH 5.0 for the Quinoline Yellow and Brilliant Blue FCF and at pH 2.0 for the Sunset Yellow FCF.
Differential pulse voltammetry was applied for quantification purposes. On the basis of dye oxidation peak current, the pulse parameters were optimized as modulation amplitude of 100 mV and modulation time of 25 ms for the Quinoline Yellow and Sunset Yellow FCF and modulation amplitude of 100 mV and modulation time of 75 ms for the Brilliant Blue FCF.
The oxidation peak current of all dyes (the first oxidation peak in the case of Quinoline Yellow) linearly increased with the concentration growth (Figure 2). Two linear ranges were obtained for each dye. The coefficients of determination of the corresponding linear plots are in the range of 0.9995–0.9998, confirming the high degree of the sensor’s response linearity. The analytical characteristics achieved are presented in Table 2, which are improved vs. existing ones [8,13,14,15,16,17,18,19] and sufficient for application to real samples. Moreover, the absence of a preconcentration step significantly simplifies the measurement and reduces its time.
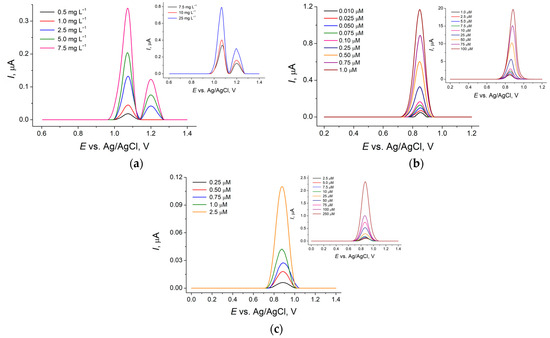
Figure 2.
Baseline-corrected differential pulse voltammograms of synthetic food dyes: (a) Quinoline Yellow at NPs CeO2–SnO2–Brij® 35/GCE in BRB pH 5.0; (b) Sunset Yellow FCF at NPs CeO2–SnO2–HDPB/GCE in BRB pH 2.0; (c) Brilliant Blue FCF at NPs CeO2·Fe2O3/GCE in BRB pH 5.0.

Table 2.
Figures of merit of the sensors based on the mixed metal oxide NPs for synthetic food dyes.
The high accuracy of the developed sensors was confirmed by recovery of 99–100% in the model solutions containing the target dye. The selectivity test in the presence of inorganic ions, carbohydrates, L-ascorbic acid, caffeine, as well as several other synthetic dyes typical for real samples was proven.
The developed sensors were applied in the beverages’ analysis. Sunset Yellow FCF and Brilliant Blue FCF were measured, and their contents were compared with the data from the independent methods (Figure 3). A good agreement of the results obtained was observed. The corresponding t-test values of 0.205–1.18 and F-test values of 1.63–6.82 were less than the critical values and indicated the absence of systematic errors and similar precision.
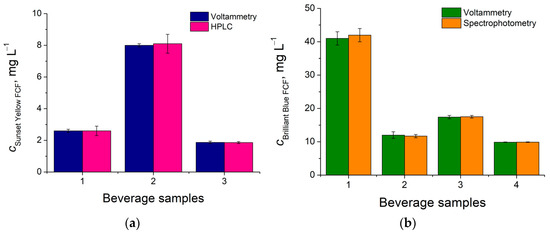
Figure 3.
Synthetic dye contents in the beverages: (a) Sunset Yellow FCF obtained by NPs CeO2–SnO2–HDPB/GCE and high-performance liquid chromatography; (b) Brilliant Blue FCF obtained by NPs CeO2·Fe2O3/GCE and spectrophotometry.
4. Conclusions
Voltammetric sensors based on the mixtures of metal oxide NPs have shown a sensitive and selective response to synthetic food dyes of various classes. The data obtained allow us to conclude that this type of electrode surface modifier is universal and can be considered as a perspective nanomaterial for organic electroanalysis. The simplicity of electrode surface modification opens perspectives on its fabrication using screen-printed technology, which can be used in combination with portable devices. Another direction of further development to be focused on is the enlargement of metal oxide nanomaterials (nanoflowers, nanowires, nanoneedles, nanoribbons, etc.), including their mixtures as modifiers.
Author Contributions
Conceptualization, G.Z.; methodology, L.G., D.B. and G.Z.; validation, D.B. and G.Z.; investigation, L.G., D.B. and G.Z.; writing—original draft preparation, G.Z.; writing—review and editing, G.Z.; visualization, L.G., D.B. and G.Z.; supervision, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank Aleksei Rogov (Laboratory of Scanning Electron Microscopy, Interdisciplinary Center for Analytical Microscopy, Kazan Federal University) for the SEM measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food colour additives: A synoptical overview on their chemical properties, applications in food products, and health side effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Alegbe, E.O.; Uthman, T.O. A review of history, properties, classification, applications and challenges of natural and synthetic dyes. Heliyon 2024, 10, e33646. [Google Scholar] [CrossRef] [PubMed]
- Mota, I.G.C.; Neves, R.A.M.D.; Nascimento, S.S.D.C.; Maciel, B.L.L.; Morais, A.H.D.A.; Passos, T.S. Artificial dyes: Health risks and the need for revision of international regulations. Food Rev. Int. 2021, 39, 1578–1593. [Google Scholar] [CrossRef]
- Soni, I.; Kumar, P.; Sharma, S.; Kudur Jayaprakash, G. A short review on electrochemical sensing of commercial dyes in real samples using carbon paste electrodes. Electrochem 2021, 2, 274–294. [Google Scholar] [CrossRef]
- Cardenas-Riojas, A.A.; Calderon-Zavaleta, S.L.; Quiroz-Aguinaga, U.; Muedas-Taipe, G.; Carhuayal-Alvarez, S.M.; Ascencio-Flores, Y.F.; Ponce-Vargas, M.; Baena-Moncada, A.M. Modified electrochemical sensors for the detection of selected food azo dyes: A review. ChemElectroChem 2024, 11, e202300490. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Fouad, F.H.; Raouf Fayek, G.; El Sayed, K.M.; Ahmed, M.N.; Mahmoud, R.Z.; El Nashar, R.M. Recent advances in electrochemical sensors based on nanomaterials for detection of red dyes in food products: A review. Food Chem. 2024, 435, 137656. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.K.; Budnikov, H.C. Voltammetric determination of tartrazine on an electrode modified with cerium dioxide nanoparticles and cetyltriphenylphosphonium bromide. J. Anal. Chem. 2022, 77, 664–670. [Google Scholar] [CrossRef]
- Gimadutdinova, L.; Ziyatdinova, G.; Davletshin, R. Selective voltammetric sensor for the simultaneous quantification of Tartrazine and Brilliant Blue FCF. Sensors 2023, 23, 1094. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Llanos, J.; García-Beltrán, O.; Nagles, E.; Hurtado, J.J. A new electrochemical method to detect Sunset Yellow, Tartrazine and Thiomersal in a pharmaceutical dose using a carbon paste electrode decorated with molybdenum oxide. Electroanalysis 2020, 32, 2174. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Gimadutdinova, L.; Antonova, T.; Grigoreva, I.; Yakupova, E. The analytical capabilities of electrochemical sensors based on transition metal oxide nanomaterials. Eng. Proc. 2023, 48, 13. [Google Scholar] [CrossRef]
- Ya, Y.; Jiang, C.; Li, T.; Liao, J.; Fan, Y.; Wei, Y.; Yan, F.; Xie, L. A zinc oxide nanoflower-based electrochemical sensor for trace detection of Sunset Yellow. Sensors 2017, 17, 545. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Varela, A.; Cardenas-Riojas, A.A.; Nagles, E.; Hurtado, J. Detection of allura red in food samples using carbon paste modified with lanthanum and titanium oxides. ChemistrySelect 2023, 8, e202204737. [Google Scholar] [CrossRef]
- Khanfar, M.F.; Abu-Nameh, E.S.M.; Azizi, N.A.; Zurayk, R.A.; Khalaf, A.; Saket, M.M.; Alnuman, N. Electrochemical determination of Sunset Yellow and Tartrazine at carbon electrodes modified by Fe-Zr oxide. Jordan J. Chem. (JJC) 2020, 15, 119–126. [Google Scholar]
- Beitollahi, H.; Tajik, S.; Dourandish, Z.; Garkani Nejad, F. Simple preparation and characterization of hierarchical flower-like NiCo2O4 nanoplates: Applications for Sunset Yellow electrochemical analysis. Biosensors 2022, 12, 912. [Google Scholar] [CrossRef] [PubMed]
- Karami, M.; Shabani-Nooshabadi, M. Electrochemical analysis of Sunset Yellow, Brilliant Blue, and Tartrazine using sensor amplified with CuNiFe2O4 hollow spheres. J. Electrochem. Soc. 2023, 170, 087510. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wu, K.; Chen, J.; Zhou, Y. Electrochemical sensor for hazardous food colourant Quinoline Yellow based on carbon nanotube-modified electrode. Food Chem. 2011, 128, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Jahani, P.M. Flower-like MoS2 screen-printed electrode based sensor for the sensitive detection of sunset yellow FCF in food samples. J. Electrochem. Sci. Eng. 2022, 12, 1099–1109. [Google Scholar] [CrossRef]
- Marquez-Mariño, K.; Penagos-Llanos, J.; García-Beltrán, O.; Nagles, E.; Hurtado, J.J. Development of a novel electrochemical sensor based on a carbon paste electrode decorated with Nd2O3 for the simultaneous detection of tartrazine and sunset yellow. Electroanalysis 2018, 30, 2760–2767. [Google Scholar] [CrossRef]
- Arvand, M.; Parhizi, Y.; Mirfathi, S.H. Simultaneous voltammetric determination of synthetic colorants in foods using a magnetic core-shell Fe3O4/SiO2/MWCNTs nanocomposite modified carbon paste electrode. Food Anal. Methods 2016, 9, 863–875. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).