Influence of Key Parameters of GDL on Performance of Anion Exchange Membrane Electrolytic Cells
Abstract
1. Introduction
2. Mathematical Models
2.1. Reversible Potential
2.2. Activation Overpotential
2.3. Ohmic Overpotential
2.4. Diffusion Overpotential
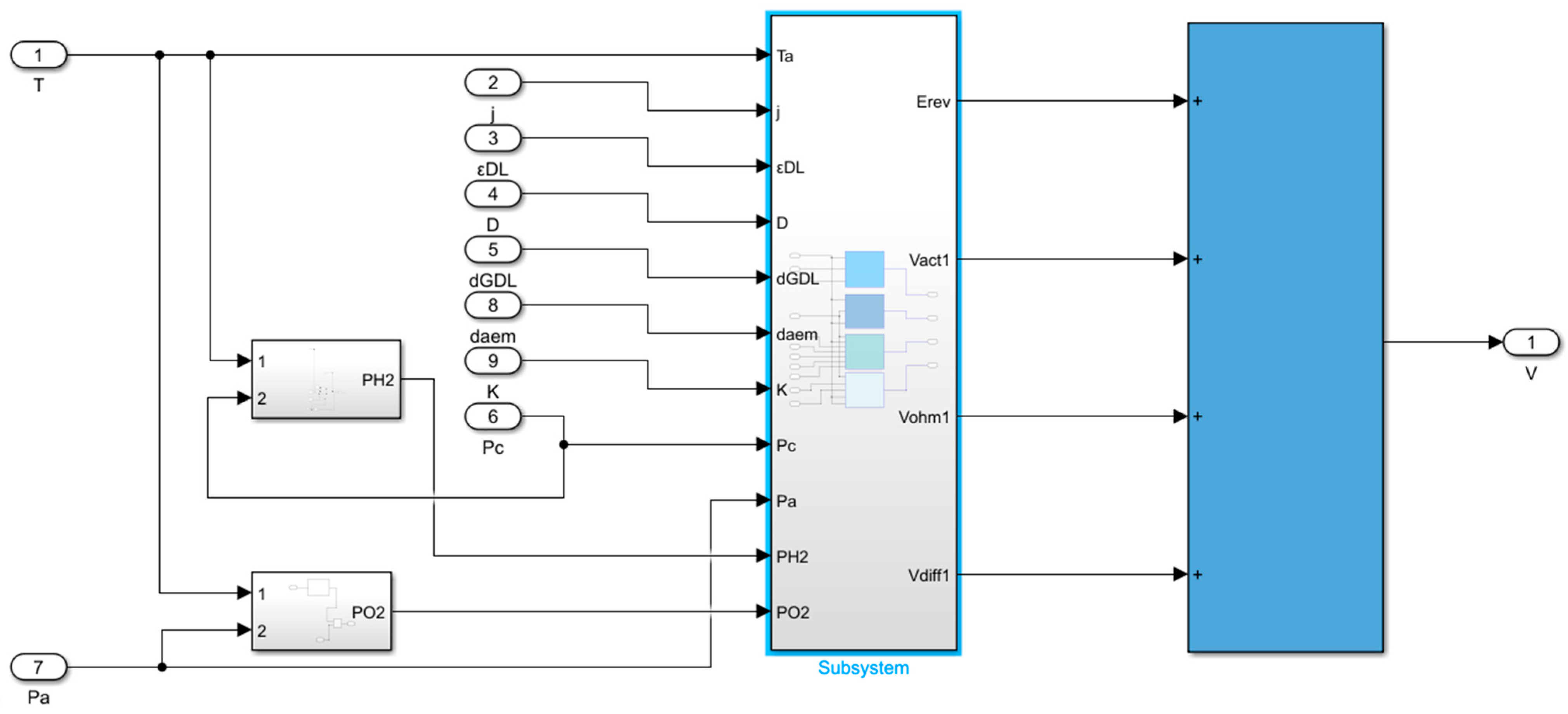
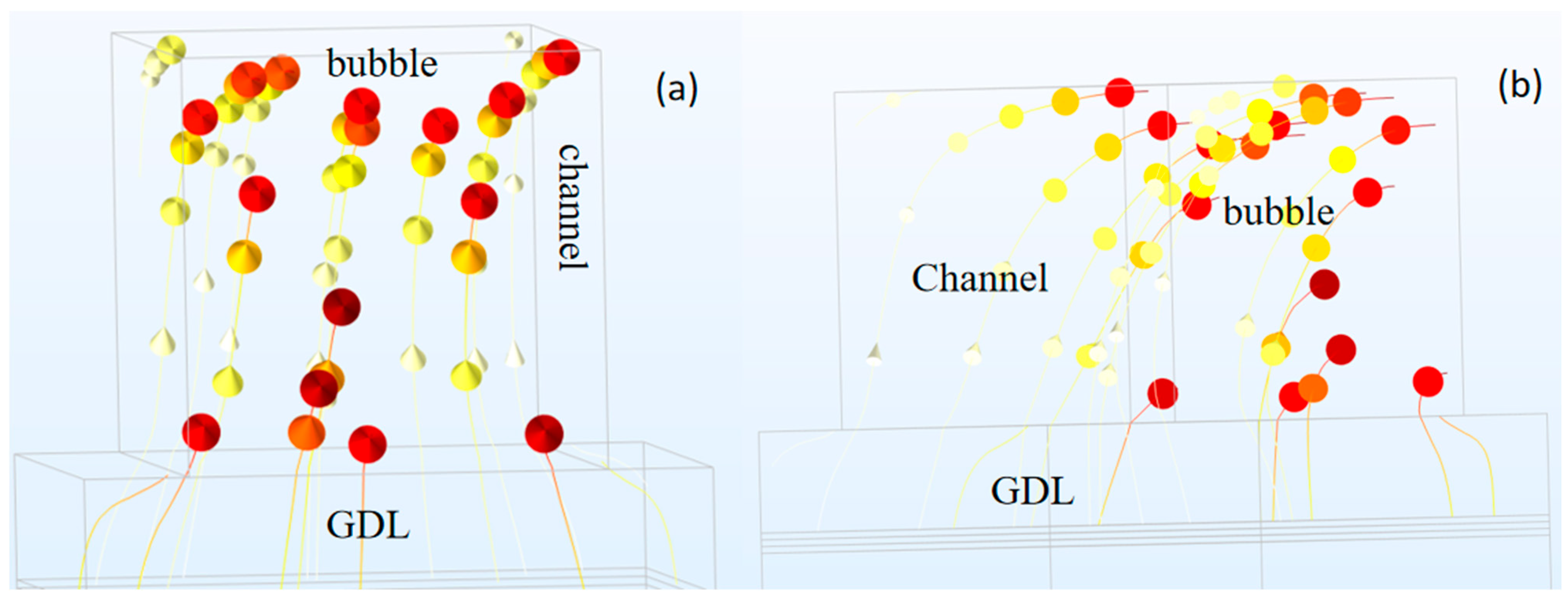
2.5. Interface Model Between Flow Channel and GDL
3. Results and Discussion
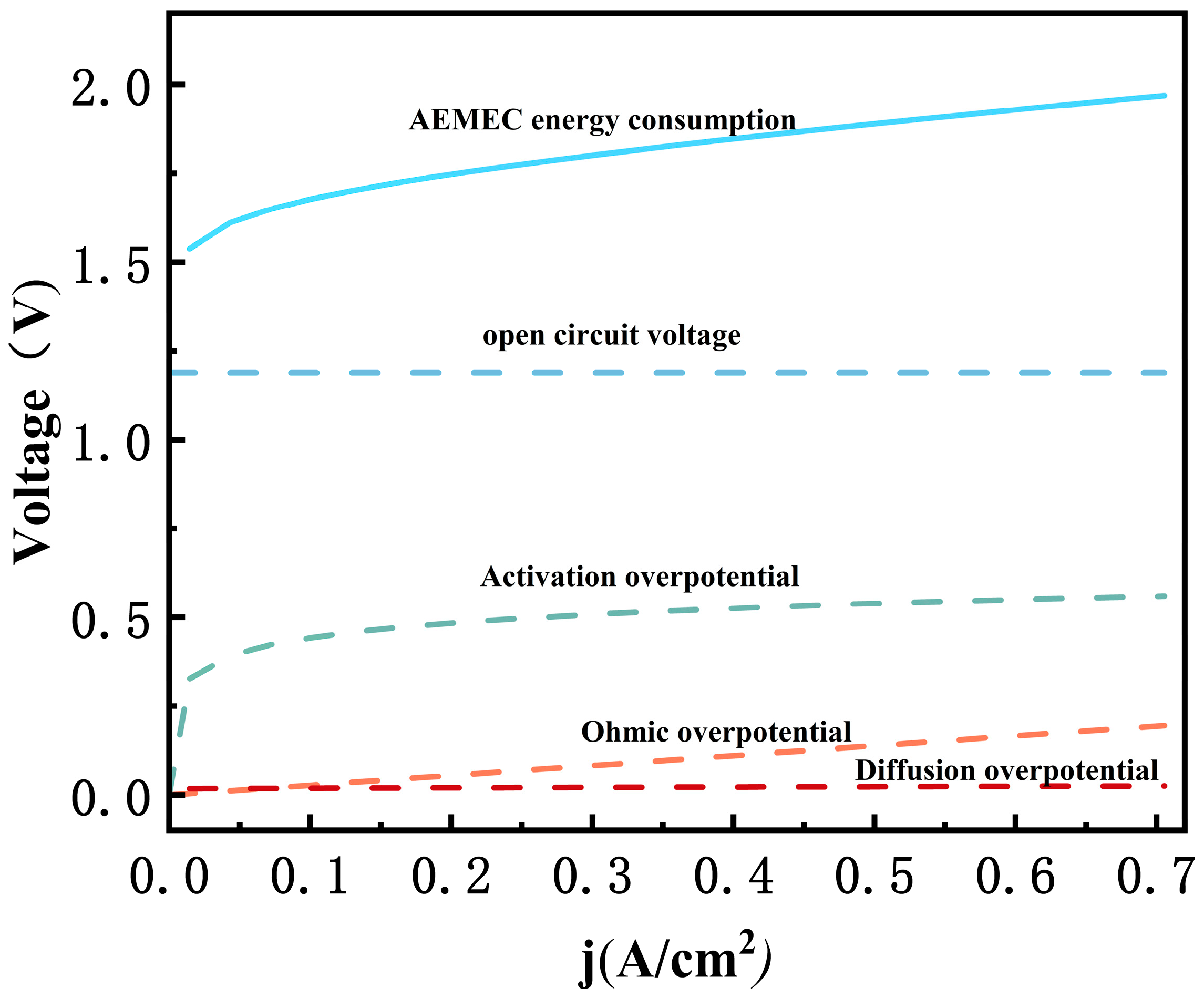
3.1. Model Validation
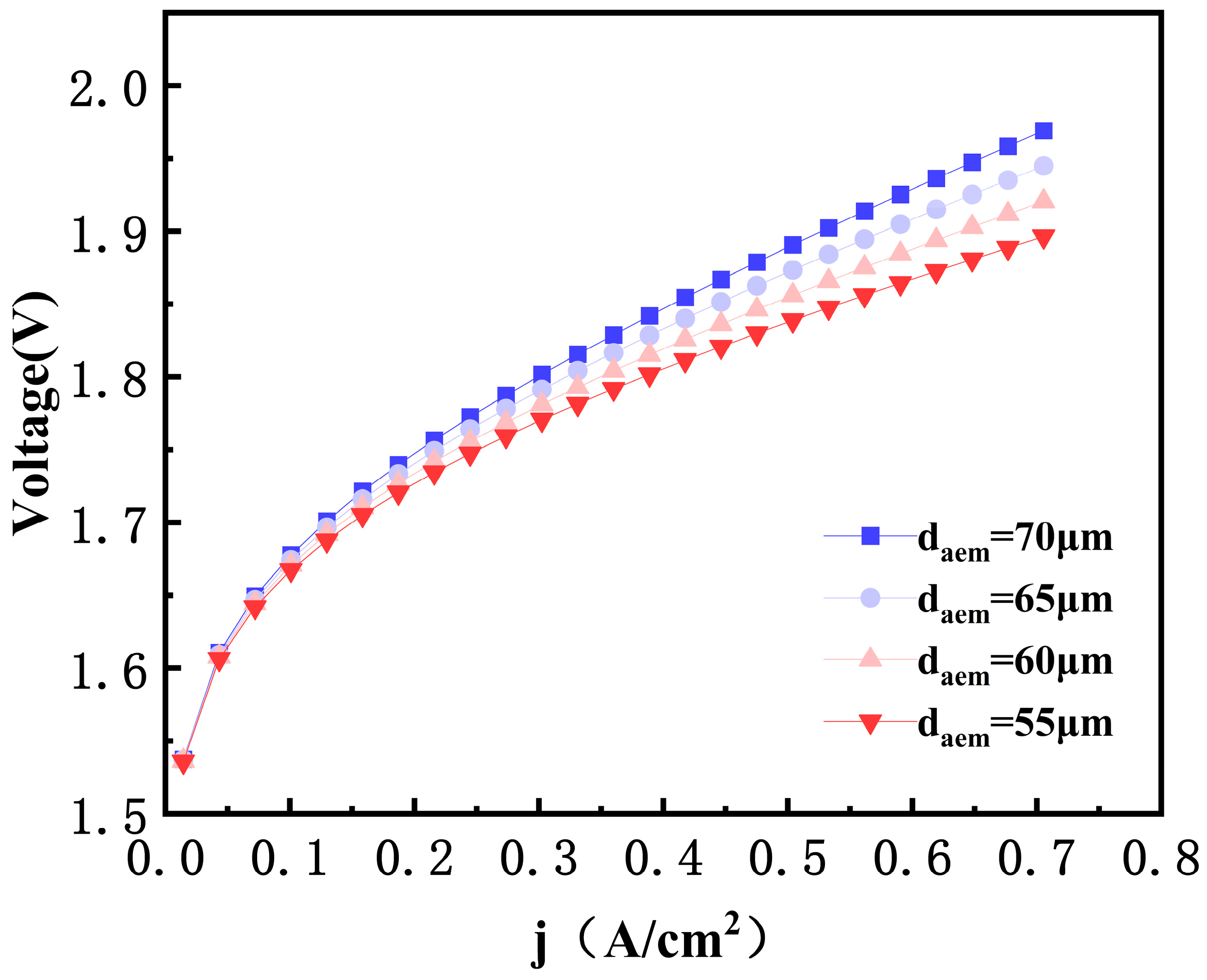
3.2. Effects of AEM Thickness
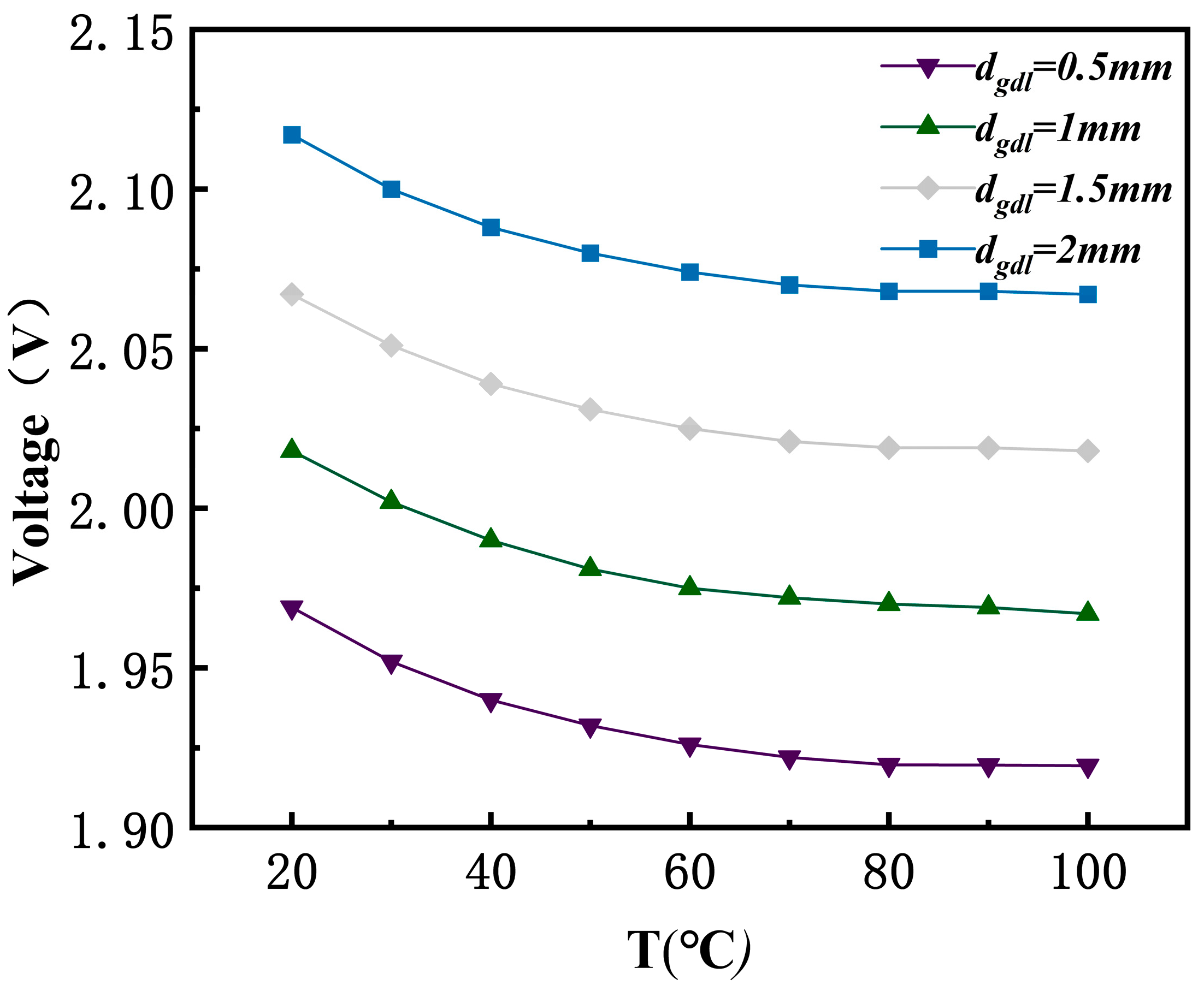
3.3. Effects of the Temperature
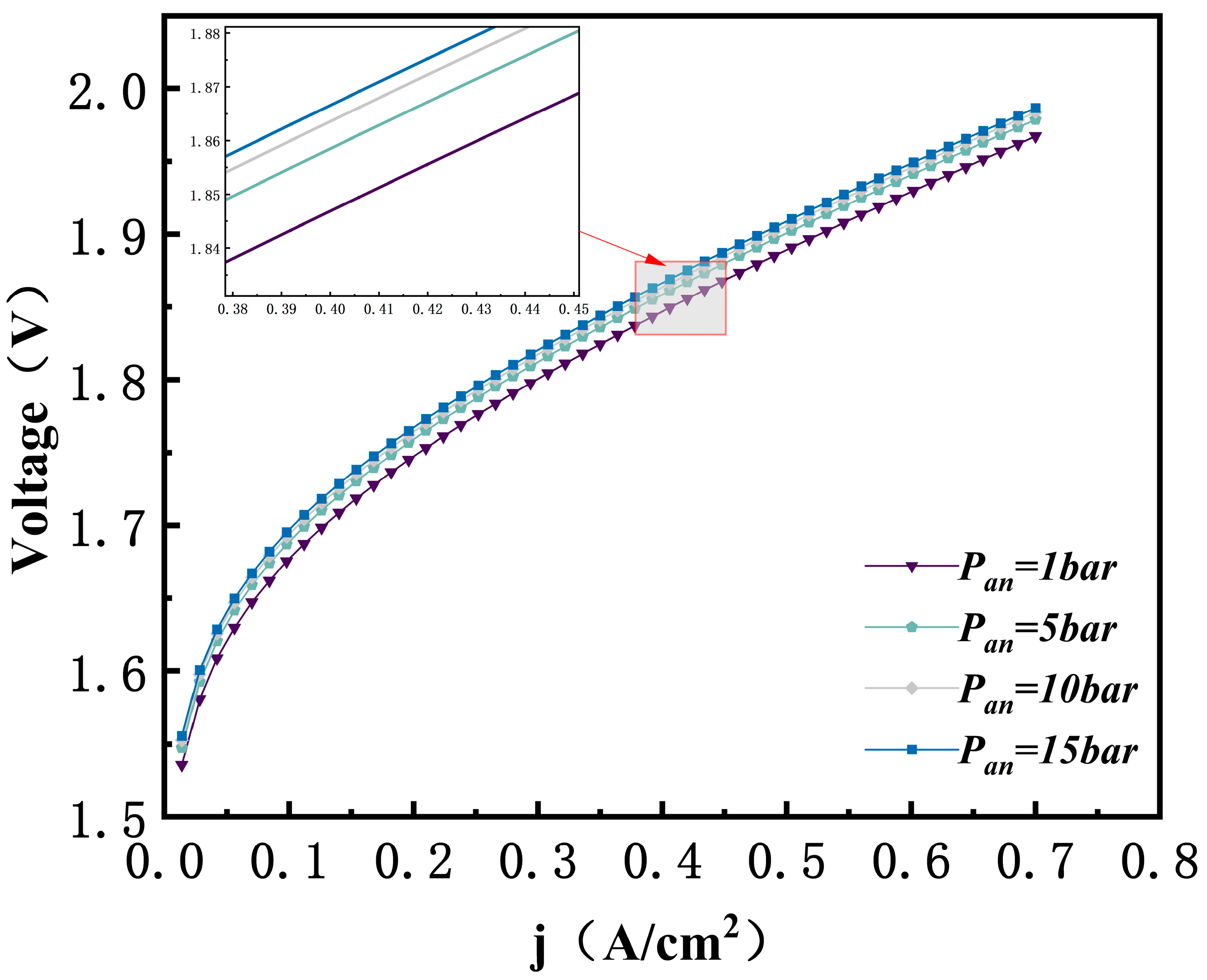
3.4. Effect of Anode Pressure
3.5. Effect of Porous GDL Thickness
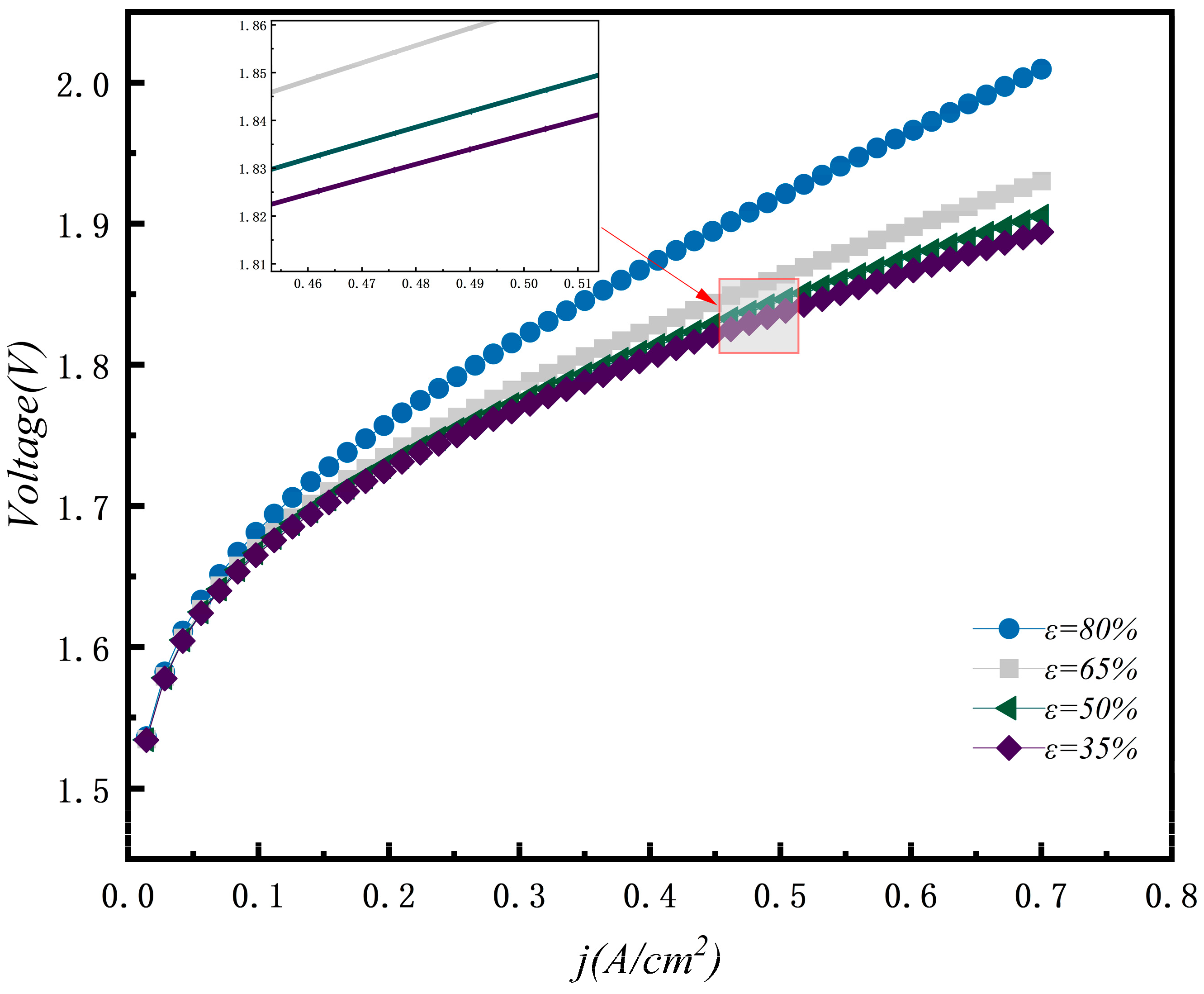
3.6. Effect of Porous GDL Porosity
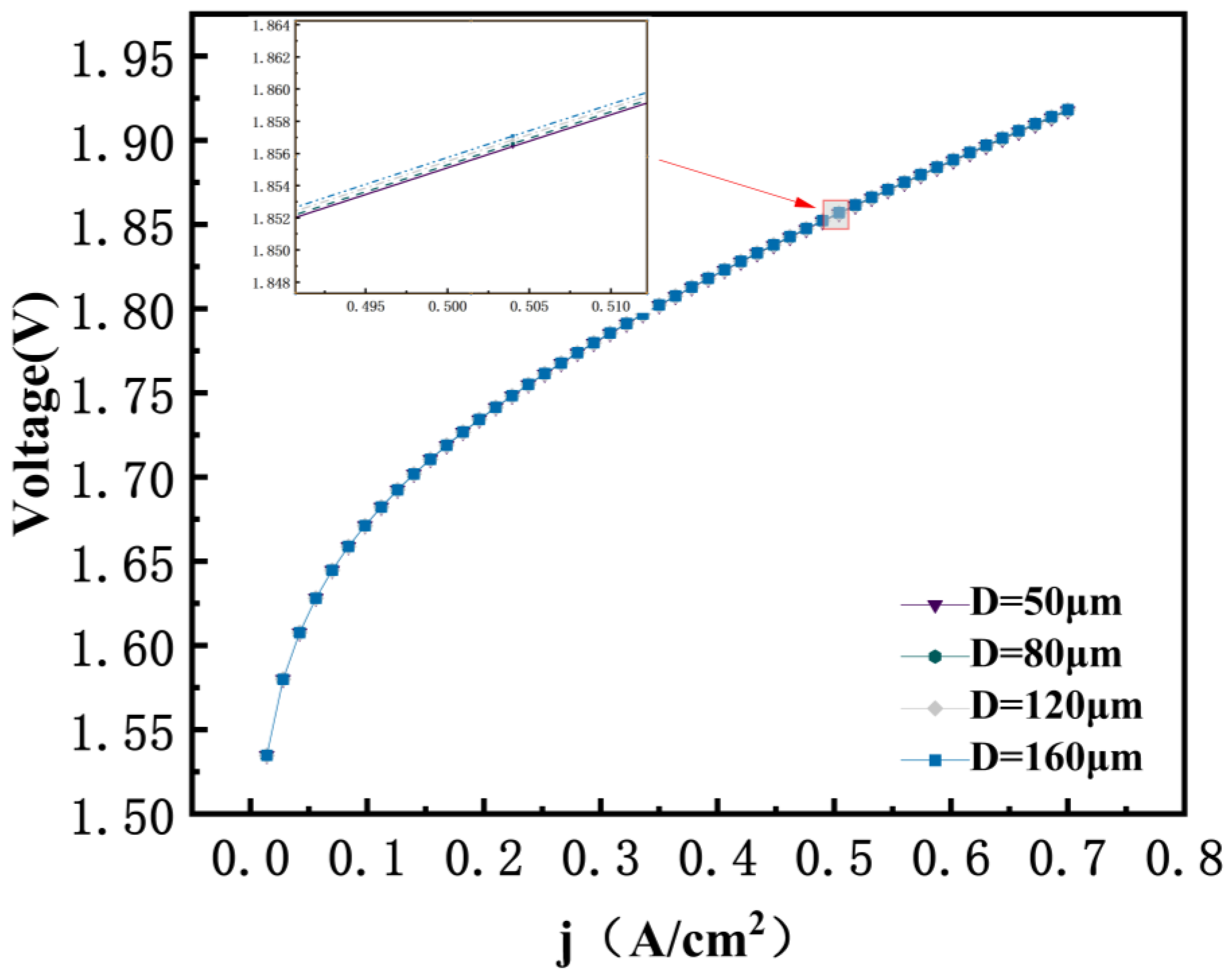
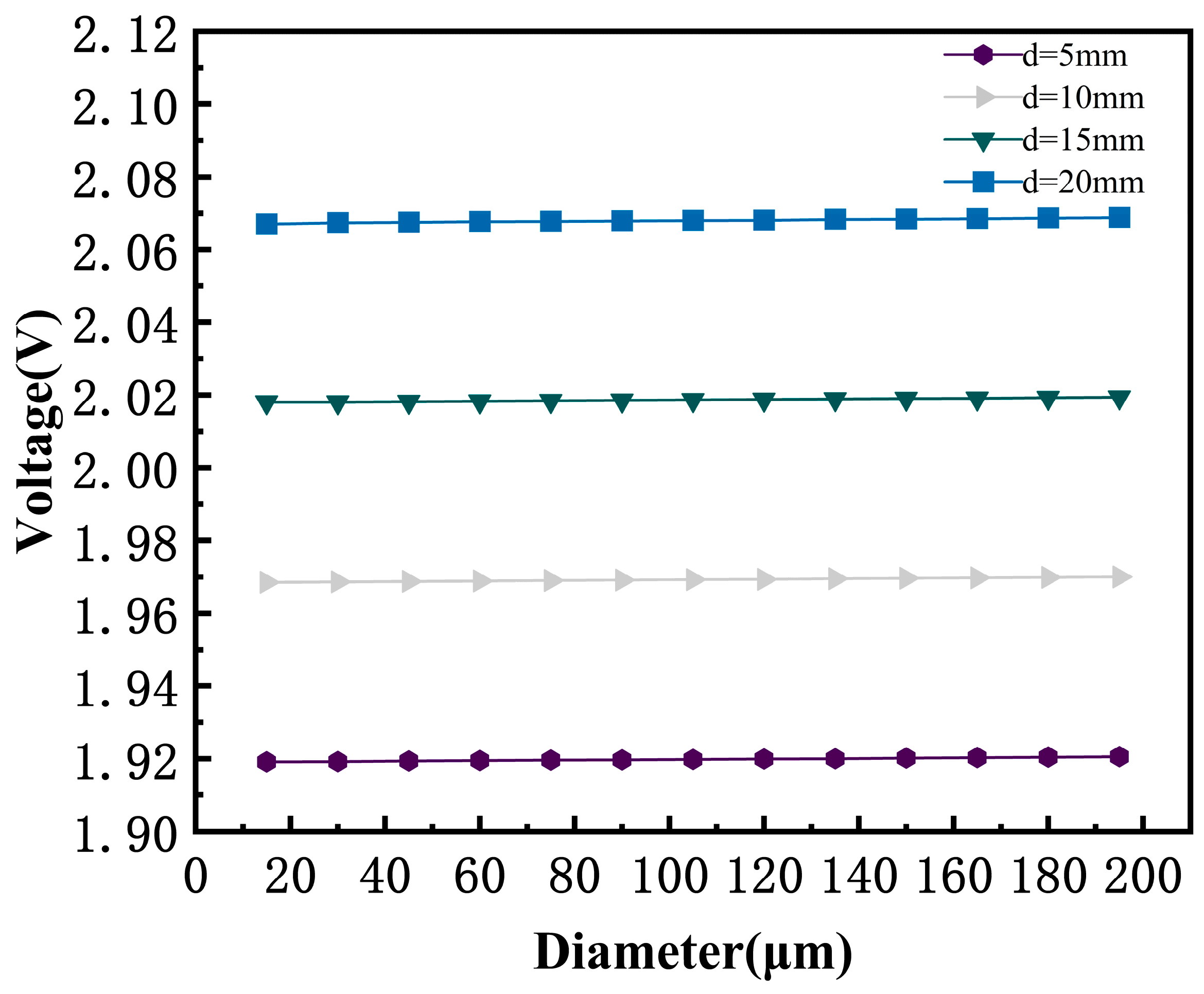
3.7. Effect of Porous GDL Pore Size
4. Conclusions
- (1)
- A reduction in AEM thickness significantly enhances electrolysis efficiency. When the AEM thickness decreased from 70 microns to 65 microns, the electrolysis voltage dropped by 24 mV. This indicates that a thinner membrane reduces ion migration resistance, which increases conductivity and reduces ohmic polarization loss.
- (2)
- Operating pressure and working temperature have a dual effect on AEMEC performance. An increase in operating pressure and working temperature has a dual effect on AEMEC performance. The increase in temperature and pressure raises the electrolysis voltage. This effect is especially noticeable at higher current densities and results in a significant rise in energy consumption. When the anode pressure increased from 1 bar to 15 bar, the electrolysis voltage increased by 19.44 mV. This suggests that excessive pressure reduces the overall efficiency of the system.
- (3)
- GDL design parameters such as thickness and porosity are key for reducing mass transfer resistance and enhancing electrolysis efficiency. A reduction in GDL thickness significantly lowers the electrolysis voltage and boosts efficiency. For instance, when the GDL thickness decreased from 2 mm to 0.5 mm, the voltage dropped from 2.068 V to 1.92 V. Furthermore, reducing GDL porosity to 0.2 helps to decrease ohmic losses and further optimize performance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| List of symbols | |
| R | Gas constant |
| T | Temperature |
| F | Faraday constant |
| j | Current density |
| x | The number of reaction sites |
| D | The pore size of GDL |
| x | The total number of reaction sites |
| A | Reaction area |
| ε | GDL porosity |
| dAEM | AEM thickness |
| dGDL | GDL thickness |
| CO2,m | The oxygen concentration at the interface between the electrode and the membrane |
| CH2,m | The hydrogen concentration at the interface between the electrode and the membrane |
| Subscripts and superscripts | |
| an | anode |
| cat | cathode |
| rev | open circuit voltage |
| act | activation overpotential |
| ohm | ohmic overpotential |
| diff | diffusion overpotential |
| AEM | Anion Exchange Membrane |
| 0 | the reversible voltage |
| Greek | |
| αan | anode charge transfer coefficient |
| αcat | Cathode charge transfer coefficient |
| σAEM | AEM conductivity |
| σGDL | GDL conductivity |
References
- Hermesmann, M.; Müllerte, T.E. Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Biswas, S.; Kaur, G.; Paul, G.; Giddey, S. A critical review on cathode materials for steam electrolysis in solid oxide electrolysis. Int. J. Hydrogen Energy 2023, 48, 12541–12570. [Google Scholar] [CrossRef]
- Lima, A.; Torrubia, J.; Valero, A.; Valero, A. Non-Renewable and Renewable Exergy Costs of Water Electrolysis in Hydrogen Production. Energies 2025, 18, 1398. [Google Scholar] [CrossRef]
- Pavel, C.C.; Cecconi, F.; Emiliani, C.; Santiccioli, S.; Scaffidi, A.; Catanorchi, S.; Comotti, M. Highly Efficient Platinum Group Metal Free Based Membrane-Electrode Assembly for Anion Exchange Membrane Water Electrolysis. Angew. Chem.-Int. Ed. 2014, 53, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.; Das, P.K.; Saha, G.; Saha, S.C. Proton Exchange Membrane Electrolysis Revisited: Advancements, Challenges, and Two-Phase Transport Insights in Materials and Modelling. Eng 2025, 6, 72. [Google Scholar] [CrossRef]
- Maksimova-Dimitrova, K.; Mladenova, B.; Borisov, G.; Slavcheva, E. Ni and Co Catalysts on Interactive Oxide Support for Anion Exchange Membrane Electrolysis Cell (AEMEC). Inorganics 2024, 12, 153. [Google Scholar] [CrossRef]
- Tang, J.Y.; Xu, X.M.; Tang, T.; Zhong, Y.J.; Shao, Z.P. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, e2201099. [Google Scholar] [CrossRef]
- Nafchi, M.; Afshari, E.; Baniasadi, E. Anion exchange membrane water electrolysis: Numerical modeling and electrochemical performance analysis. Int. J. Hydrogen Energy 2024, 52, 306–321. [Google Scholar] [CrossRef]
- Abdelhafiz, A.; Mohammed, M.H.; Abed, J. Tri-Metallic Catalyst for Oxygen Evolution Reaction Enables Continuous Operation of Anion Exchange Membrane Electrolyzer at 1A cm−2 for Hundreds of Hours. Adv. Energy Mater. 2024, 14, 2303350. [Google Scholar] [CrossRef]
- Li, L.X.; Jiang, W.X.; Wan, Q.Q.; Zhang, E.D.; Li, B.; Yuan, L.; Li, G.F.; Hou, J.B.; Zhuang, X.D.; Zhang, J.L. Production of the porous transport layer based on stainless steel felt for anion exchange membrane water electrolysis. Electrochim. Acta 2025, 513, 145598. [Google Scholar] [CrossRef]
- Tricker, A.W.; Ertugrul, T.Y.; Lee, J.K.; Shin, J.R.; Kushner, D.; Wang, G.Z.; Lang, J.; Zenyuk, I.V.; Weber, A.Z. Pathways Toward Efficient and Durable Anion Exchange Membrane Water Electrolyzers Enabled by Electro-Active Porous Transport Layers. Adv. Energy Mater. 2024, 14, 2303629. [Google Scholar] [CrossRef]
- Sampathkumar, S.N.; Ferriday, T.B.; Middleton, P.H.; Van Herle, J. Activation of stainless steel 316L anode for anion exchange membrane water electrolysis. Electrochem. Commun. 2023, 146, 107418. [Google Scholar] [CrossRef]
- Wang, R.X.; Inoguchi, K.; Ohashi, M.; Someya, S.; Munakata, T.; Ishida, M.; Ito, H. Effect of porous transport layer properties on the anode electrode in anion exchange membrane electrolyzers. J. Power Sources 2023, 555, 232371. [Google Scholar]
- Singh, A.; Gupta, V.K.; Kumar, A. Chaotic convection in Rivlin-Ericksen fluid flowing through highly permeable porous medium. Phys. Fluids 2024, 36, 114117. [Google Scholar] [CrossRef]
- Dubey, R.; Murthy, P.V.S.N. The onset of convective instability of horizontal throughflow in a porous layer with inclined thermal and solutal gradients. Phys. Fluids 2018, 30, 074104. [Google Scholar] [CrossRef]
- Barman, P.; Srinivasacharya, D. Linear and nonlinear stability analyses of micropolar fluid flow in horizontal porous layers. Phys. Fluids 2024, 36, 033122. [Google Scholar] [CrossRef]
- Montaño, D.L.C.; García, P.R.; Navarro, A.J. Effect of the Cathodic Gas Diffusion Layer on the Performance of a Proton Exchange Membrane Electrolyzer. Energies 2024, 17, 5298. [Google Scholar] [CrossRef]
- Wang, W.T.; Ding, L.; Xie, Z.Q.; Yu, S.L.; Capuano, C.B.; Keane, A.; Ayers, K.; Zhang, F.Y. 3D structured liquid/gas diffusion layers with flow enhanced microchannels for proton exchange membrane electrolyzers. Energy Convers. Manag. 2023, 296, 117665. [Google Scholar] [CrossRef]
- Kang, Z.Y.; Yu, S.L.; Yang, G.Q.; Li, Y.F.; Bender, G.; Pivovar, B.S.; Green, J.B.; Zhang, F.Y. Performance improvement of proton exchange membrane electrolyzer cells by introducing in-plane transport enhancement layers. Electrochim. Acta 2019, 316, 43–51. [Google Scholar] [CrossRef]
- Roenning, F.H.; Roy, A.; Aaron, D.S.; Mench, M.M. Mass transport limitations in polymer electrolyte water electrolyzers using spatially-resolved current measurement. J. Power Sources 2022, 542, 231749. [Google Scholar] [CrossRef]
- Zhu, X.H.; Zhang, J.Y.; Du, S.J.; Dang, J.; Yang, M.Y.; Liu, B.; Ma, J.G.; Yang, F.Y.; Ouyang, M.G. Exploring the Influence Mechanism of Porous Transport Layer Structure and Type on Performance in Proton Exchange Membrane Electrolyzer. J. Electrochem. Soc. 2023, 170, 124510. [Google Scholar] [CrossRef]
- Li, K.; Yu, S.L.; Li, D.G.; Ding, L.; Wang, W.T.; Xie, Z.Q.; Park, E.J.; Fujimoto, C.; Cullen, D.A.; Kim, Y.S.; et al. Engineered Thin Diffusion Layers for Anion-Exchange Membrane Electrolyzer Cells with Outstanding Performance. ACS Appl. Mater. Interfaces 2021, 13, 50957–50964. [Google Scholar] [CrossRef]
- Lawand, K.; Sampathkumar, S.N.; Mury, Z.; Van Herle, J. Membrane electrode assembly simulation of anion exchange membrane water electrolysis. J. Power Sources 2024, 595, 234047. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Yang, F.; Xu, H. Two-dimensional multi-physics modeling of porous transport layer in polymer electrolyte membrane electrolyzer for water splitting. Int. J. Hydrogen Energy 2020, 45, 32984–32994. [Google Scholar] [CrossRef]
- Yigit, T.; Selamet, O.F. Mathematical modeling and dynamic Simulink simulation of high-pressure PEM electrolyzer system. Int. J. Hydrogen Energy 2016, 41, 13901–13914. [Google Scholar] [CrossRef]
- Zou, L.; Shen, Q.W.; Liao, J.D.; Xu, L.Y.; Yang, G.G.; Li, S. Effect of porous transport layer parameters on the proton exchange membrane electrolyzer performance. Chem. Phys. Lett. 2023, 823, 140570. [Google Scholar] [CrossRef]
- Ojong, E.T.; Kwan, J.T.H.; Nouri-Khorasani, A.; Bonakdarpour, A.; Wilkinson, D.P.; Smolinka, T. Development of an experimentally validated semi-empirical fully-coupled performance model of a PEM electrolysis cell with a 3-D structured porous transport layer. Int. J. Hydrogen Energy 2017, 42, 25831–25847. [Google Scholar] [CrossRef]
- Kang, Z.Y.; Mo, J.K.; Yang, G.Q.; Retterer, S.T.; Cullen, D.A.; Toops, T.J.; Green, J.B.; Mench, M.M.; Zhang, F.Y. Investigation of thin/well-tunable liquid/gas diffusion layers exhibiting superior multifunctional performance in low-temperature electrolytic water splitting. Energy Environ. Sci. 2017, 10, 166–175. [Google Scholar] [CrossRef]
- Marangio, F.; Santarelli, M.; Cali, M. Theoretical model and experimental analysis of a high pressure PEM water electrolyser for hydrogen production. Int. J. Hydrogen Energy 2009, 34, 1143–1158. [Google Scholar] [CrossRef]
- Shen, M.Z.; Bennett, N.; Ding, Y.L.; Scott, K. A concise model for evaluating water electrolysis. Int. J. Hydrogen Energy 2011, 36, 14335–14341. [Google Scholar] [CrossRef]
- Diaz, D.F.R.; Valenzuela, E.; Wang, Y. A component-level model of polymer electrolyte membrane electrolysis cells for hydrogen production. Appl. Energy 2022, 321, 119398. [Google Scholar] [CrossRef]




| Current Density (A/cm2) | |
|---|---|
| 0 | 1 |
| 0.1 | 0.989 |
| 0.2 | 0.978 |
| 0.3 | 0.967 |
| 0.4 | 0.956 |
| 0.5 | 0.945 |
| 0.6 | 0.934 |
| 0.7 | 0.923 |
| Description | Value, Unit |
|---|---|
| Faraday constant | 96,485 C/mol |
| Gas constant | 8.314 J/mol K |
| The operating temperature | 353.15 K, 333.15 K, 323.15 K |
| Anode and cathode working pressure | 1 bar, 1 bar |
| The maximum current density | 0.7 A/cm2 |
| Anode and cathode charge transfer coefficient | 0.5, 0.5 |
| Anode and cathode exchange current density | 6.815/0.0000572 A/cm2 |
| Porosity | 0.6 |
| Pore diameter | 50 μm |
| Interface contact resistance coefficient | 1.4 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Shen, Q.; Zhang, Z.; Li, H.; Li, S. Influence of Key Parameters of GDL on Performance of Anion Exchange Membrane Electrolytic Cells. Eng 2025, 6, 111. https://doi.org/10.3390/eng6060111
Zhao X, Shen Q, Zhang Z, Li H, Li S. Influence of Key Parameters of GDL on Performance of Anion Exchange Membrane Electrolytic Cells. Eng. 2025; 6(6):111. https://doi.org/10.3390/eng6060111
Chicago/Turabian StyleZhao, Xinyue, Qiuwan Shen, Zhaoyang Zhang, Hongda Li, and Shian Li. 2025. "Influence of Key Parameters of GDL on Performance of Anion Exchange Membrane Electrolytic Cells" Eng 6, no. 6: 111. https://doi.org/10.3390/eng6060111
APA StyleZhao, X., Shen, Q., Zhang, Z., Li, H., & Li, S. (2025). Influence of Key Parameters of GDL on Performance of Anion Exchange Membrane Electrolytic Cells. Eng, 6(6), 111. https://doi.org/10.3390/eng6060111







