Calotropis Gigantea Latex-Derived Zinc Oxide Nanoparticles: Biosynthesis, Characterization, and Biofunctional Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of ZnO NPs Using C. gigantea Latex
2.3. Characterization of ZnO NPs
2.4. Determination of Antibacterial Activity of ZnO NPs
2.5. Determination of Antioxidant Activity of ZnO NPs
3. Results and Discussion
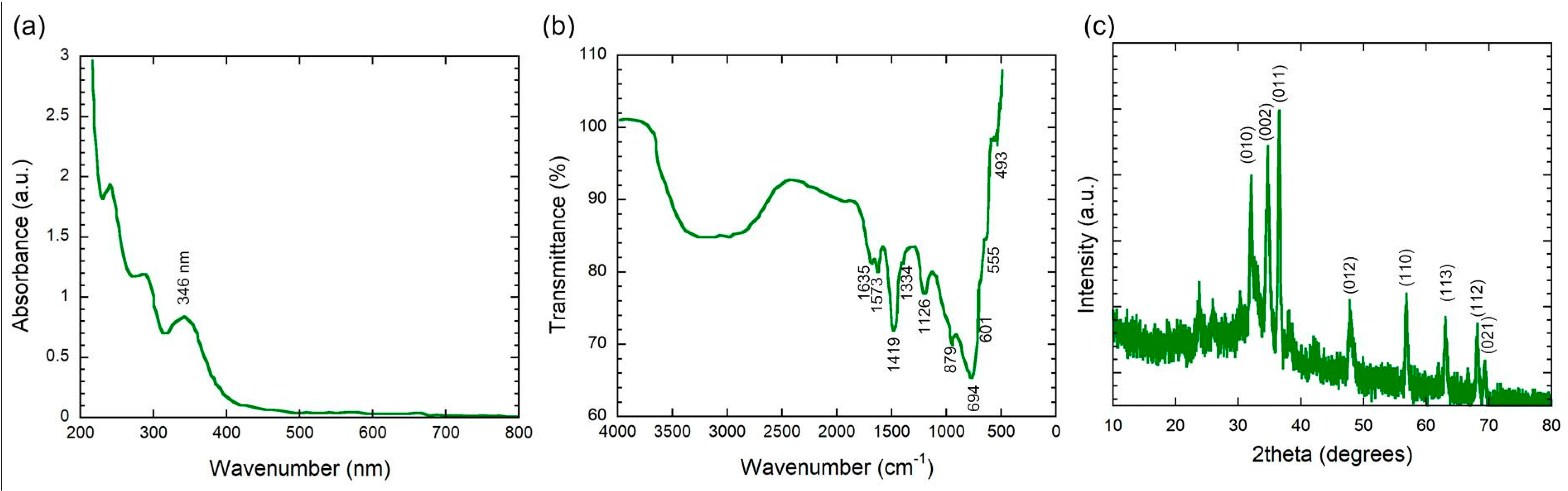
3.1. Characterization of C. gigantea Latex-Mediated ZnO NPs
3.2. Analysis of Antioxidant Activity
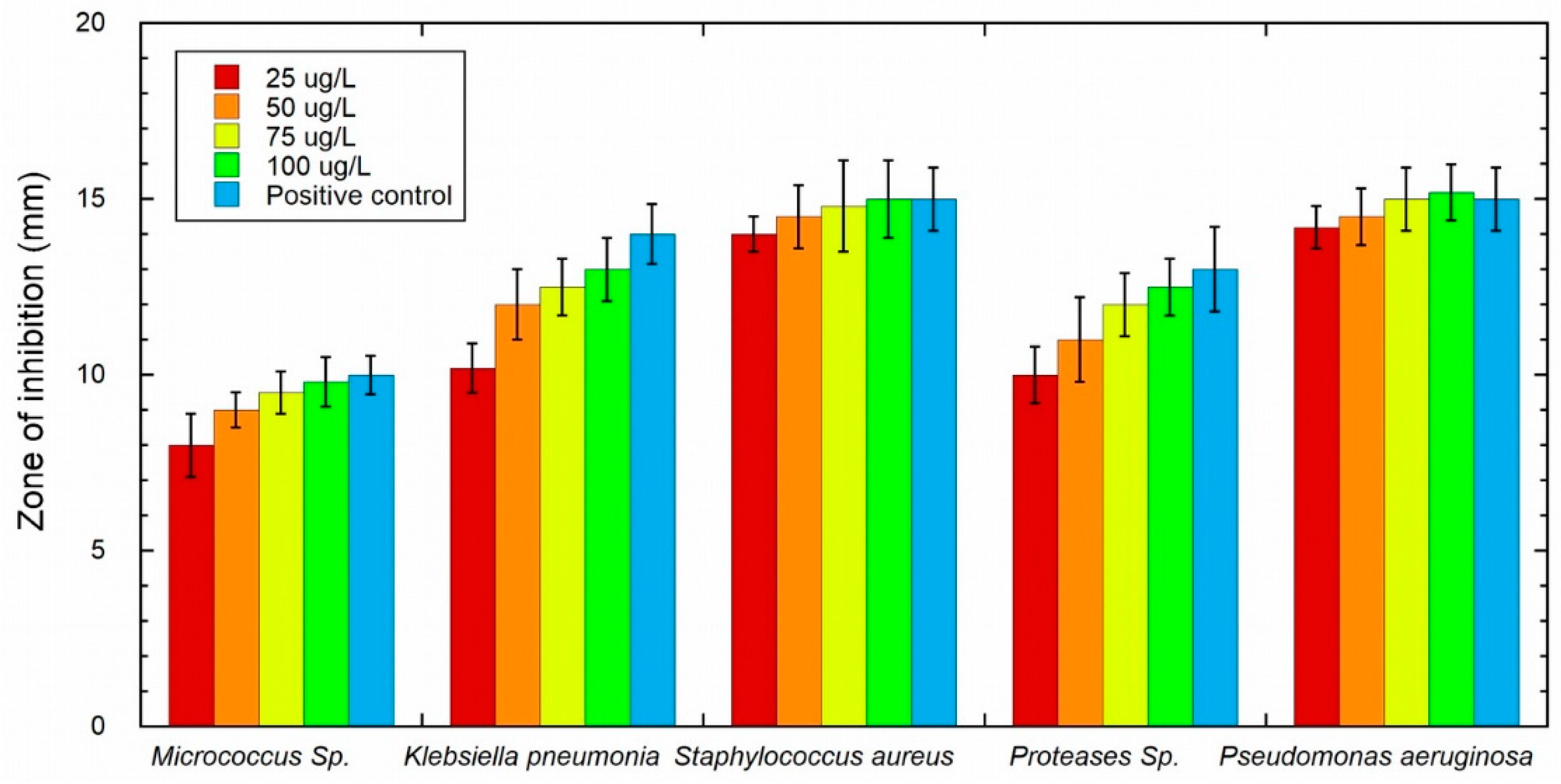
3.3. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romanovski, V.; Dubina, A.; Sehat, A.A.; Su, X.; Moskovskikh, D. Green and Bio-waste-based Materials for Energy Production, Conversion, Storage, and Hybrid Technologies. In Materials for Energy Production, Conversion, and Storage; CRC Press: Boca Raton, FL, USA, 2024; pp. 118–135. [Google Scholar]
- Periakaruppan, R.; Romanovski, V.; Thirumalaisamy, S.K.; Palanimuthu, V.; Sampath, M.P.; Anilkumar, A.; Sivaraj, D.K.; Ahamed, N.A.; Murugesan, S.; Chandrasekar, D. Innovations in Modern Nanotechnology for the Sustainable Production of Agriculture. ChemEngineering 2023, 7, 61. [Google Scholar] [CrossRef]
- Romanovski, V.; Matsukevich, I.; Romanovskaia, E.; Periakaruppan, R. Nano metal oxide as nanosensors in agriculture and environment. In Nanometal Oxides in Horticulture and Agronomy; Academic Press: Cambridge, MA, USA, 2023; pp. 321–352. [Google Scholar]
- Romanovski, V.; Periakaruppan, R. Why metal oxide nanoparticles are superior to other nanomaterials for agricultural application? In Nanometal Oxides in Horticulture and Agronomy; Academic Press: Cambridge, MA, USA, 2023; pp. 7–18. [Google Scholar]
- Kumari, S.; Sarkar, L. A review on nanoparticles: Structure, classification, synthesis & applications. J. Sci. Res. 2021, 65, 42–46. [Google Scholar]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P.; Smith, J.E. Sources of pathogenic microorganisms and their fate during land application of wastes. J. Environ. Qual. 2005, 34, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-Anbr, M.N.; Khaled, J.M.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.K.; Malodia, L. Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: Characterization and its evaluation on tree seedling growth in nursery stage. Appl. Nanosci. 2017, 7, 501–512. [Google Scholar] [CrossRef]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fu, L.; Han, F.; Wang, A.; Cai, W.; Yu, J.; Yang, J.; Peng, F. Green biosynthesis and characterization of zinc oxide nanoparticles using Corymbia citriodora leaf extract and their photocatalytic activity. Green Chem. Lett. Rev. 2015, 8, 59–63. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ.-Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Supraja, N.; Prasad, T.N.V.K.V.; Krishna, T.G.; David, E. Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated zinc oxide nanoparticles. Appl. Nanosci. 2016, 6, 581–590. [Google Scholar] [CrossRef]
- Rajabairavi, N.; Raju, C.S.; Karthikeyan, C.; Varutharaju, K.; Nethaji, S.; Hameed, A.S.H.; Shajahan, A. Biosynthesis of novel zinc oxide nanoparticles (ZnO NPs) using endophytic bacteria Sphingobacterium thalpophilum. In Recent Trends in Materials Science and Applications: Nanomaterials, Crystal Growth, Thin Films, Quantum Dots, Spectroscopy (Proceedings ICRTMSA 2016); Springer International Publishing: Cham, Switzerland, 2017; pp. 245–254. [Google Scholar]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A.A. Sustainable microbial cell nanofactory for zinc oxide nanoparticles production by zinc-tolerant probiotic Lactobacillus plantarum strain TA4. Microb. Cell Factories 2020, 19, 10. [Google Scholar] [CrossRef]
- Puay, N.Q.; Qiu, G.; Ting, Y.P. Effect of Zinc oxide nanoparticles on biological wastewater treatment in a sequencing batch reactor. J. Clean. Prod. 2015, 88, 139–145. [Google Scholar] [CrossRef]
- Roy, A.; Gauri, S.S.; Bhattacharya, M.; Bhattacharya, J. Antimicrobial activity of CaO nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 1570–1578. [Google Scholar] [CrossRef]
- Vanathi, P.; Karungan Selvaraj, V.S.; Abed, S.A.; Periakaruppan, R. Production and characterization of Azadirachtaindica oil-based iron oxide nanoparticles with antibacterial potential. Biomass Convers. Biorefinery 2024, 1–10. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Romanovski, V.; Roslyakov, S.; Trusov, G.; Periakaruppan, R.; Romanovskaia, E.; Chan, H.L.; Moskovskikh, D. Synthesis and effect of CoCuFeNi high entropy alloy nanoparticles on seed germination, plant growth, and microorganisms inactivation activity. Environ. Sci. Pollut. Res. 2023, 30, 23363–23371. [Google Scholar] [CrossRef]
- Romanovski, V.; Paspelau, A.; Kamarou, M.; Likhavitski, V.; Korob, N.; Romanovskaia, E. Comparative Analysis of the Disinfection Efficiency of Steel and Polymer Surfaces with Aqueous Solutions of Ozone and Sodium Hypochlorite. Water 2024, 16, 793. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, R.; El-Kady, A.A.; Oranj, B.T.; Ahmed, R.; Valentin, R.; Hu, X.; Wu, W.; Wang, D.; Mao, J.; et al. Nanomaterials enabled photoelectrocatalysis for removing pollutants in the environment and food. TrAC Trends Anal. Chem. 2023, 166, 117187. [Google Scholar]
- Zhou, Z.; Zhang, L.; Yan, B.; Wu, J.; Kong, D.; Romanovski, V.; Ivanets, A.; Li, H.; Chu, S.; Su, X. Removal of chromium from electroplating sludge by roasting-acid leaching and catalytic degradation of antibiotics by its residue. J. Environ. Chem. Eng. 2024, 12, 111754. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Dwivedi, A.; Pratap, S.; Awasthi, S.; Gautam, P.; Kadir, A. Calotropis gigantea: An in-depth review of its therapeutic potential. J. Pharmacogn. Phytochem. 2024, 13, 715–721. [Google Scholar] [CrossRef]
- Rajiv, P.; Rajeshwari, S.; Venckatesh, R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 112, 384–387. [Google Scholar] [CrossRef]
- Vanathi, P.; Rajiv, P.; Narendhran, S.; Rajeshwari, S.; Rahman, P.K.; Venckatesh, R. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: A green chemistry approach. Mater. Lett. 2014, 134, 13–15. [Google Scholar] [CrossRef]
- Ganeshan, A.; Periakaruppan, R.; Vanathi, P.; Thirumalaisamy, S.K.; Vijai Selvaraj, K.S.; Moskovskikh, D. Ulv arigida–mediated silver nanoparticles: Synthesis, characterization, and antibacterial activity. Biomass Convers. Biorefinery 2024, 1–8. [Google Scholar] [CrossRef]
- Sivaraj, R.; Rahman, P.K.; Rajiv, P.; Narendhran, S.; Venckatesh, R. Biosynthesis and characterization of Acalyphaindica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 255–258. [Google Scholar] [CrossRef]
- Periakaruppan, R.; Ariuthayan, B.; Vanathi, P.; Abed, S.A.; Al-Awsi, G.R.L.; Al-Dayan, N.; Dhanasekaran, S. Ocimumtenuiflorum-Assisted Fabrication of Iron-Oxide Nanoparticles and Its Use in Wastewater Treatment of the Textile Industry. JOM 2023, 75, 5273–5280. [Google Scholar] [CrossRef]
- Jayappa, M.D.; Ramaiah, C.K.; Kumar, M.A.P.; Suresh, D.; Prabhu, A.; Devasya, R.P.; Sheikh, S. Green synthesis of zinc oxide nanoparticles from the leaf, stem and in vitro grown callus of Mussaenda frondosa L.: Characterization and their applications. Appl. Nanosci. 2020, 10, 3057–3074. [Google Scholar] [CrossRef]
- Alamdari, S.; Sasani Ghamsari, M.; Lee, C.; Han, W.; Park, H.H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and characterization of zinc oxide nanoparticles using leaf extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Chamkouri, N.; Jomehzadeh, N.; Naserzadeh, N. Rapid biosynthesis and antibacterial activity of zinc oxide nanoparticles using fruit peel of Punica granatum L as cellulose. Curr. Res. Green Sustain. Chem. 2023, 6, 100366. [Google Scholar] [CrossRef]
- Manokari, M.; Latha, R.; Priyadharshini, S.; Cokul, R.M.; Beniwal, P.; Shekhawat, M.S. Green synthesis of zinc oxide nanoparticles from aqueous extracts of Sesamum indicum L. and their characterization. World News Nat. Sci. 2019, 23, 200–210. [Google Scholar]
- Kalaiselvi, A.; Roopan, S.M.; Madhumitha, G.; Ramalingam, C.; Al-Dhabi, N.A.; Arasu, M.V. Catharanthus roseus-mediated zinc oxide nanoparticles against photocatalytic application of phenol red under UV@ 365 nm. Curr. Sci. 2016, 111, 1811–1815. [Google Scholar] [CrossRef]
- Geetha, M.S.; Nagabhushana, H.; Shivananjaiah, H.N. Green mediated synthesis and characterization of ZnO nanoparticles using Euphorbia Jatropa latex as reducing agent. J. Sci. Adv. Mater. Devices 2016, 1, 301–310. [Google Scholar] [CrossRef]
- Miri, A.; Mahdinejad, N.; Ebrahimy, O.; Khatami, M.; Sarani, M. Zinc oxide nanoparticles: Biosynthesis, characterization, antifungal and cytotoxic activity. Mater. Sci. Eng. C 2019, 104, 109981. [Google Scholar] [CrossRef]
- Al-darwesh, M.Y.; Ibrahim, S.S.; Hamid, L.L. Ficus carica latex mediated biosynthesis of zinc oxide nanoparticles and assessment of their antibacterial activity and biological safety. Nano-Struct. Nano-Objects 2024, 38, 101163. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dhananjaya, N.; Yadav, L.R. E. tirucalli plant latex mediated green combustion synthesis of ZnO nanoparticles: Structure, photoluminescence and photo-catalytic activities. J. Sci. Adv. Mater. Devices 2018, 3, 303–309. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Kumar, S.V.; Ramaiah, A.; Agarwal, H.; Lakshmi, T.; Roopan, S.M. Biosynthesis of zinc oxide nanoparticles using Mangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzym. Microb. Technol. 2018, 117, 91–95. [Google Scholar] [CrossRef]
- Abdelhakim, H.K.; El-Sayed, E.R.; Rashidi, F.B. Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. J. Appl. Microbiol. 2020, 128, 1634–1646. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst. Eng. 2017, 40, 943–957. [Google Scholar] [CrossRef]
- Chemingui, H.; Missaoui, T.; Mzali, J.C.; Yildiz, T.; Konyar, M.; Smiri, M.; Saidi, N.; Hafiane, A.; Yatmaz, H.C. Facile green synthesis of zinc oxide nanoparticles (ZnO NPs): Antibacterial and photocatalytic activities. Mater. Res. Express 2019, 6, 1050b4. [Google Scholar] [CrossRef]
- Rathnayake, W.G.I.U.; Ismail, H.; Baharin, A.; Bandara, I.M.C.C.D.; Rajapakse, S. Enhancement of the antibacterial activity of natural rubber latex foam by the incorporation of zinc oxide nanoparticles. J. Appl. Polym. Sci. 2014, 131, 39601. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Sandiya, K.; Santhiya, S.; Pradeep, R.S.; Kumar, N.M.; Suriyanarayanan, N.; Thirumarimurugan, M. Biosynthesis, characterization, and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J. Nanostruct. Chem. 2018, 8, 293–299. [Google Scholar] [CrossRef]
- Li, T.; Zhang, C.; Jia, J.; Cheng, X. Nano zinc oxide decorated latex drainage: A promising antibacterial material prevent retrograde infection associated with drainage. J. Biomater. Appl. 2022, 37, 795–804. [Google Scholar]

| Concentration (µg/mL) | % of Inhibition | IC50 (µg/mL) |
|---|---|---|
| 10 | 58.20 ± 1.29 | 12.52 |
| 50 | 65.57 ± 1.58 | |
| 150 | 68.85 ± 1.47 | |
| 250 | 77.21 ± 1.36 | |
| 350 | 83.11 ± 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
C, J.; Periakaruppan, R.; Romanovski, V.; Vijai Selvaraj, K.S.; Al-Dayan, N. Calotropis Gigantea Latex-Derived Zinc Oxide Nanoparticles: Biosynthesis, Characterization, and Biofunctional Applications. Eng 2024, 5, 1399-1406. https://doi.org/10.3390/eng5030073
C J, Periakaruppan R, Romanovski V, Vijai Selvaraj KS, Al-Dayan N. Calotropis Gigantea Latex-Derived Zinc Oxide Nanoparticles: Biosynthesis, Characterization, and Biofunctional Applications. Eng. 2024; 5(3):1399-1406. https://doi.org/10.3390/eng5030073
Chicago/Turabian StyleC, Jayalekshmi, Rajiv Periakaruppan, Valentin Romanovski, Karungan Selvaraj Vijai Selvaraj, and Noura Al-Dayan. 2024. "Calotropis Gigantea Latex-Derived Zinc Oxide Nanoparticles: Biosynthesis, Characterization, and Biofunctional Applications" Eng 5, no. 3: 1399-1406. https://doi.org/10.3390/eng5030073
APA StyleC, J., Periakaruppan, R., Romanovski, V., Vijai Selvaraj, K. S., & Al-Dayan, N. (2024). Calotropis Gigantea Latex-Derived Zinc Oxide Nanoparticles: Biosynthesis, Characterization, and Biofunctional Applications. Eng, 5(3), 1399-1406. https://doi.org/10.3390/eng5030073








