Supercritical CO2 Sizing and Desizing of Cotton Yarns
Abstract
1. Introduction
2. Experiments
2.1. Materials
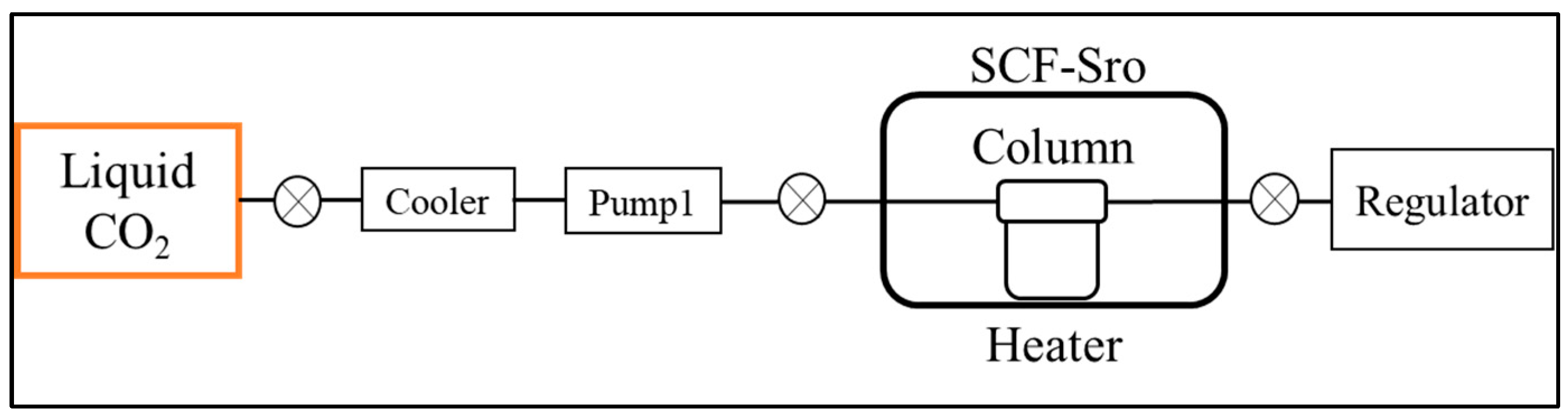
2.2. Equipment
2.3. Methods
2.3.1. Solubility Test
2.3.2. Sizing
2.3.3. Desizing
Batch Method
Continuous Method
- (i)
- Temperature and Pressure:
- Temperature and Pressure: (40 °C, 10 MPa), (40 °C, 20 MPa), and (100 °C, 10 MPa).
- The flow rate of CO2 was 1 mL/min, and acetone was 0.2 mL/min.
- Treatment duration was 300 min.
- (ii)
- Mixing:
- The wire stand was removed to allow the cotton thread to contact the stirrer.
- Ten stainless-steel metal balls (1/4 inch in size) were placed in the high-pressure vessel to facilitate further stirring and size removal.
- The treatment conditions were 40 °C and 10 MPa, with CO2 flowing at 1 mL/min and acetone at 0.2 mL/min for 300 min.
- (iii)
- Flow Velocity: The flow rates and supercritical treatment times were set as follows:
- (CO2 5 mL/min, acetone 1 mL/min, 60 min),
- (CO2 1 mL/min, acetone 0.2 mL/min, 300 min),
- (CO2 0.5 mL/min, acetone 0.1 mL/min, 600 min).

2.3.4. Analysis
Evaluation of Solubility of Hydrophobic Polymers
Adhesion Rate of the Sizing Agent
Desizing Rate
Tensile Strength
FE-SEM Analysis
Friction Test
3. Results and Discussion
3.1. Solubility of Sizing Agent
3.2. Rate of Sizing
3.2.1. Effects of Temperature and Pressure
3.2.2. Effect of Acetone as a Co-Solvent on Sizing Paste Solubility
- Absence of Acetone (0 mol%):
- The sizing rate remains at 1.2%, indicating minimal solubility and adhesion. This aligns with the inherent characteristics of supercritical carbon dioxide as a nearly non-polar solvent, which generally exhibits low solubility for high-molecular-weight substances like cellulose acetate.
- Acetone at 10 mol%:
- Initially, the adhesion rate peaks at 62.6%, demonstrating a substantial enhancement in solubility when acetone is present.
- The sizing rate drops steadily from 62.6% down to 31.4%, 20.6%, and 5.4% as the amount of sizing agent decreases, confirming a clear downward trend in solubility under otherwise identical conditions.
- 3.
- Lower Acetone Concentrations (5 mol% and below):
- At 5 mol%, the adhesion rate drops to 2.4%, showing diminished enhancement compared to 10 mol%.
- Further reduction to 2.5 mol% leads to a negligible sizing rate of 0.3%. This confirms that a higher co-solvent concentration is necessary to improve solubility significantly.
3.2.3. Influence of Adhesive Quantity on Adhesion Performance
3.2.4. Effect of Polymer Molecular Weight
3.3. Rate of Desizing
3.3.1. Comparison Between Batch and Continuous Desizing Methods
3.3.2. Influence of Temperature and Pressure
3.3.3. Role of Physical Contact and Mechanical Agitation
3.3.4. Effect of Flow Rate and Treatment Duration
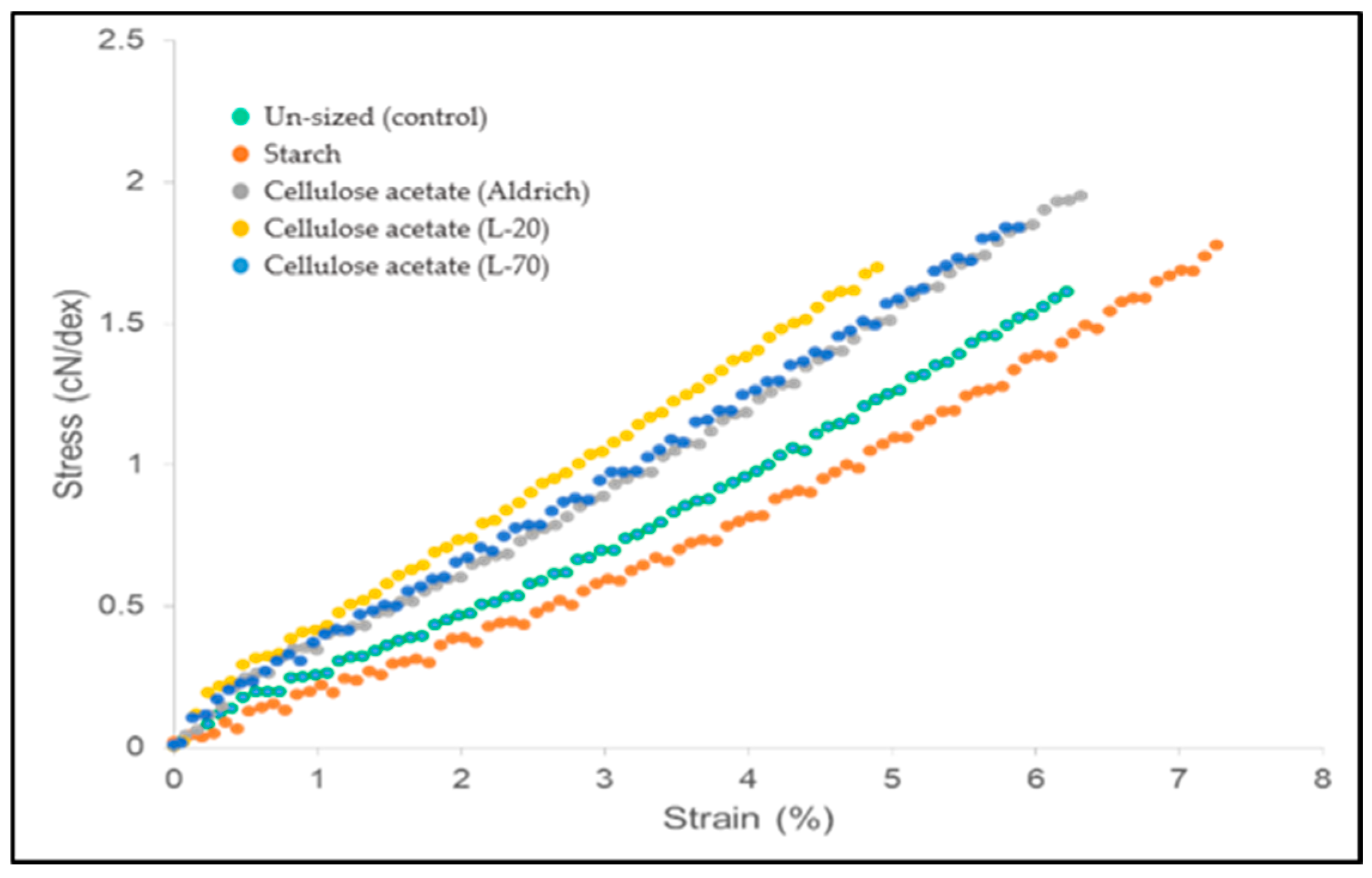
3.4. Tensile Strength and Friction Characteristics
3.5. Yarn Surface Characterization by FE-SEM
4. Conclusions
5. Patent
- Publication number: JP2021-121700 (P2021-121700A)
- Publication Date: 26 August 2021
- Applicants: Izawa Towel Co., Ltd. (Tokyo), National University Corporation Kyoto Institute of Technology (Kyoto)
- Inventors: Shoji IZAWA (Tokyo), Satoko OKUBAYASHI (Kyoto)
- Issuing Authority: Japan Patent Office (JP)
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | Cellulose acetate |
| CMC | Carboxymethylcellulose |
| D% | Desizing rate |
| FE-SEM | Field-emission Scanning electron microscope |
| PVA | Polyvinyl alcohol |
| S% | Sizing rate |
| ScCO2 | Supercritical carbon dioxide |
| SD | Standard deviation |
References
- Lord, P.R.; Mohamed, M.H. Weaving: Conversion of Yarn to Fabric; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar]
- Lewin, M. (Ed.) Handbook of Fiber Chemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Goswami, B.C.; Anandjiwala, R.D.; Hall, D. Textile Sizing; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Trotman, E.R. Dyeing and Chemical Technology of Textile Fibres, 6th ed.; Wiley: Hoboken, NJ, USA, 1985. [Google Scholar]
- Rafikov, A.S.; Fayzullaeva, K.; Shonakhunov, T.E.; Soyibova, D.B.Q.; Yasinskaya, N.N. Enzymatic treatment of cotton fabric for desizing. J. Chem. Eng. Res. Updates 2023, 10, 31–41. [Google Scholar] [CrossRef]
- Jhatial, A.K.; Yesuf, H.M.; Wagaye, B.T. Pretreatment of cotton. In Cotton Science and Processing Technology; Wang, H., Memon, H., Eds.; Springer: Singapore, 2020; pp. 333–353. [Google Scholar] [CrossRef]
- Choudhury, A.K.R. Sustainable textile wet processing: Applications of enzymes. In Roadmap to Sustainable Textiles and Clothing; Blackburn, R.S., Ed.; Springer: Singapore, 2014; pp. 127–168. [Google Scholar]
- Madhav, S.; Ahamad, A.; Singh, P.; Mishra, P.K. A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods. Environ. Qual. Manag. 2018, 27, 31–41. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L. A technical review of emerging technologies for energy and water efficiency and pollution reduction in the textile industry. J. Clean. Prod. 2015, 95, 30–44. [Google Scholar] [CrossRef]
- Deng, D.; Lamssali, M.; Aryal, N.; Ofori-Boadu, A.; Jha, M.K.; Samuel, R.E. Textiles wastewater treatment technology: A review. Water Environ. Res. 2020, 92, 1805–1810. [Google Scholar] [CrossRef]
- Zeeshan, M.H.; Ruman, U.E.; He, G.; Sabir, A.; Shafiq, M.; Zubair, M. Environmental issues concerned with poly (vinyl alcohol) (PVA) in textile wastewater. In Polymer Technology in Dye-Containing Wastewater; Khadir, A., Muthu, S.S., Eds.; Springer: Singapore, 2022; pp. 225–236. [Google Scholar]
- Correia, V.M.; Stephenson, T.; Judd, S.J. Characterization of textile wastewaters—A review. Environ. Technol. 1994, 15, 917–929. [Google Scholar] [CrossRef]
- Gandhi, K.L. Yarn preparation for weaving: Sizing. In Woven Textiles: Principles, Technologies and Applications, 2nd ed.; Woodhead Publishing: Delhi, India, 2020; The Textile Institute Book Series; pp. 119–166. [Google Scholar]
- Knittel, D.; Saus, W.; Schollmeyer, E. Application of Supercritical Carbon Dioxide in Finishing Processes. J. Text. Inst. 1993, 84, 534–552. [Google Scholar] [CrossRef]
- Kazarian, S.G. Polymer processing with supercritical fluids. Polym. Sci. Ser. C 2000, 42, 78–101. [Google Scholar]
- Var, C.; Palamutcu, S. Sustainable approaches in textile-sizing process. In Sustainable Manufacturing Practices in the Textiles and Fashion Sector; Muthu, S.S., Ed.; Springer: Cham, Switzerland, 2024; Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; pp. 55–74. [Google Scholar]
- Yiğit, İ.; Akarsu Özenç, A.; Eren, S. The application of scCO2 medium in cellulosic fibers: A sustainable approach in textile production. Clean Techn Env. Policy 2025, 27, 3959–3968. [Google Scholar] [CrossRef]
- Kang, X.; Mao, L.; Shi, J.; Liu, Y.; Zhai, B.; Xu, J.; Jiang, Y.; Lichtfouse, E.; Jin, H.; Guo, L. Supercritical carbon dioxide systems for sustainable and efficient dissolution of solutes: A review. Environ. Chem. Lett. 2024, 22, 815–839. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.A.; El-Aziz, E.A.; Ma, J.; El-Taweel, F.; Okubayashi, S. Eco-Friendly Disperse Dyeing and Functional Finishing of Nylon 6 Using Supercritical Carbon Dioxide. Fibers 2015, 3, 309–322. [Google Scholar] [CrossRef]
- Montero, G.A.; Smith, C.B.; Hendrix, W.A.; Butcher, D.L. Supercritical fluid technology in textile processing: An overview. Ind. Eng. Chem. Res. 2000, 39, 4806–4812. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Abd El-Aziz, E. Supercritical carbon dioxide as a green media in textile dyeing: A review. Text. Res. J. 2018, 88, 1184–1212. [Google Scholar] [CrossRef]
- Abate, M.T.; Ferri, A.; Guan, J.; Chen, G.; Nierstrasz, V. Colouration and bio-activation of polyester fabric with curcumin in supercritical CO2: Part I—Investigating colouration properties. J. Supercrit. Fluids 2019, 152, 104548. [Google Scholar] [CrossRef]
- Antony, M.; Sakthivel, M.; Raghavendran, N.; Kumar, R.; Subramanian, B. Sizing and desizing of cotton and polyester yarns using liquid and supercritical carbon dioxide with nonfluorous CO2-philes as size compounds. ACS Sustain. Chem. Eng. 2018, 6, 3724–3733. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Martini, R.E. Supercritical CO2-assisted dyeing and functionalization of polymeric materials: A review of recent advances (2015–2020). J. CO2 Util. 2021, 54, 101760. [Google Scholar] [CrossRef]
- Eren, H.A.; Yiğit, İ.; Eren, S.; Avinc, O. Sustainable textile processing with zero water utilization using supercritical carbon dioxide technology. In Sustainability in the Textile and Apparel Industries: Sustainable Textiles—Production, Processing, Manufacturing & Chemistry; Muthu, S., Gardetti, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 179–196. [Google Scholar]
- World Intellectual Property Organization. Enzyme-assisted Desizing Process in Supercritical Carbon Dioxide (WO2016058252A1). 2016. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017135792 (accessed on 26 October 2025).
- Abd-Elaal, L.S.; El-Wekil, A.N.; Marey, A.G.; Allam, L.N.; Hassabo, A.G. Supercritical Carbon Dioxide as an Impregnation Medium for Producing Functional Materials in Textile Finishing. J. Text. Color. Polym. Sci. 2025, 22, 187–195. [Google Scholar] [CrossRef]
- Ghanayem, H.M.; Okubayashi, S. Water-free dewaxing of grey cotton fabric using supercritical carbon dioxide. J. Supercrit. Fluids 2021, 174, 105264. [Google Scholar] [CrossRef]
- Ghanayem, H.M.; Okubayashi, S. Improvement of water wettability of gray cotton fabric using electron beam irradiation and supercritical carbon dioxide. J. Supercrit. Fluids 2022, 181, 105506. [Google Scholar] [CrossRef]
- Ghanayem, H.M.; Okubayashi, S. Coloration of gray cotton fabric pre-treated with electron beam irradiation and supercritical carbon dioxide. J. Supercrit. Fluids 2023, 200, 105974. [Google Scholar] [CrossRef]
- Schmidt-Przewozna, K.; Rój, E. Green sustainable textile supercritical dyeing process using CO2 Madder (Rubia tinctorum L.) extract. J. Nat. Fibers 2023, 20, 2277836. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Zheng, L.J.; Ye, F.; Qian, Y.F.; Yan, J.; Xiong, X.Q. Water/oil repellent property of polyester fabrics after supercritical carbon dioxide finishing. Therm. Sci. 2015, 19, 1273–1277. [Google Scholar] [CrossRef]
- Abate, M.T.; Zhou, Y.; Guan, J.; Chen, G.; Ferri, A.; Nierstrasz, V. Colouration and bio-activation of polyester fabric with curcumin in supercritical CO2: Part II—Effect of dye concentration on the colour and functional properties. J. Supercrit. Fluids 2020, 157, 104703. [Google Scholar] [CrossRef]
- Abou Elmaaty, T. Recent advances in textile wet processing using supercritical carbon dioxide. In Green Chemistry for Sustainable Textiles: Modern Design and Approaches; Woodhead Publishing: Cambridge, UK; The Textile Institute: Manchester, UK, 2021; pp. 279–299. [Google Scholar]
- JIS L 1096:2010; Testing Methods for Woven and Knitted Fabrics. Japanese Standards Association: Tokyo, Japan, 2010. Available online: https://www.intertekinform.com/en-gb/standards/jis-l-1096-2010-628343_saig_jsa_jsa_1455668/ (accessed on 26 October 2025).
- ISO 13934-1:1999; Textiles—Tensile Properties of Fabrics—Part 1: Determination of Maximum Force and Elongation at Maximum Force Using the Strip Method. International Organization for Standardization: Geneva, Switzerland, 1999. Available online: https://www.iso.org/standard/23363.html (accessed on 26 October 2025).
- Sarkodie, B.; Feng, Q.; Xu, C.; Xu, Z. Desizability and biodegradability of textile warp sizing materials and their mechanism: A review. J. Polym. Environ. 2023, 31, 10924–10945. [Google Scholar] [CrossRef]
- Catarino, M.L.; Sampaio, F.; Gonçalves, A.L. Sustainable Wet Processing Technologies for the Textile Industry: A Comprehensive Review. Sustainability 2025, 17, 3041. [Google Scholar] [CrossRef]
- Teli, M.D.; Adere, T.T. Short and efficient desizing and scouring process of cotton textile materials. Int. J. Eng. Trends Technol. 2016, 35, 257–269. [Google Scholar] [CrossRef]
- Shanmugasundaram, O.L. (Ed.) Biotechnological approaches in desizing of textile materials. In Applications of Biotechnology for Sustainable Textile Production; Woodhead Publishing: Duxford, UK, 2022; pp. 47–73. [Google Scholar] [CrossRef]
- Körlü, A. Use of Ozone in the Textile Industry; IntechOpen: London, UK, 2019. [Google Scholar]
- Perinçek, S.; Duran, K.; Körlü, A.E. Ozonation: A new method which can take place of enzymatic desizing. In Proceedings of the XIIIth International Izmir Textile and Apparel Symposium, Izmir, Turkey, 2–5 April 2014; Ege University: İzmir, Turkey; p. 506. [Google Scholar]
- Tari, S.; Athalye, A. Valorising desizing textile effluent. Indian J. Fibre Text. Eng. 2023, 3, 7–12. [Google Scholar] [CrossRef]
- Saxena, S.; Raja, A.S.M.; Arputharaj, A. Challenges in Sustainable Wet Processing of Textiles. In Textiles and Clothing Sustainability; Muthu, S., Ed.; Springer: Singapore, 2017. [Google Scholar]
- Patil, H.; Athalye, A. Sustainable enzymatic desizing of cotton with bio-surfactant extracted from soapnut. Text. Leather Rev. 2024, 7, 327–339. [Google Scholar] [CrossRef]


| No. | Supplier | Brand | Acetyl Content (% w/w) | Viscosity (×10−3 Pa·s) |
|---|---|---|---|---|
| 1 | Aldrich | 56 | ||
| 2 | Daicel | L-20 | 55 | 50 |
| 3 | Daicel | L-70 | 55 | 140 |
| Sizing Agent | Supplier | Chemical Structure | MW |
|---|---|---|---|
| Sodium alginate | Nacalai tesque, Inc., Kyoto, Japan. |  | |
| Starch | Nacalai tesque | 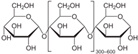 | |
| Methyl methacrylate | Nacalai tesque | 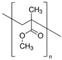 | 8000 (n) |
| Polyethylene glycol | Nacalai tesque | 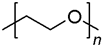 | 950~1050 (MW) |
| Polyvinyl alcohol | Nacalai tesque | 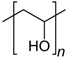 | 500 (n) |
| Polyvinyl acetate | Alfa Aesar, Johnson Matthey Japan Inc., Tokyo, Japan. | 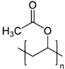 | 50,000 (MW) |
| Cellulose acetate | Aldrich | 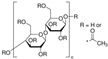 | ~30,000 (Mn) |
| Polystyrene | Polyscience.inc |  | 800~5000 (MW) |
| Sizing Agent | Solubility (%) |
|---|---|
| Sodium alginate | 4.8 |
| Starch | 4.7 |
| Methyl methacrylate | 2.5 |
| Polyethylene glycol | 0.2 |
| Polyvinyl alcohol | −0.7 |
| Polyvinyl acetate | 0.9 |
| Cellulose acetate | 19.5 |
| polystyrene | 1.6 |
| Temperature (°C) | Pressure (MPa) | Amount of Sizing Agent (g) | Co-Solvent Acetone (mol%) | Sizing Rate S (%) |
|---|---|---|---|---|
| 40 | 10 | 1.000 | 0 | 1.2 |
| 100 | 20 | 1.000 | 0 | 1.2 |
| 40 | 10 | 1.000 | 10 | 62.6 |
| 40 | 10 | 0.500 | 10 | 21.4 |
| 40 | 10 | 0.325 | 10 | 10.0 |
| 40 | 10 | 0.250 | 10 | 5.4 |
| 40 | 10 | 0.325 | 5 | 2.4 |
| 40 | 10 | 0.325 | 2.5 | 0.3 |
| Supplier | Brand | Acetyl Content (% w/w) | Viscosity (×10−3 Pa·s) | Acetone (mol%) | Sizing Rate S (%) |
|---|---|---|---|---|---|
| Aldrich | - | 56 | - | 10 | 10.0 |
| - | 56 | - | 7 | 6.9 | |
| Daicel | L-20 | 55 | 50 | 10 | 24.3 |
| L-20 | 55 | 50 | 7 | 7.2 | |
| L-70 | 55 | 140 | 10 | 4.5 | |
| L-70 | 55 | 140 | 7 | 4.9 |
| S0 (%) | S1 (%) | D1 (%) | S2 (%) | D2 (%) | S3 (%) | D3 (%) | S4 (%) | D4 (%) | S5 (%) | D5 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 15.5 | 15.8 | −1.9 | 9.7 | 37.8 | 4.1 | 74.1 | 3.0 | 81.0 | 2.8 | 82.4 |
| No. | Temperature (°C) | Pressure (MPa) | Time (min) | CO2 (mL/min) | Acetone (mL/min) | S1 (%) | S2 (%) | D (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 10 | 60 | 5 | 1 | 12.3 | 10.1 | 17.7 |
| 2 | 40 | 10 | 300 | 1 | 0.2 | 14.5 | 8.1 | 43.9 |
| 3 | 40 | 20 | 300 | 1 | 0.2 | 13.4 | 8.3 | 38.3 |
| 4 | 100 | 10 | 300 | 1 | 0.2 | 13.4 | 13.1 | 2.24 |
| 5 * | 40 | 10 | 300 | 1 | 0.2 | 12.3 | 3.4 | 72.4 |
| 6 * | 40 | 10 | 300 | 1 | 0.2 | 21.2 | 4.0 | 81.1 |
| 7 * | 40 | 10 | 600 | 0.5 | 0.1 | 19.1 | 4.6 | 75.9 |
| Sizing Agent | S (%) | Average Strain (%) | Average Strain SD | Average Strength (cN/dtex) | Strength SD |
|---|---|---|---|---|---|
| - | 0 | 5.95 | 0.510 | 1.63 | 0.158 |
| Starch | 3 | 7.46 | 0.784 | 1.75 | 0.193 |
| Starch (friction) | 3 | 3.08 | 0.647 | 0.81 | 0.127 |
| Cellulose acetate (Aldrich) | 17.7 | 6.13 | 0.562 | 1.94 | 0.164 |
| Cellulose acetate (Aldrich) (friction) | 11.8 | 3.17 | 1.011 | 1.11 | 0.367 |
| Cellulose acetate (L-20) | 14.8 | 4.75 | 0.258 | 1.71 | 0.097 |
| Cellulose acetate (L-70) | 17.8 | 5.30 | 0.663 | 1.83 | 0.168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukasa, I.; Okubayashi, S.; Yoshiharu, M.; Ghanayem, H.M. Supercritical CO2 Sizing and Desizing of Cotton Yarns. Eng 2025, 6, 300. https://doi.org/10.3390/eng6110300
Tsukasa I, Okubayashi S, Yoshiharu M, Ghanayem HM. Supercritical CO2 Sizing and Desizing of Cotton Yarns. Eng. 2025; 6(11):300. https://doi.org/10.3390/eng6110300
Chicago/Turabian StyleTsukasa, Ito, Satoko Okubayashi, Masuda Yoshiharu, and Heba Mehany Ghanayem. 2025. "Supercritical CO2 Sizing and Desizing of Cotton Yarns" Eng 6, no. 11: 300. https://doi.org/10.3390/eng6110300
APA StyleTsukasa, I., Okubayashi, S., Yoshiharu, M., & Ghanayem, H. M. (2025). Supercritical CO2 Sizing and Desizing of Cotton Yarns. Eng, 6(11), 300. https://doi.org/10.3390/eng6110300









