Exploiting the Fruitfly, Drosophila melanogaster, to Identify the Molecular Basis of Cryptochrome-Dependent Magnetosensitivity
Abstract
1. Main Text
2. Drosophila Magnetosensitivity Requires the Presence of Cryptochrome
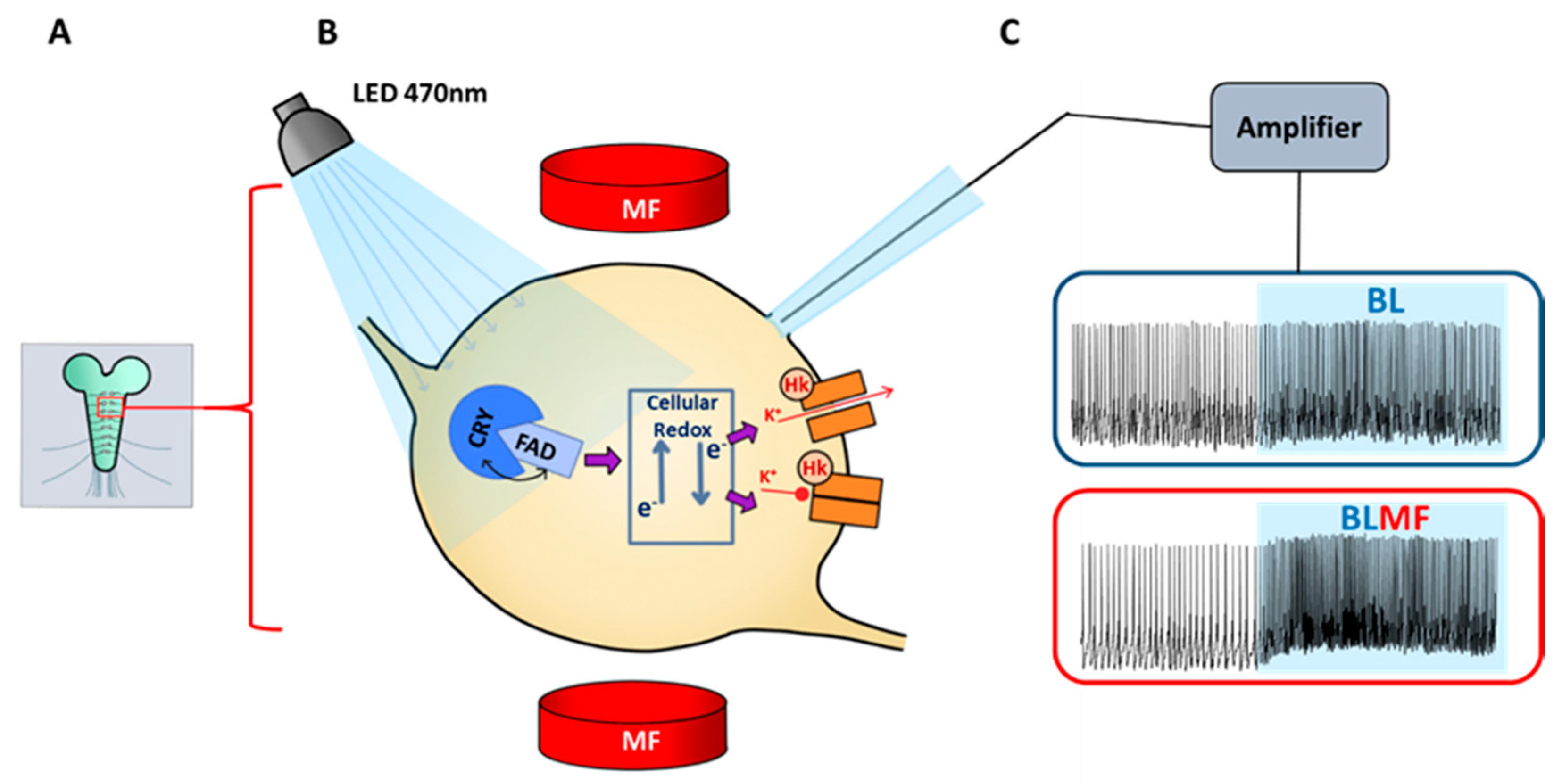
3. Mechanistic Basis of Magnetosensitivity in Drosophila
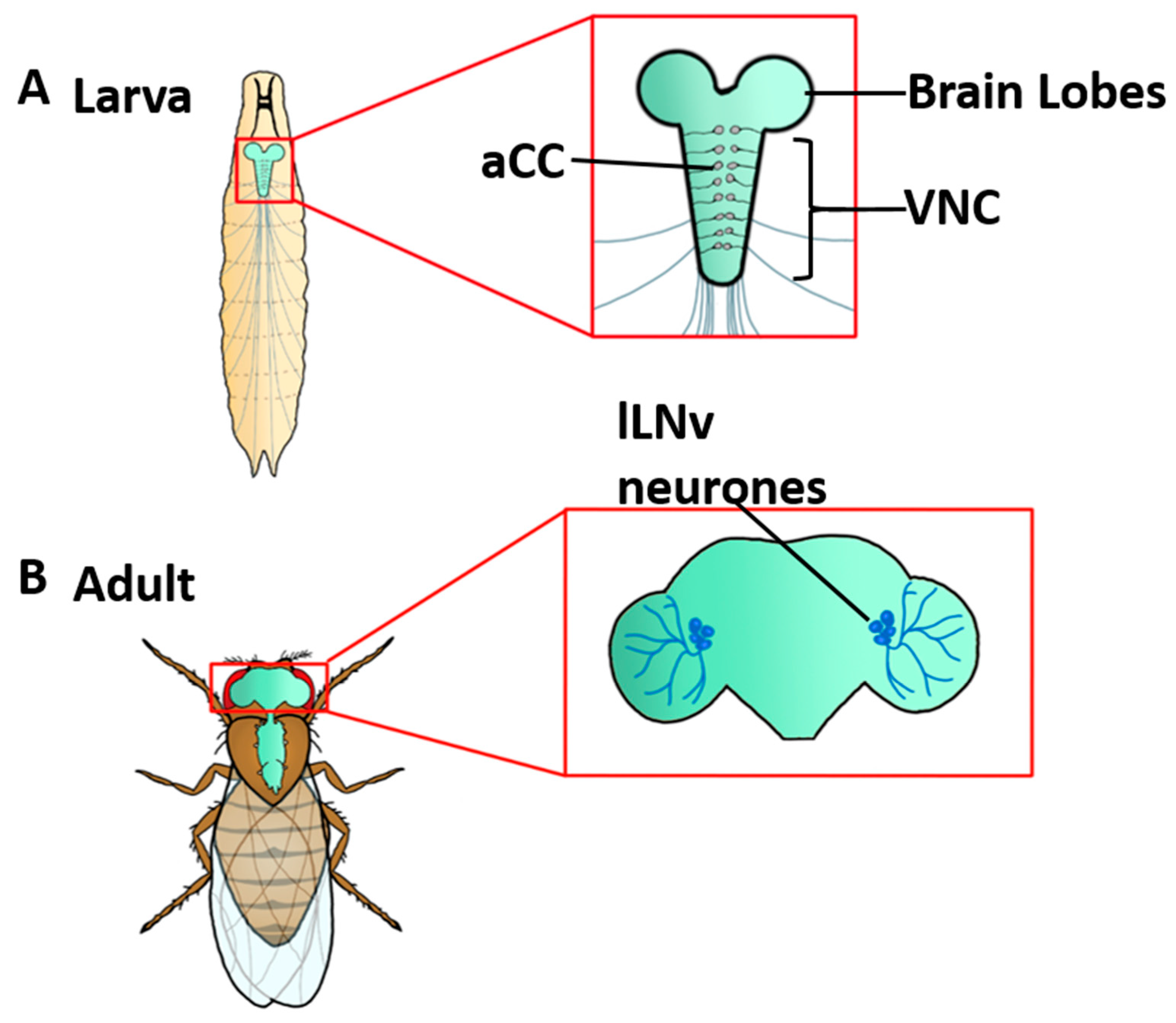
4. CRY Mediates Magnetosensitivity in Drosophila Neurons
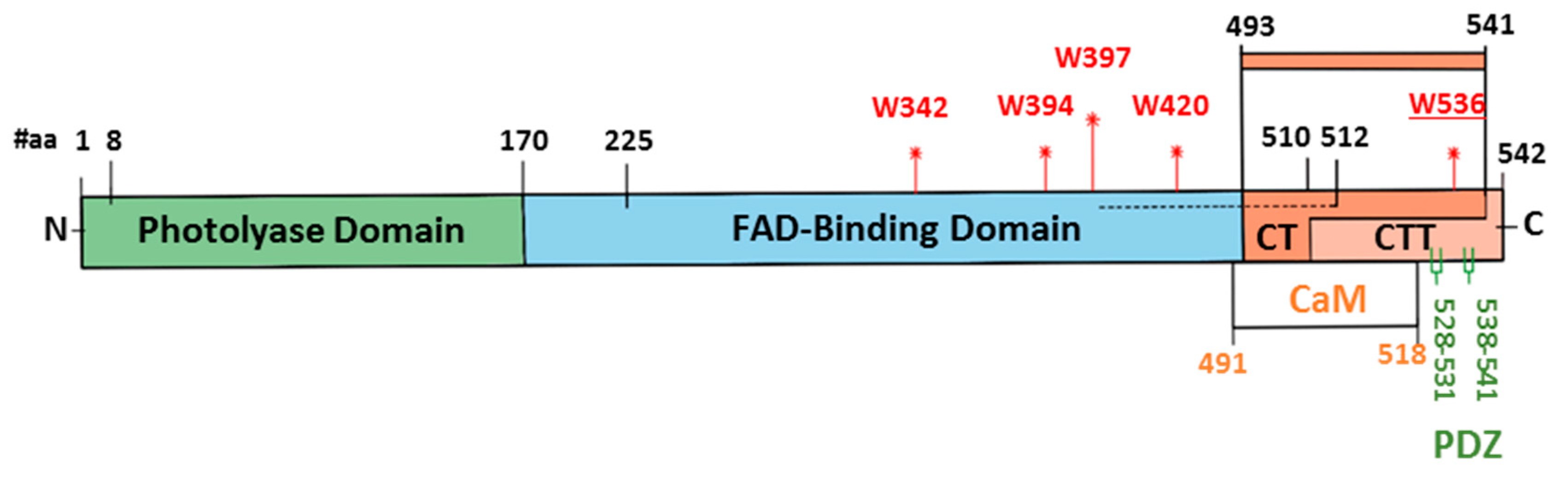
5. Is Full-Length CRY Essential for Magnetosensitivity in Drosophila?
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulten, K.; Staerk, H.; Weller, A.; Werner, H.J.; Nickel, B. Magnetic field dependence of the geminate recombination of radical ion pairs in polar solvents. Z. Phys. Chem. 1976, 101, 371–390. [Google Scholar] [CrossRef]
- Ritz, T.; Adem, S.; Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000, 78, 707–718. [Google Scholar] [CrossRef]
- Hore, P.J.; Mouritsen, H. The Radical-Pair Mechanism of Magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.T.; Top, D.; Manahan, C.C.; Tokuda, J.M.; Zhang, S.; Pollack, L.; Young, M.W.; Crane, B.R. Flavin reduction activates Drosophila cryptochrome. Proc. Natl. Acad. Sci. USA 2013, 110, 20455–20460. [Google Scholar] [CrossRef]
- Vacha, M. Magnetoreception of Invertebrates; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Damulewicz, M.; Mazzotta, G.M. One Actor, Multiple Roles: The Performances of Cryptochrome in Drosophila. Front. Physiol. 2020, 11, 99. [Google Scholar] [CrossRef]
- Foley, L.E.; Emery, P. Drosophila Cryptochrome: Variations in Blue. J. Biol. Rhythms. 2020, 35, 16–27. [Google Scholar] [CrossRef]
- Wehner, R.; Labhart, T. Perception of the geomagnetic field in the fly Drosophila melanogaster. Experientia 1970, 26, 967–968. [Google Scholar] [CrossRef]
- Phillips, J.B.; Sayeed, O. Wavelength-dependent effects of light on magnetic compass orientation in Drosophila melanogaster. J. Comp. Physiol. A 1993, 172, 303–308. [Google Scholar] [CrossRef]
- Gegear, R.J.; Casselman, A.; Waddell, S.; Reppert, S.M. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 2008, 454, 1014–1018. [Google Scholar] [CrossRef]
- Gegear, R.J.; Foley, L.E.; Casselman, A.; Reppert, S.M. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature 2010, 463, 804–807. [Google Scholar] [CrossRef]
- Foley, L.E.; Gegear, R.J.; Reppert, S.M. Human cryptochrome exhibits light-dependent magnetosensitivity. Nat. Commun. 2011, 2, 356. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Ahmad, M.; Helfrich-Forster, C. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol. 2009, 7, e1000086. [Google Scholar] [CrossRef] [PubMed]
- Fedele, G.; Edwards, M.D.; Bhutani, S.; Hares, J.M.; Murbach, M.; Green, E.W.; Dissel, S.; Hastings, M.H.; Rosato, E.; Kyriacou, C.P. Genetic analysis of circadian responses to low frequency electromagnetic fields in Drosophila melanogaster. PLoS Genet. 2014, 10, e1004804. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.B.; Jorge, P.E.; Muheim, R. Light-dependent magnetic compass orientation in amphibians and insects: Candidate receptors and candidate molecular mechanisms. J. R. Soc. Interface 2010, 7 (Suppl. S2), S241–S256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fedele, G.; Green, E.W.; Rosato, E.; Kyriacou, C.P. An electromagnetic field disrupts negative geotaxis in Drosophila via a CRY-dependent pathway. Nat. Commun. 2014, 5, 4391. [Google Scholar] [CrossRef]
- Bae, J.E.; Bang, S.; Min, S.; Lee, S.H.; Kwon, S.H.; Lee, Y.; Lee, Y.H.; Chung, J.; Chae, K.S. Positive geotactic behaviors induced by geomagnetic field in Drosophila. Mol. Brain 2016, 9, 55. [Google Scholar] [CrossRef]
- Liang, C.H.; Chuang, C.L.; Jiang, J.A.; Yang, E.C. Magnetic Sensing through the Abdomen of the Honey bee. Sci Rep. 2016, 6, 23657. [Google Scholar] [CrossRef]
- Bazalova, O.; Kvicalova, M.; Valkova, T.; Slaby, P.; Bartos, P.; Netusil, R.; Tomanova, K.; Braeunig, P.; Lee, H.J.; Sauman, I.; et al. Cryptochrome 2 mediates directional magnetoreception in cockroaches. Proc. Natl. Acad. Sci. USA 2016, 113, 1660–1665. [Google Scholar] [CrossRef]
- Slaby, P.; Bartos, P.; Karas, J.; Netusil, R.; Tomanova, K.; Vacha, M. How Swift Is Cry-Mediated Magnetoreception? Conditioning in an American Cockroach Shows Sub-second Response. Front. Behav. Neurosci. 2018, 12, 107. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005, 191, 675–693. [Google Scholar] [CrossRef]
- Johnsen, S.; Mattern, E.; Ritz, T. Light-dependent magnetoreception: Quantum catches and opponency mechanisms of possible photosensitive molecules. J. Exp. Biol. 2007, 210, 3171–3178. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Robertson, D.E.; Ahmad, M.; Raibekas, A.A.; Jorns, M.S.; Dutton, P.L.; Cashmore, A.R. Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science 1995, 269, 968–970. [Google Scholar] [CrossRef]
- Berndt, A.; Kottke, T.; Breitkreuz, H.; Dvorsky, R.; Hennig, S.; Alexander, M.; Wolf, E. A novel photoreaction mechanism for the circadian blue light photoreceptor Drosophila cryptochrome. J. Biol. Chem. 2007, 282, 13011–13021. [Google Scholar] [CrossRef] [PubMed]
- Bouly, J.P.; Schleicher, E.; Dionisio-Sese, M.; Vandenbussche, F.; Van Der Straeten, D.; Bakrim, N.; Meier, S.; Batschauer, A.; Galland, P.; Bittl, R.; et al. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 2007, 282, 9383–9391. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.; Bouly, J.P.; Ahmad, M. Evidence of a light-sensing role for folate in Arabidopsis cryptochrome blue-light receptors. Mol. Plant. 2008, 1, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Schleicher, E.; Meier, S.; Viana, R.M.; Pokorny, R.; Ahmad, M.; Bittl, R.; Batschauer, A. The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J. Biol. Chem. 2007, 282, 14916–14922. [Google Scholar] [CrossRef] [PubMed]
- Dissel, S.; Hansen, C.N.; Ozkaya, O.; Hemsley, M.; Kyriacou, C.P.; Rosato, E. The logic of circadian organization in Drosophila. Curr. Biol. 2014, 24, 2257–2266. [Google Scholar] [CrossRef]
- Busza, A.; Emery-Le, M.; Rosbash, M.; Emery, P. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science 2004, 304, 1503–1506. [Google Scholar] [CrossRef]
- Peschel, N.; Chen, K.F.; Szabo, G.; Stanewsky, R. Light-dependent interactions between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless. Curr. Biol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Hemsley, M.J.; Mazzotta, G.M.; Mason, M.; Dissel, S.; Toppo, S.; Pagano, M.A.; Sandrelli, F.; Meggio, F.; Rosato, E.; Costa, R.; et al. Linear motifs in the C-terminus of D. melanogaster cryptochrome. Biochem. Biophys. Res. Commun. 2007, 355, 531–537. [Google Scholar] [CrossRef]
- Mazzotta, G.M.; Bellanda, M.; Minervini, G.; Damulewicz, M.; Cusumano, P.; Aufiero, S.; Stefani, M.; Zambelli, B.; Mammi, S.; Costa, R.; et al. Calmodulin Enhances Cryptochrome Binding to INAD in Drosophila Photoreceptors. Front. Mol. Neurosci. 2018, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Ahmad, M. Light-activated cryptochrome reacts with molecular oxygen to form a flavin-superoxide radical pair consistent with magnetoreception. J. Biol. Chem. 2011, 286, 21033–21040. [Google Scholar] [CrossRef] [PubMed]
- Consentino, L.; Lambert, S.; Martino, C.; Jourdan, N.; Bouchet, P.E.; Witczak, J.; Castello, P.; El-Esawi, M.; Corbineau, F.; d′Harlingue, A.; et al. Blue-light dependent reactive oxygen species formation by Arabidopsis cryptochrome may define a novel evolutionarily conserved signaling mechanism. New Phytol. 2015, 206, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Fogle, K.J.; Baik, L.S.; Houl, J.H.; Tran, T.T.; Roberts, L.; Dahm, N.A.; Cao, Y.; Zhou, M.; Holmes, T.C. CRYPTOCHROME-mediated phototransduction by modulation of the potassium ion channel beta-subunit redox sensor. Proc. Natl. Acad. Sci. USA 2015, 112, 2245–2250. [Google Scholar] [CrossRef]
- Pooam, M.; Arthaut, L.D.; Burdick, D.; Link, J.; Martino, C.F.; Ahmad, M. Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 2019, 249, 319–332. [Google Scholar] [CrossRef]
- El-Esawi, M.; Arthaut, L.D.; Jourdan, N.; d′Harlingue, A.; Link, J.; Martino, C.F.; Ahmad, M. Blue-light induced biosynthesis of ROS contributes to the signaling mechanism of Arabidopsis cryptochrome. Sci. Rep. 2017, 7, 13875. [Google Scholar] [CrossRef]
- Arthaut, L.D.; Jourdan, N.; Mteyrek, A.; Procopio, M.; El-Esawi, M.; d′Harlingue, A.; Bouchet, P.E.; Witczak, J.; Ritz, T.; Klarsfeld, A.; et al. Blue-light induced accumulation of reactive oxygen species is a consequence of the Drosophila cryptochrome photocycle. PLoS ONE 2017, 12, e0171836. [Google Scholar] [CrossRef]
- Maeda, K.; Robinson, A.J.; Henbest, K.B.; Hogben, H.J.; Biskup, T.; Ahmad, M.; Schleicher, E.; Weber, S.; Timmel, C.R.; Hore, P.J. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4774–4779. [Google Scholar] [CrossRef]
- Sheppard, D.M.; Li, J.; Henbest, K.B.; Neil, S.R.; Maeda, K.; Storey, J.; Schleicher, E.; Biskup, T.; Rodriguez, R.; Weber, S.; et al. Millitesla magnetic field effects on the photocycle of an animal cryptochrome. Sci. Rep. 2017, 7, 42228. [Google Scholar] [CrossRef]
- Fogle, K.J.; Parson, K.G.; Dahm, N.A.; Holmes, T.C. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science 2011, 331, 1409–1413. [Google Scholar] [CrossRef]
- Buhl, E.; Bradlaugh, A.; Ogueta, M.; Chen, K.F.; Stanewsky, R.; Hodge, J.J. Quasimodo mediates daily and acute light effects on Drosophila clock neuron excitability. Proc. Natl. Acad. Sci. USA 2016, 113, 13486–13491. [Google Scholar] [CrossRef] [PubMed]
- Giachello, C.N.G.; Scrutton, N.S.; Jones, A.R.; Baines, R.A. Magnetic Fields Modulate Blue-Light-Dependent Regulation of Neuronal Firing by Cryptochrome. J. Neurosci. 2016, 36, 10742–10749. [Google Scholar] [CrossRef] [PubMed]
- Chouinard, S.W.; Wilson, G.F.; Schlimgen, A.K.; Ganetzky, B. A potassium channel beta subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila hyperkinetic locus. Proc. Natl. Acad. Sci. USA 1995, 92, 6763–6767. [Google Scholar] [CrossRef]
- Kempf, A.; Song, S.M.; Talbot, C.B.; Miesenbock, G. A potassium channel beta-subunit couples mitochondrial electron transport to sleep. Nature 2019, 568, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Marley, R.; Giachello, C.N.; Scrutton, N.S.; Baines, R.A.; Jones, A.R. Cryptochrome-dependent magnetic field effect on seizure response in Drosophila larvae. Sci. Rep. 2014, 4, 5799. [Google Scholar] [CrossRef]
- Steiner, U.E.; Ulrich, T. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 1989, 89, 51–147. [Google Scholar] [CrossRef]
- Muller, P.; Brettel, K.; Grama, L.; Nyitrai, M.; Lukacs, A. Photochemistry of Wild-Type and N378D Mutant E. coli DNA Photolyase with Oxidized FAD Cofactor Studied by Transient Absorption Spectroscopy. Chemphyschem 2016, 17, 1329–1340. [Google Scholar] [CrossRef]
- Nohr, D.; Franz, S.; Rodriguez, R.; Paulus, B.; Essen, L.O.; Weber, S.; Schleicher, E. Extended Electron-Transfer in Animal Cryptochromes Mediated by a Tetrad of Aromatic Amino Acids. Biophys. J. 2016, 111, 301–311. [Google Scholar] [CrossRef]
- Lin, C.; Top, D.; Manahan, C.C.; Young, M.W.; Crane, B.R. Circadian clock activity of cryptochrome relies on tryptophan-mediated photoreduction. Proc. Natl. Acad. Sci. USA 2018, 115, 3822–3827. [Google Scholar] [CrossRef]
- Baik, L.S.; Au, D.D.; Nave, C.; Foden, A.J.; Enrriquez-Villalva, W.K.; Holmes, T.C. Distinct mechanisms of Drosophila CRYPTOCHROME-mediated light-evoked membrane depolarization and in vivo clock resetting. Proc. Natl. Acad. Sci. USA 2019, 116, 23339–23344. [Google Scholar] [CrossRef]
- Solov′yov, I.A.; Schulten, K. Magnetoreception through cryptochrome may involve superoxide. Biophys. J. 2009, 96, 4804–4813. [Google Scholar] [CrossRef] [PubMed]
- Wiltschko, R.; Ahmad, M.; Niessner, C.; Gehring, D.; Wiltschko, W. Light-dependent magnetoreception in birds: The crucial step occurs in the dark. J. R. Soc. Interface 2016, 13, 20151010. [Google Scholar] [CrossRef] [PubMed]
- Kutta, R.J.; Archipowa, N.; Johannissen, L.O.; Jones, A.R.; Scrutton, N.S. Vertebrate Cryptochromes are Vestigial Flavoproteins. Sci. Rep. 2017, 7, 44906. [Google Scholar] [CrossRef] [PubMed]
- Antill, L.M.; Woodward, J.R. Flavin Adenine Dinucleotide Photochemistry Is Magnetic Field Sensitive at Physiological pH. J. Phys. Chem. Lett. 2018, 9, 2691–2696. [Google Scholar] [CrossRef]
- Ikeya, N.; Woodward, J.R. Cellular autofluorescence is magnetic field sensitive. Proc. Natl. Acad. Sci. USA 2021, 118, e2018043118. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradlaugh, A.; Munro, A.L.; Jones, A.R.; Baines, R.A. Exploiting the Fruitfly, Drosophila melanogaster, to Identify the Molecular Basis of Cryptochrome-Dependent Magnetosensitivity. Quantum Rep. 2021, 3, 127-136. https://doi.org/10.3390/quantum3010007
Bradlaugh A, Munro AL, Jones AR, Baines RA. Exploiting the Fruitfly, Drosophila melanogaster, to Identify the Molecular Basis of Cryptochrome-Dependent Magnetosensitivity. Quantum Reports. 2021; 3(1):127-136. https://doi.org/10.3390/quantum3010007
Chicago/Turabian StyleBradlaugh, Adam, Anna L. Munro, Alex R. Jones, and Richard A. Baines. 2021. "Exploiting the Fruitfly, Drosophila melanogaster, to Identify the Molecular Basis of Cryptochrome-Dependent Magnetosensitivity" Quantum Reports 3, no. 1: 127-136. https://doi.org/10.3390/quantum3010007
APA StyleBradlaugh, A., Munro, A. L., Jones, A. R., & Baines, R. A. (2021). Exploiting the Fruitfly, Drosophila melanogaster, to Identify the Molecular Basis of Cryptochrome-Dependent Magnetosensitivity. Quantum Reports, 3(1), 127-136. https://doi.org/10.3390/quantum3010007




