Investigation of the First Hydrogenation of LaNi5
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Effect of Particle Size on Incubation Time
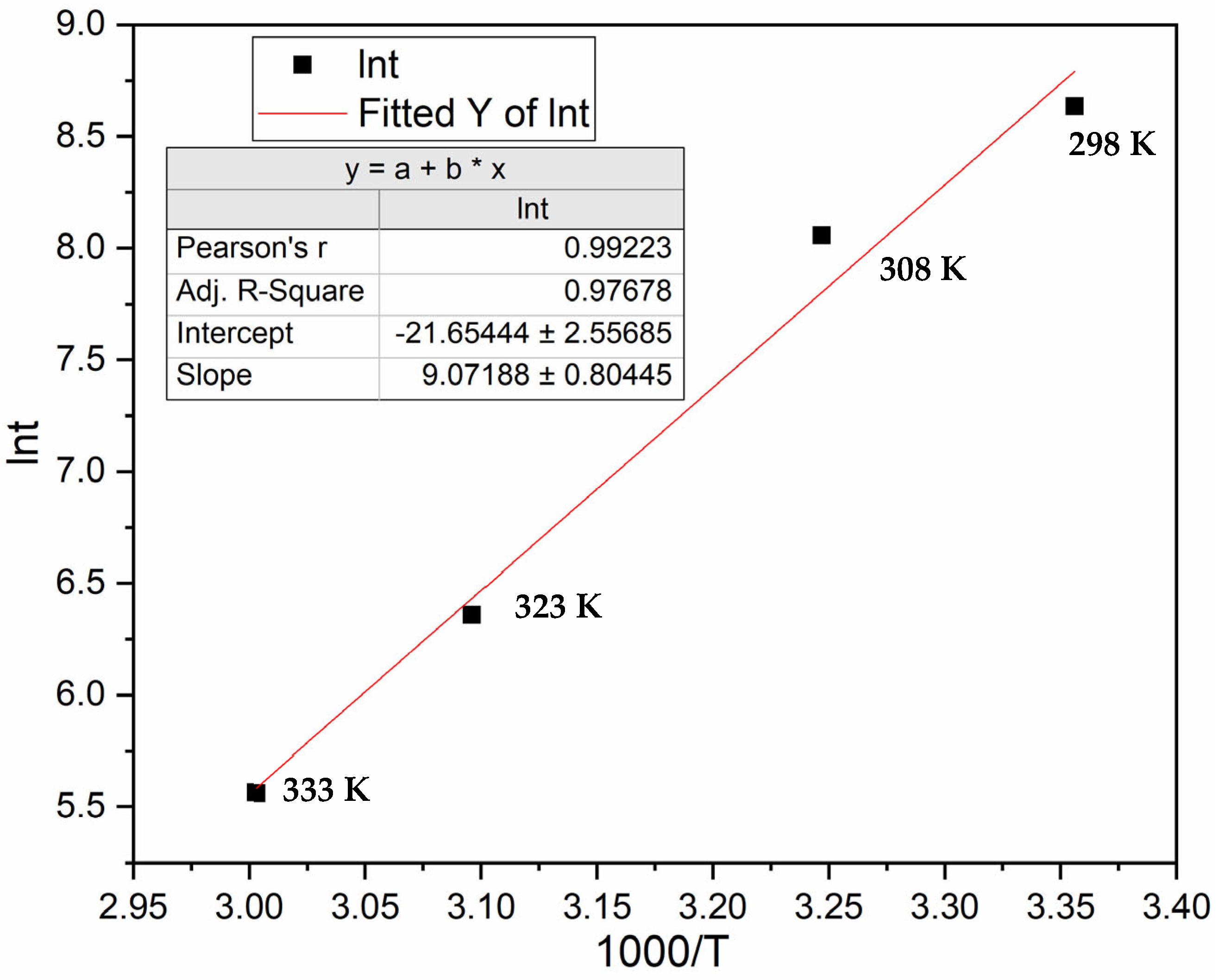
3.2. Effect of Temperature on Incubation Time
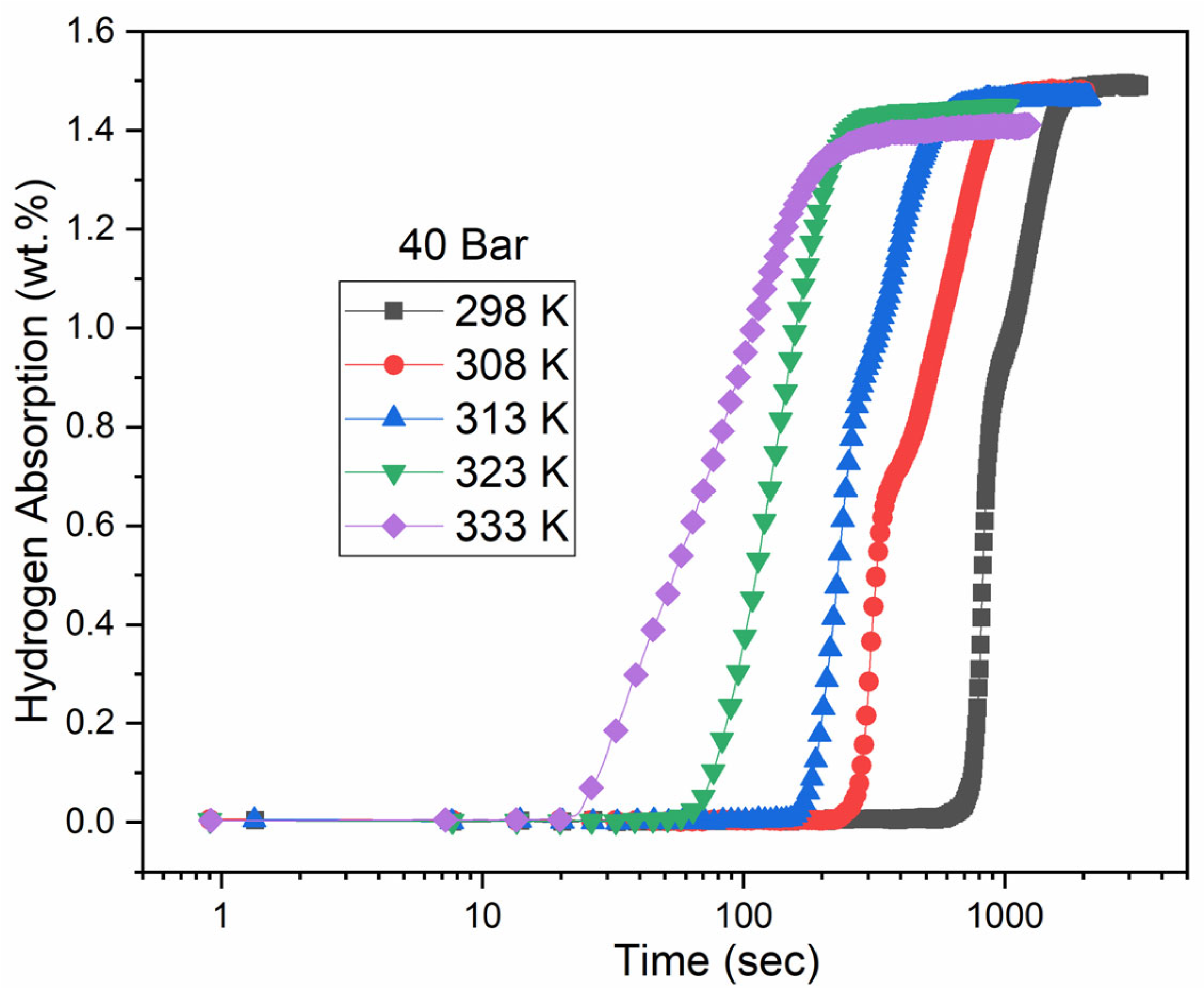
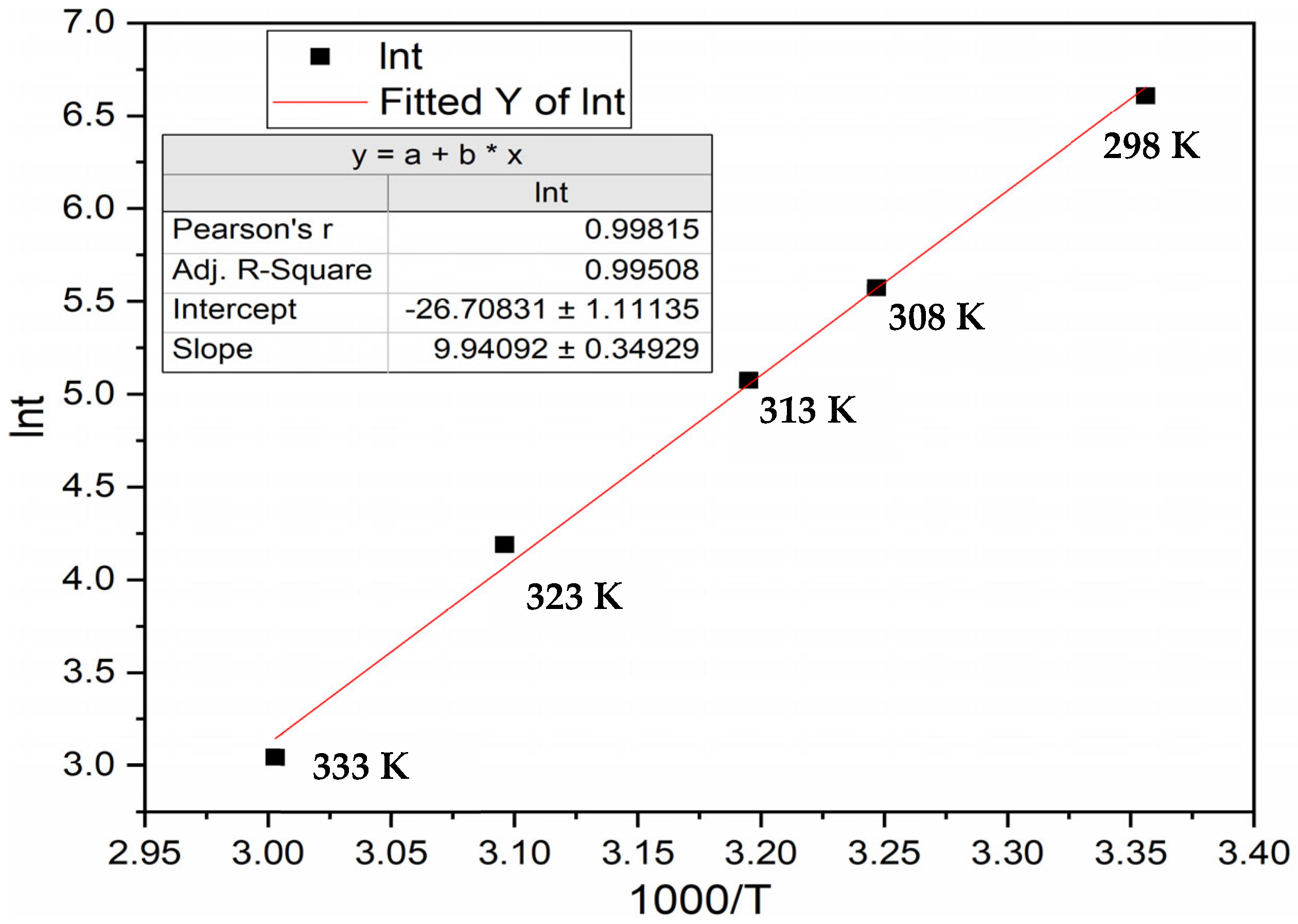
3.2.1. Hydrogen Pressure of 40 Bars
3.2.2. Hydrogen Pressure of 50 Bars
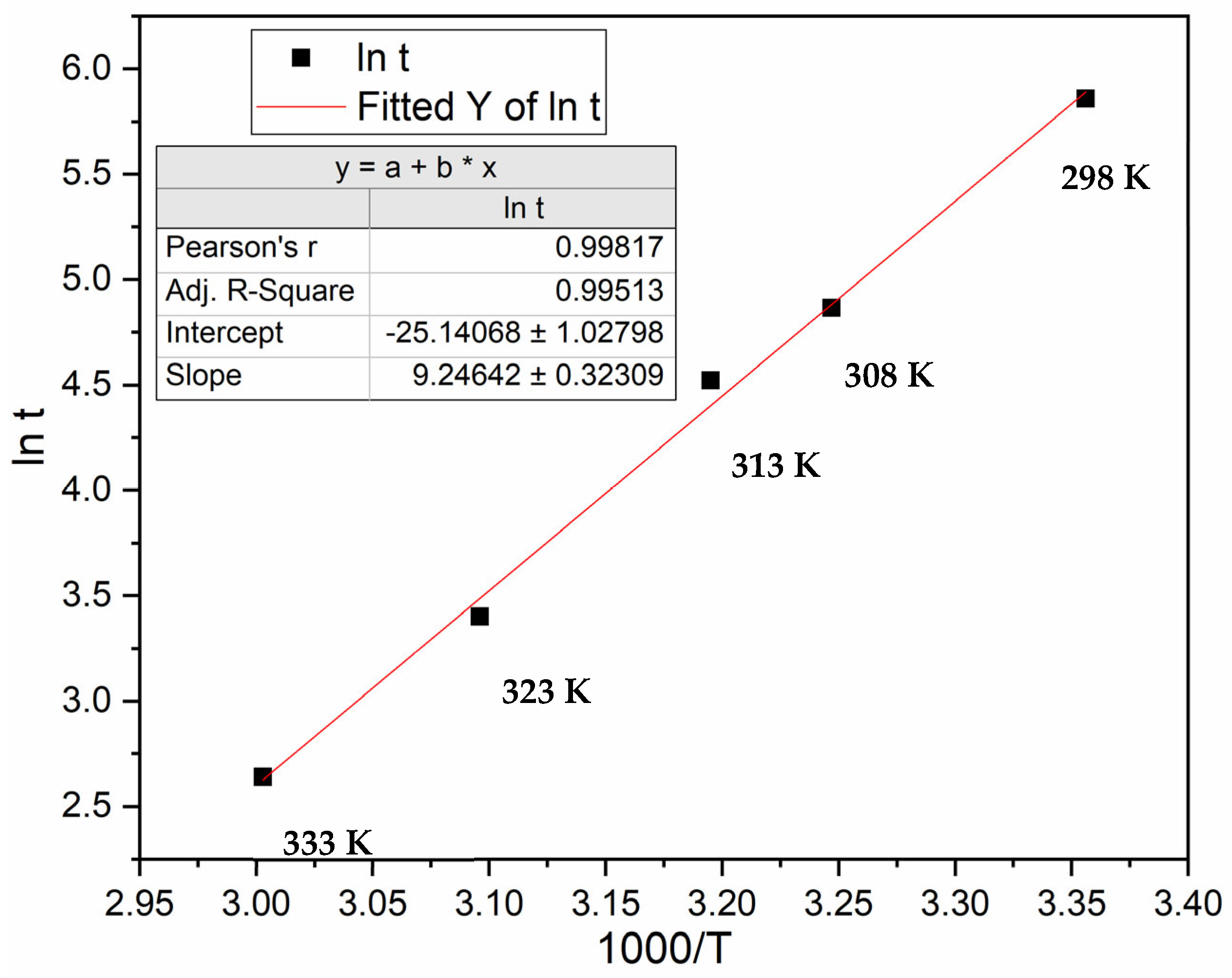
3.3. Incubation Time under Constant Driving Force
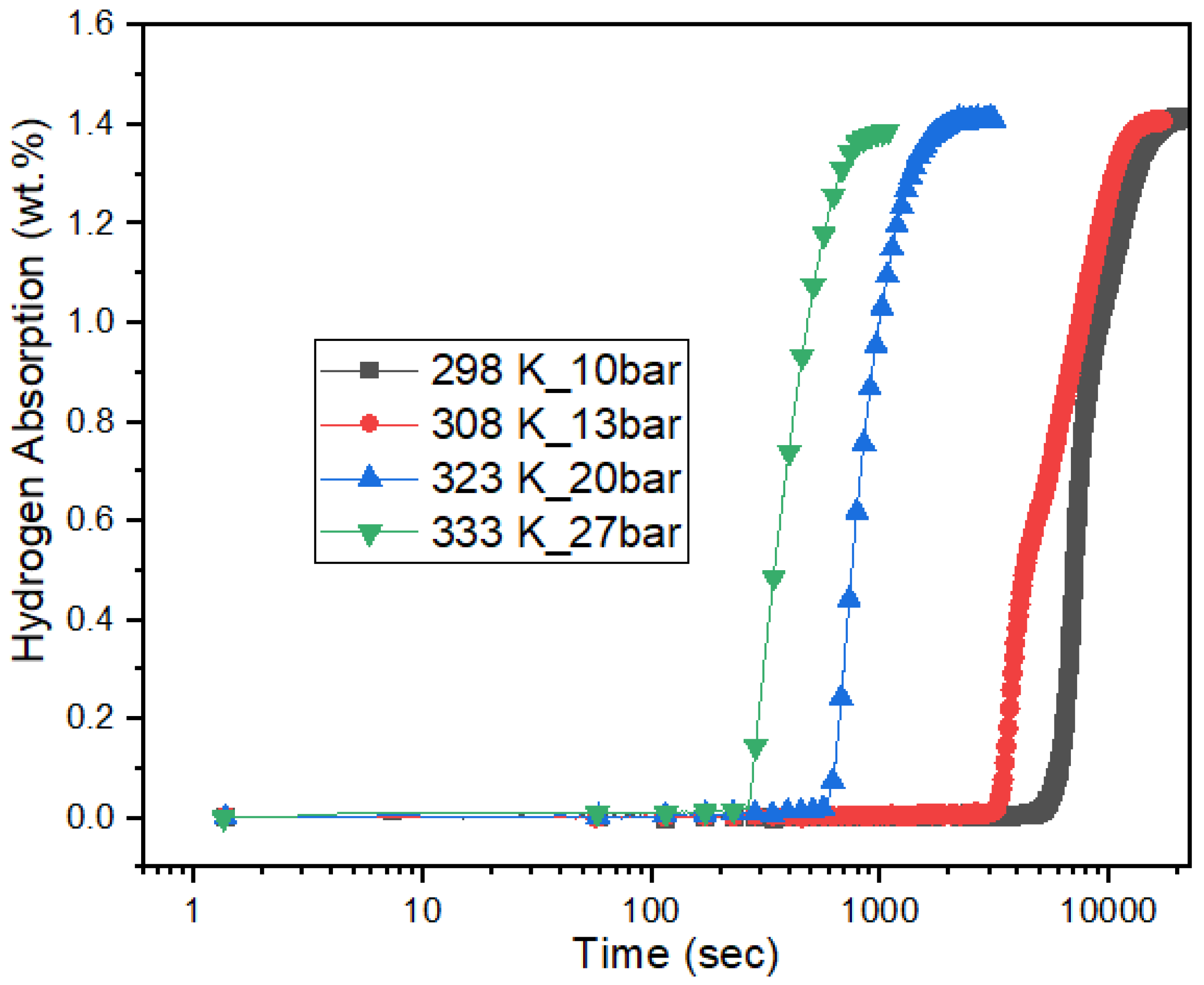
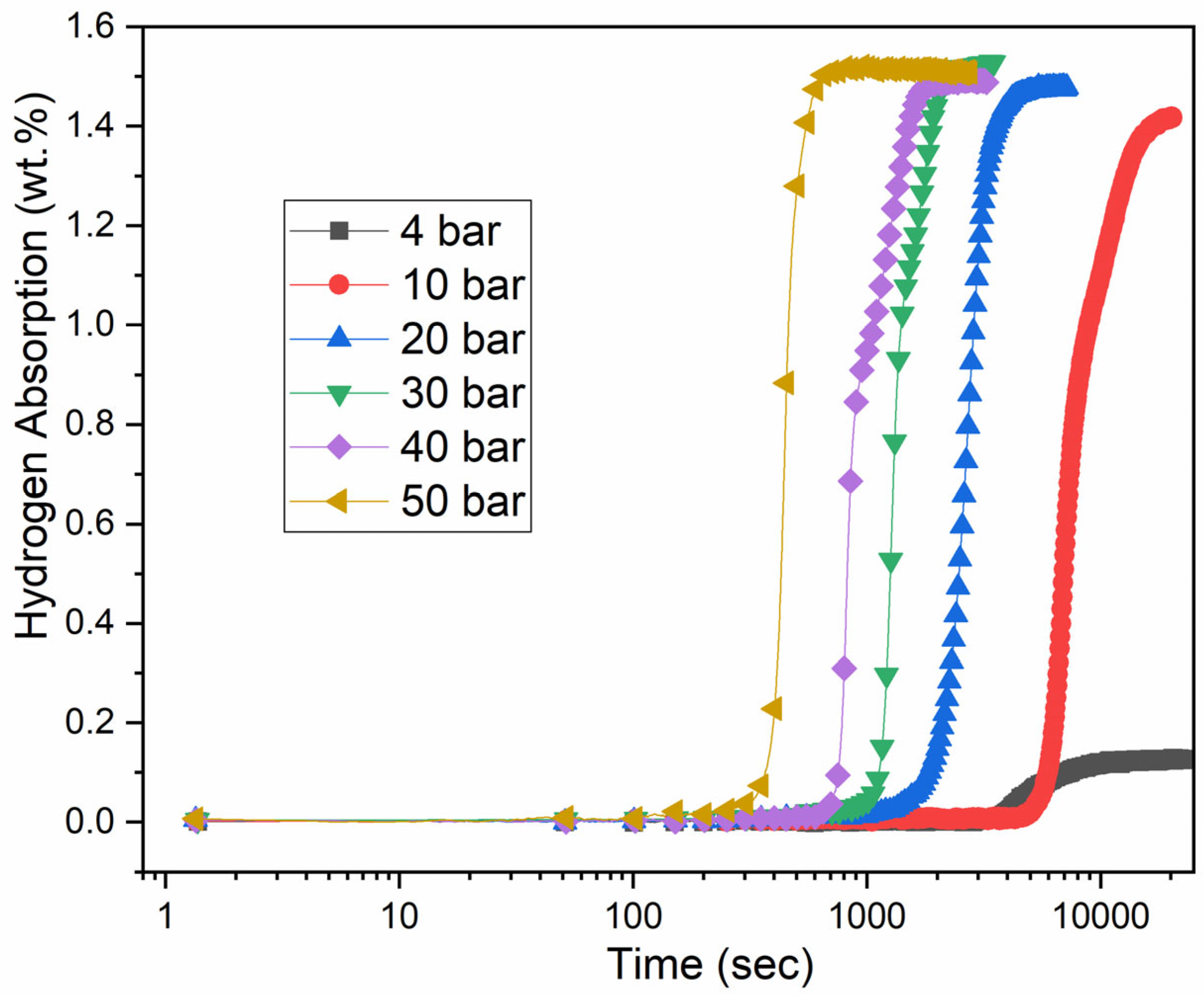
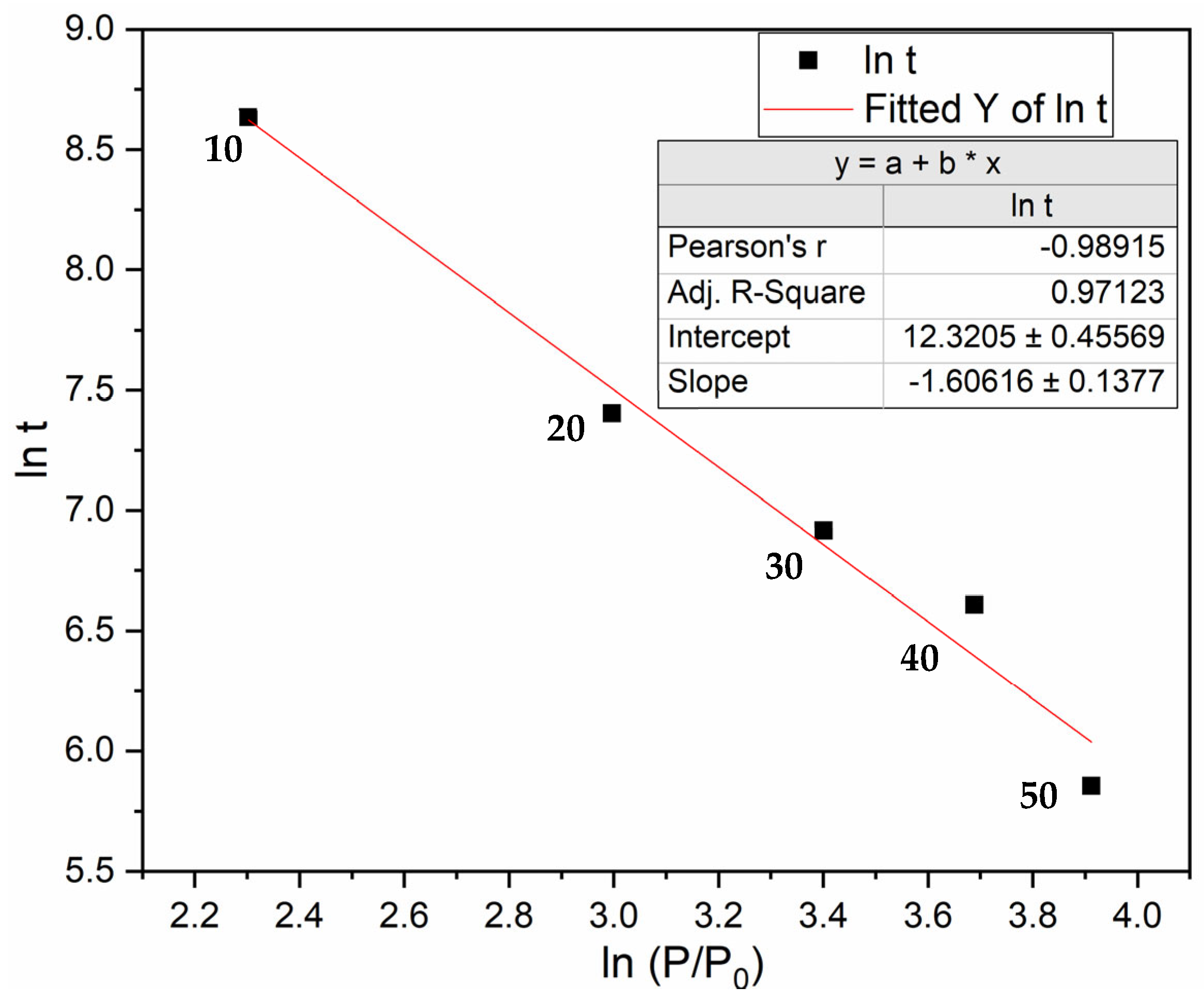
3.4. Effect of Pressure on Incubation Time
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Hydrogen Review 2023. Available online: https://www.iea.org/energy-system/low-emission-fuels/hydrogen (accessed on 31 July 2023).
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C., Jr.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P. Materials for hydrogen-based energy storage–past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Züttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: The future energy carrier. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2010, 368, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Yartys, V.A.; Chidziva, S.; Pasupathi, S.; Lototskyy, M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int. J. Hydrogen Energy 2021, 46, 13647–13657. [Google Scholar] [CrossRef]
- Van Vucht, J.H.; Kuijpers, F.; Bruning, H.C. Reversible room-temperature absorption of large quantities of hydrogen by intermetallic compounds. Philips Res. Rep. 1970, 25, 133–140. [Google Scholar]
- Joubert, J.-M.; Paul-Boncour, V.; Cuevas, F.; Zhang, J.; Latroche, M. LaNi5 related AB5 compounds: Structure, properties and applications. J. Alloys Compd. 2021, 862, 158163. [Google Scholar] [CrossRef]
- Joubert, J.M.; Latroche, M.; Percheron-Guégan, A. Metallic hydrides ii: Materials for electrochemical storage. MRS Bull. 2002, 27, 694–698. [Google Scholar] [CrossRef]
- Miura, S.; Fujisawa, A.; Ishida, M. A hydrogen purification and storage system using metal hydride. Int. J. Hydrogen Energy 2012, 37, 2794–2799. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Yaakob, Z.; Mat Jalil, N.; Tasirin, S.M. Synthesis of high-surface-area hexagonal LaNi5 nanofibers via electrospinning. J. Alloys Compd. 2012, 541, 335–337. [Google Scholar] [CrossRef]
- Ikeda, K.; Ohshita, H.; Otomo, T.; Sakaki, K.; Kim, H.; Nakamura, Y.; Machida, A.; Von Dreele, R.B. Pressure cells for in situ neutron total scattering: Time and real-space resolution during deuterium absorption. J. Appl. Crystallogr. 2022, 55, 1631–1639. [Google Scholar] [CrossRef]
- Joubert, J.M.; Latroche, M.; Černý, R.; Percheron-Guégan, A.; Yvon, K. Hydrogen cycling induced degradation in LaNi5-type materials. J. Alloys Compd. 2002, 330–332, 208–214. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Schulz, R. Hydrogen storage properties of the mechanically alloyed LaNi5-based materials. J. Alloys Compd. 2001, 320, 133–139. [Google Scholar] [CrossRef]
- Van Mal, H.H.; Buschow, K.H.J.; Miedema, A.R. Hydrogen absorption in LaNi5 and related compounds: Experimental observations and their explanation. J. Less Common Met. 1974, 35, 65–76. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Tousignant, M.; Huot, J. Hydrogen sorption enhancement in cold rolled LaNi5. J. Alloys Compd. 2014, 595, 22–27. [Google Scholar] [CrossRef]
- Lv, P.; Huot, J. Hydrogenation improvement of tife by adding ZrMn2. Energy 2017, 138, 375–382. [Google Scholar] [CrossRef]
- Montero, J.; Ek, G.; Sahlberg, M.; Zlotea, C. Improving the hydrogen cycling properties by Mg addition in Ti-V-Zr-Nb refractory high entropy alloy. Scr. Mater. 2021, 194, 113699. [Google Scholar] [CrossRef]
- Sleiman, S.; Huot, J. Effect of particle size, pressure and temperature on the activation process of hydrogen absorption in TiVZrHfNb high entropy alloy. J. Alloys Compd. 2021, 861, 158615. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, X.; Nyahuma, F.M.; Yan, N.; Xiao, J.; Su, S.; Zhang, L. Enhancing hydrogen storage properties of MgH2 by transition metals and carbon materials: A brief review. Front. Chem. 2020, 8, 552. [Google Scholar] [CrossRef]
- Huot, J. Enhancing Hydrogen Storage Properties of Metal Hybrides: Enhancement by Mechanical Deformations; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Sleiman, S.; Aliouat, A.; Huot, J. Enhancement of first hydrogenation of Ti1V0.9Cr1.1 BCC alloy by cold rolling and ball milling. Materials 2020, 13, 3106. [Google Scholar] [CrossRef]
- Dixit, V.; Huot, J. Investigation of the microstructure, crystal structure and hydrogenation kinetics of Ti-V-Cr alloy with Zr addition. J. Alloys Compd. 2019, 785, 1115–1120. [Google Scholar] [CrossRef]
- Shelyapina, M.; Dost, A.; Skryabina, N.; Privalov, A.; Vogel, M.; Fruchart, D. Effect of Zr7Ni10 additive on hydrogen mobility in (TiCr1.8)1-xVx (x= 0.2, 0.4, 0.6, 0.8): An 1H NMR SFG study. Int. J. Hydrogen Energy 2020, 45, 7929–7937. [Google Scholar] [CrossRef]
- Sleiman, S.; Huot, J. Microstructure and hydrogen storage properties of Ti1V0.9Cr1.1 alloy with addition of x wt % Zr (x = 0, 2, 4, 8, and 12). Inorganics 2017, 5, 86. [Google Scholar] [CrossRef]
- Balcerzak, M. Hydrogenation properties of nanocrystalline TiVMn body-centered-cubic alloys. Int. J. Hydrogen Energy 2020, 45, 15521–15529. [Google Scholar] [CrossRef]
- Strozi, R.B.; Ivanisenko, J.; Koudriachova, N.; Huot, J. Effect of HPT on the first hydrogenation of LaNi5 metal hydride. Energies 2021, 14, 6710. [Google Scholar] [CrossRef]
- Broom, D.P. Hydrogen Storage Materials: The Characterisation of Their Storage Properties; Springer: Berlin/Heidelberg, Germany, 2011; Volume 1. [Google Scholar]
- Rudman, P. Hydrogen-diffusion-rate-limited hydriding and dehydriding kinetics. J. Appl. Phys. 1979, 50, 7195–7199. [Google Scholar] [CrossRef]
- Kumar, S.; Kojima, Y.; Dey, G.K. Thermodynamics and kinetics of hydrogen absorption–desorption of highly crystalline laNi5. J. Therm. Anal. Calorim. 2018, 134, 889–894. [Google Scholar] [CrossRef]


| 40 Bars | 50 Bars | C = 170 | |
|---|---|---|---|
| EA (kJ/mol H2) | 83 ± 3 | 77 ± 3 | 75 ± 7 |
| A (s−1) | 3.9 × 1011 | 8.2 × 1010 | 2.5 × 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sleiman, S.; Shahgaldi, S.; Huot, J. Investigation of the First Hydrogenation of LaNi5. Reactions 2024, 5, 419-428. https://doi.org/10.3390/reactions5030021
Sleiman S, Shahgaldi S, Huot J. Investigation of the First Hydrogenation of LaNi5. Reactions. 2024; 5(3):419-428. https://doi.org/10.3390/reactions5030021
Chicago/Turabian StyleSleiman, Salma, Samaneh Shahgaldi, and Jacques Huot. 2024. "Investigation of the First Hydrogenation of LaNi5" Reactions 5, no. 3: 419-428. https://doi.org/10.3390/reactions5030021
APA StyleSleiman, S., Shahgaldi, S., & Huot, J. (2024). Investigation of the First Hydrogenation of LaNi5. Reactions, 5(3), 419-428. https://doi.org/10.3390/reactions5030021









