Synthesis of Nanostructured Mg2Ni for Hydrogen Storage by Mechanical Alloying via High-Pressure Torsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPT Procedure and Equipment
2.3. Characterization Methods
3. Results and Discussion
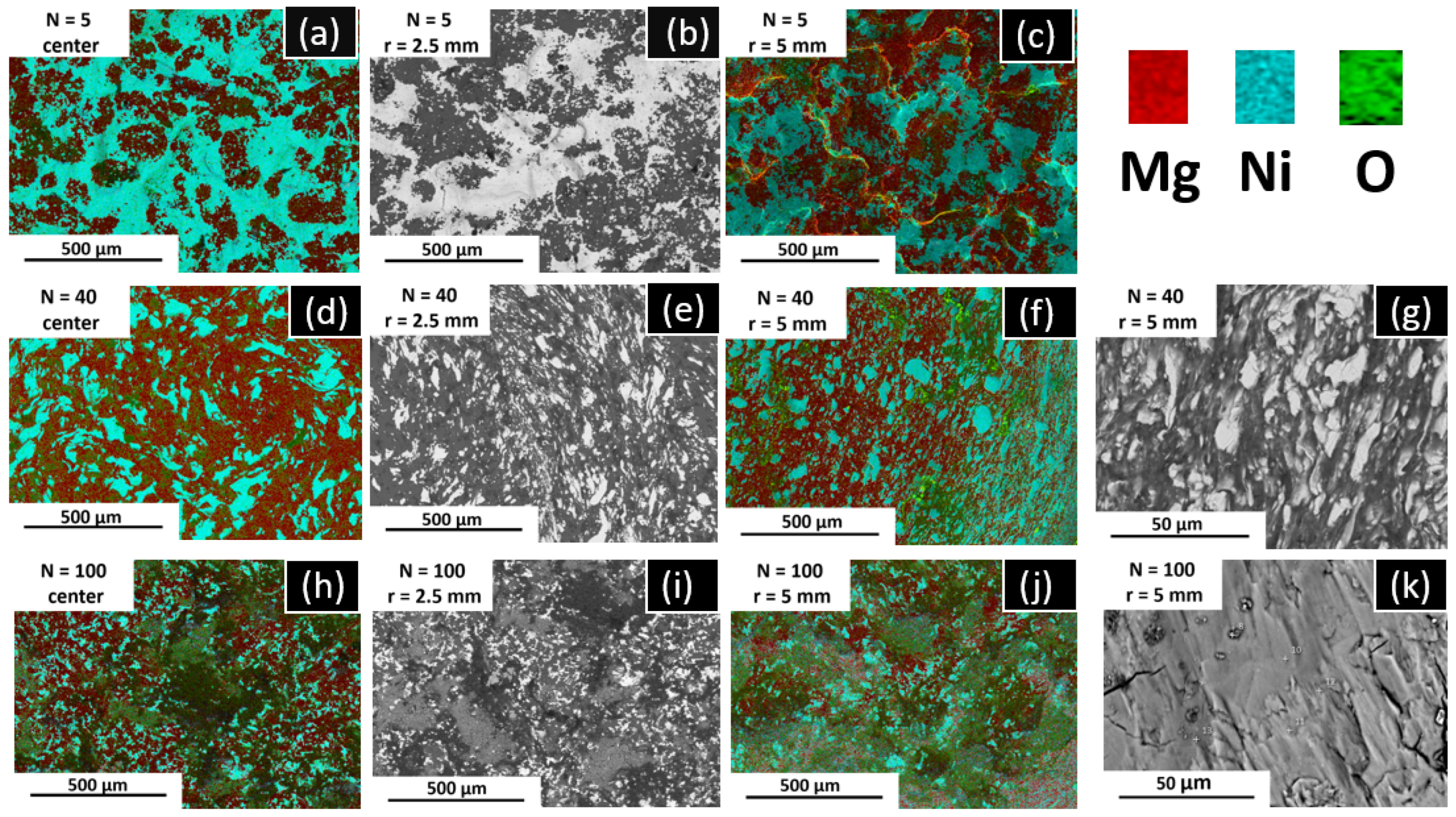
3.1. Synthesis and Characterization of Mg2Ni by HPT
3.2. Hydrogen Storage Properties of Mg-Ni Processed by HPT
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| a | a lattice parameter in x,y,y (a,b,c) system | h | thickness of the disc |
| Abs | absorption | hcp | hexagonal compact structure |
| Activ | activation | HT | heat treatment |
| Å | Angström unit | HEBM | high-energy ball milling |
| at% | atomic percentage | Kα | k alpha radiation |
| bcc | body-cubic center structure | N | number of HPT turns |
| c | c lattice parameter in x,y,y (a,b,c) system | r | radius |
| CP | commercially pure | RT | room temperature |
| ECAP | equal-channel angular processing | wt% | weight percentage |
| fcc | face-center cubic structure | γ | equivalent shear strain |
References
- James, B.D. Overview of Hydrogen Storage Technologies. 2008, pp. 568–569. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=2b733c606694f44cf0dcb744c94c3161d3aca4bb (accessed on 22 August 2024).
- Paster, M.D.; Ahluwalia, R.K.; Berry, G.; Elgowainy, A.; Lasher, S.; McKenney, K.; Gardiner, M. Hydrogen Storage Technology Options for Fuel Cell Vehicles: Well-to-Wheel Costs, Energy Efficiencies, and Greenhouse Gas Emissions. Int. J. Hydrogen Energy 2011, 36, 14534–14551. [Google Scholar] [CrossRef]
- Arjona, V.T.; Ast, C.; Thompson, S.; Fellow, O. Hydrogen and Fuel Cells Overview. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-and-fuel-cell-technologies-office-information-resources (accessed on 14 July 2024).
- Yamashita, A.; Kondo, M.; Goto, S.; Ogami, N. Development of High-Pressure Hydrogen Storage System for the Toyota “Mirai.”; SAE Technical Paper 2015-01-1169; SAE International: Warrendale, PA, USA, 2015. [Google Scholar]
- Latroche, M. Structural and Thermodynamic Properties of Metallic Hydrides Used for Energy Storage☆. J. Phys. Chem. Solids 2004, 65, 517–522. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A Review on the Current Progress of Metal Hydrides Material for Solid-State Hydrogen Storage Applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Young, K. Metal Hydrides☆. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Srinivasan, S.S.; Demirocak, D.E. Metal Hydrides Used for Hydrogen Storage. In Nanostructured Materials for Next-Generation Energy Storage and Conversion: Hydrogen Production, Storage, and Utilization; Springer: Berlin/Heidelberg, Germany, 2017; pp. 225–255. ISBN 9783662535141. [Google Scholar]
- Dornhel, M. Thermodynamics of Metal Hydrides: Tailoring Reaction Enthalpies of Hydrogen Storage Materials. In Thermodynamics—Interaction Studies—Solids, Liquids and Gases; IntechOpen: London, UK, 2011; pp. 891–911. [Google Scholar]
- Tanaka, T.; Ketta, M.; Azofeifa, D.E. Theory of Hydrogen Absorption in Metal Hydrides. Phys. Rev. B 1981, 24, 1771. [Google Scholar] [CrossRef]
- Huot, J.; Edalati, K.; Stefano, D.; Yaroslav, F. Mechanochemistry of Metal Hydrides: Recent Advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- Grataetz, J.; Reilly, J.J.; Wegrzyn, J. Metal Hydrides for Hydrogen Storage. In Materials Research Society Symposium—Proceedings; Materials Research Society: Warrendale, PA, USA, 2008; Volume 1041. [Google Scholar]
- Shelyapina, M.G. Metal Hydrides for Energy Storage. In Handdbook of Evomaterials; Martínez, L., Kharissova, O., Kharisov, B., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar]
- Liu, H.; Zhang, J.; Sun, P.; Zhou, C.; Liu, Y.; Fang, Z.Z. Effect of Oxygen on the Hydrogen Storage Properties of TiFe Alloys. J. Energy Storage 2022, 55, 105543. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Sun, P.; Zhou, C.; Liu, Y.; Fang, Z.Z. Effect of Oxygen Addition on Phase Composition and Activation Properties of TiFe Alloy. Int. J. Hydrogen Energy 2022, 48, 8563–8572. [Google Scholar] [CrossRef]
- Yadav, D.K.; Chawla, K.; Pooja; Lal, N.; Choudhary, B.L.; Lal, C. Catalytic Effect of TiO2 on Hydrogen Storage Properties of MgH2. Mater. Today Proc. 2021, 46, 2326–2329. [Google Scholar] [CrossRef]
- Lobo, N.; Klimkowicz, A.; Takasaki, A. Effect of TiO2 + Nb2O5 + TiH2 Catalysts on Hydrogen Storage Properties of Magnesium Hydride. MRS Adv. 2020, 5, 1059–1069. [Google Scholar] [CrossRef]
- Korablov, D.; Nielsen, T.K.; Bessencacher, F.; Jensen, T.R. Mechanism and Kinetics of Early Transition Metal Hydrides, Oxides, and Chlorides to Enhance Hydrogen Release and Uptake Properties of MgH2. Powder Diffr. 2015, 30, S9–S15. [Google Scholar] [CrossRef]
- Borgschulte, A.; Rector, J.H.; Dam, B.; Grissen, R.; Zuttel, A. The Role of Niobium Oxide as a Surface Catalyst for Hydrogen Absorption. J. Catal. 2005, 235, 353–358. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Rudiger Bormann, T.K. Fast Hydrogen Sorption Kinetics of Nanocrystalline Mg Using Nb2O5 as Catalyst. Scr. Mater. 2003, 49, 213–217. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, R.; Klassen, R. Comparison of the Catalytic Effects of V, V2O5, VN, and VC on the Hydrogen Sorption of Nanocrystalline Mg. J. Alloys Compd. 2001, 322, 5–9. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, R.; Bormann, R. Metal Oxides as Catalysts for Improved Hydrogen Sorption in Nanocrystalline Mg-Based Materials. J. Alloys Compd. 2000, 315, 237–242. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Berti, N.; Cuevas, F.; Latroche, M.; Baricco, M. Substitutional Effects in TiFe for Hydrogen Storage: A Comprehensive Review. Mater. Adv. 2021, 2, 2524–2560. [Google Scholar] [CrossRef]
- Park, K.B.; Na, T.W.; Kim, Y.D.; Park, J.Y.; Kang, J.W.; Kang, H.S.; Park, K. Characterization of Microstructure and Surface Oxide of Ti1.2Fe Hydrogen Storage Alloy. Int. J. Hydrogen Energy 2021, 46, 13082–13087. [Google Scholar] [CrossRef]
- Sun, D.; Enoki, H.; Gingl, F.; Akiba, E. New Approach for Synthesizing Mg-Based Alloys; Elsevier: Amsterdam, The Netherlands, 1999; Volume 285. [Google Scholar]
- Janot, R.; Aymard, L.; Rougier, A.; Tarascon, J.M. Fast Hydrogen Sorption Kinetics for Ball-Milled Mg2Ni Alloys. J. Phys. Chem. Solids 2004, 65, 529–534. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural Study and Hydrogen Sorption Kinetics of Ball-Milled Magnesium Hydride. J. Alloys Compd. 1999, 295, 495–500. [Google Scholar] [CrossRef]
- Sapassov, T.; Solsana, P.; Suriñach, S.; Baró, M.D. Optimisation of the Ball-Milling and Heat Treatment Parameters for Syntesis of Amorphous and Nanocrystalline Mg2Ni-Based Alloys. J. Alloys Compd. 2003, 349, 242–254. [Google Scholar] [CrossRef]
- Lototskyy, M.; Sibanyoni, J.M.; Denys, R.V.; Williams, M.; Pollet, B.G.; Yartys, V.A. Magnesium—Carbon Hydrogen Storage Hybrid Materials Produced by Reactive Ball Milling in Hydrogen Magnesium—Carbon Hydrogen Storage Hybrid Materials Produced by Reactive Ball Milling in Hydrogen. Carbon 2013, 57, 146–160. [Google Scholar] [CrossRef]
- Wu, Y.; Han, W.; Zhou, S.X.; Lototsky, M.V.; Solberg, J.K.; Yartys, V.A. Microstructure and Hydrogenation Behavior of Ball-Milled and Melt-Spun Mg—10Ni–2Mm Alloys. J. Alloys Compd. 2008, 466, 176–181. [Google Scholar] [CrossRef]
- Kurnia-Dewa, M.D.; Wiryolukito, S.; Suwarno, H. Hydrogen Absorption Capacity of Fe-Ti-Al Alloy Prepared by High Energy Ball Milling. Energy Procedia 2015, 68, 316–325. [Google Scholar] [CrossRef]
- Iturbe-García, J.L.; García-Núñez, M.R.; López-Muñoz, B.E. Synthesis of the Mg2Ni Alloy Prepared by Mechanical Alloying Using a High Energy Ball Mill. J. Mex. Chem. Soc. 2010, 54, 46–50. [Google Scholar] [CrossRef]
- Phasha, M.; Maweja, K.; Babst, C. Mechanical Alloying by Ball Milling of Ti and Mg Elemental Powders: Operation Condition Considerations. J. Alloys Compd. 2010, 492, 201–207. [Google Scholar] [CrossRef]
- Leiva, D.R.; De Almeida Costa, H.C.; Huot, J.; Santos Pinhero, T.; Jorge, M., Jr.; Tomishimi Ishikawa, T.; Botta, W.J. Magnesium-Nickel Alloy for Hydrogen Storage Produced by Melt Spinning Followed by Cold Rolling. Mater. Res. 2012, 15, 813–817. [Google Scholar] [CrossRef][Green Version]
- Kudriashova, N.; Huot, J. Effect of Cold Rolling on Magnesium-Based Metal Hydrides. Mater. Trans. 2023, 64, 1879–1885. [Google Scholar] [CrossRef]
- Emami, H.; Edalati, K.; Staykov, A.; Hongo, T.; Iwaoka, H.; Horita, Z.; Akiba, E. Solid-State Reactions and Hydrogen Storage in Magnesium Mixed with Various Elements by High-Pressure Torsion: Experiments and First-Principles Calculations. RSC Adv. 2016, 6, 11665–11674. [Google Scholar] [CrossRef]
- López Gómez, E.I.; Edalati, K.; Antiqueira, F.J.; Coimbrão, D.D.; Zepon, G.; Leiva, D.R.; Ishikawa, T.T.; Cubero-Sesin, J.M.; Botta, W.J. Synthesis of Nanostructured TiFe Hydrogen Storage Material by Mechanical Alloying via High-Pressure Torsion. Adv. Eng. Mater. 2020, 22, 2000011. [Google Scholar] [CrossRef]
- Edalati, K.; Emami, H.; Ikeda, Y.; Iwaoka, H.; Tanaka, I.; Akiba, E.; Horita, Z. New Nanostructured Phases with Reversible Hydrogen Storage Capability in Immiscible Magnesium-Zirconium System Produced by High-Pressure Torsion. Acta Mater. 2016, 108, 293–303. [Google Scholar] [CrossRef]
- Edalati, K.; Emami, H.; Staykov, A.; Smith, D.J.; Akiba, E.; Horita, Z. Formation of Metastable Phases in Magnesium-Titanium System by High-Pressure Torsion and Their Hydrogen Storage Performance. Acta Mater. 2015, 99, 150–156. [Google Scholar] [CrossRef]
- López-Gómez, E.I.; Edalati, K.; Coimbrão, D.D.; Antiqueira, F.J.; Zepon, G.; Cubero-Sesin, J.M.; Botta, W.J. FCC Phase Formation in Immiscible Mg-Hf (Magnesium-Hafnium) System by High-Pressure Torsion. AIP Adv. 2020, 10, 055222. [Google Scholar] [CrossRef]
- Edalati, K. Metallurgical Alchemy by Ultra-Severe Plastic Deformation via High-Pressure Torsion Process. Mater. Trans. 2019, 60, 1221–1229. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. A Review on High-Pressure Torsion (HPT) from 1935 to 1988. Mater. Sci. Eng. A 2016, 652, 325–352. [Google Scholar] [CrossRef]
- Oh-ishi, K.; Edalati, K.; Kim, H.S.; Hono, K.; Horita, Z. High-Pressure Torsion for Enhanced Atomic Diffusion and Promoting Solid-State Reactions in the Aluminum–Copper System. Acta Mater. 2013, 61, 3482–3489. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M.; Varga, L.K.; Krállics, G.; Péter, L. Hydrogen Storage of Nanocrystalline Mg-Ni Alloy Processed by Equal-Channel Angular Pressing and Cold Rolling. Int. J. Hydrogen Energy 2014, 39, 9911–9917. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Ma, A.; Li, Y.; Song, D. A Critical Review of Mg-Based Hydrogen Storage Materials Processed by Equal Channel Angular Pressing. Metals 2017, 7, 324. [Google Scholar] [CrossRef]
- Krystian, M.; Zehetbauer, M.J.; Kropik, H.; Mingler, B.; Krexner, G. Hydrogen Storage Properties of Bulk Nanostructured ZK60 Mg Alloy Processed by Equal Channel Angular Pressing. J. Alloys Compd. 2011, 5095, 5449–5455. [Google Scholar] [CrossRef]
- Skripnyuk, V.M.; Rabkin, E.; Estrin, Y.; Lapovok, R. Improving Hydrogen Storage Properties of Magnesium Based Alloys by Equal Channel Angular Pressing. Int. J. Hydrogen Energy 2009, 34, 6320–6324. [Google Scholar] [CrossRef]
- Asselli, A.A.C.; Leiva, D.R.; Huot, J.; Kawasaki, M.; Langdon, T.G.; Botta, W.J. Effects of Equal-Channel Angular Pressing and Accumulative Roll-Bonding on Hydrogen Storage Properties of a Commercial ZK60 Magnesium Alloy. Int. J. Hydrogen Energy 2015, 40, 16971–16976. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Yanagida, A.; Akiba, E.; Horita, Z. Activation of TiFe for Hydrogen Storage by Plastic Deformation Using Groove Rolling and High-Pressure Torsion: Similarities and Differences. Int. J. Hydrogen Energy 2014, 39, 15589–15594. [Google Scholar] [CrossRef]
- Edalati, K.; Matsuda, J.; Iwaoka, H.; Toh, S.; Akiba, E.; Horita, Z. High-Pressure Torsion of TiFe Intermetallics for Activation of Hydrogen Storage at Room Temperature with Heterogeneous Nanostructure. Int. J. Hydrogen Energy 2013, 38, 4622–4627. [Google Scholar] [CrossRef]
- Edalati, K.; Shao, H.; Emami, H.; Iwaoka, H.; Akiba, E.; Horita, Z. Activation of Titanium-Vanadium Alloy for Hydrogen Storage by Introduction of Nanograins and Edge Dislocations Using High-Pressure Torsion. Int. J. Hydrogen Energy 2016, 41, 8917–8924. [Google Scholar] [CrossRef]
- Edalati, K.; Akiba, E.; Horita, Z. High-Pressure Torsion for New Hydrogen Storage Materials. Sci. Technol. Adv. Mater. 2018, 19, 185–193. [Google Scholar] [CrossRef]
- Edalati, K.; Yamamoto, A.; Horita, Z.; Ishihara, T. High Pressure Torsion of Pure Magnesium: Evolution of Mechanical Properties, Microstructure and Hydrogen Storage Capacity with Equivalent Strain. Scr. Mater. 2011, 64, 880–883. [Google Scholar] [CrossRef]
- Kitabayashi, K.; Edalati, K.; Li, H.-W.; Akiba, E.; Horita, Z. Phase Transformations in MgH2–TiH2 Hydrogen Storage System by High-Pressure Torsion Process. Adv. Eng. Mater. 2018, 22, 1900027. [Google Scholar] [CrossRef]
- He, L.; Shi, X.; Li, X.; Huang, J.; Cheng, T.; Wang, X.; Li, Y.; Lin, H.; Edalati, K.; Li, H.-W. Severe Plastic Deformation through High-Pressure Torsion for Preparation of Hydrogen Storage Materials—A Review. Mater. Trans. 2023, 64, 1575–1584. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M. The Effect of Severe Plastic Deformation on the Hydrogen Storage Properties of Metal Hydrides. Mater. Trans. 2023, 64, 1387–1400. [Google Scholar] [CrossRef]
- Edalati, K.; Ahmed, A.Q.; Akrami, S.; Ameyama, K.; Aptukov, V.; Asfandiyarov, R.N.; Ashida, M.; Astanin, V.; Bachmaier, A.; Beloshenko, V.; et al. Severe Plastic Deformation for Producing Superfunctional Ultrafine-Grained and Heterostructured Materials: An Interdisciplinary Review. J. Alloys Compd. 2024, 1002, 174667. [Google Scholar] [CrossRef]
- Hongo, T.; Edalati, K.; Arita, M.; Matsuda, J.; Akiba, E.; Horita, Z. Significance of Grain Boundaries and Stacking Faults on Hydrogen Storage Properties of Mg 2 Ni Intermetallics Processed by High-Pressure Torsion. Acta Mater. 2015, 92, 46–54. [Google Scholar] [CrossRef]
- Edalati, K.; Uehiro, R.; Ikeda, Y.; Li, H.-W.; Emami, H.; Filinchuk, Y.; Arita, M.; Sauvage, X.; Tanaka, I.; Akiba, E.; et al. Design and Synthesis of a Magnesium Alloy for Room Temperature Hydrogen Storage. Acta Mater. 2018, 149, 88–96. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Li, L. Structure and Hydrogenation Properties of Nanocrystalline Mg2Ni Prepared by Hydriding Combustion Synthesis and Mechanical Milling. J. Alloys Compd. 2008, 455, 197–202. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Liu, Y.; Chabane, D.; Elkedim, O. Intermetallic Compounds Synthesized by Mechanical Alloying for Solid-State Hydrogen Storage: A Review. Energies 2021, 14, 5758. [Google Scholar] [CrossRef]
- Ruslan, Z.; Valiev, R.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y.T. Producing Bulk Ultrafine-Grained Meterials by Severe Plastic Deformation. JOM 2006, 58, 33–39. [Google Scholar] [CrossRef]
- Faddegon, B.; Ramos-Méndez, J.; Schuemann, J.; McNamara, A.; Shin, J.; Perl, J.; Paganetti, H. The TOPAS Tool for Particle Simulation, a Monte Carlo Simulation Tool for Physics, Biology and Clinical Research. Phys. Med. 2020, 72, 114–121. [Google Scholar] [CrossRef]
- Perl, J.; Shin, J.; Schümann, J.; Faddegon, B.; Paganetti, H. TOPAS: An Innovative Proton Monte Carlo Platform for Research and Clinical Applications. Med. Phys. 2012, 39, 6818–6837. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Asfandiyarov, R.N.; Astanin, V.V.; Sharafutdinov, A.V. Slippage during High-Pressure Torsion: Accumulative High-Pressure Torsion—Overview of the Latest Results. Metals 2023, 13, 1340. [Google Scholar] [CrossRef]
- Edalati, K.; Uehiro, R.; Fujiwara, K.; Ikeda, Y.; Li, H.-W.; Sauvage, X.; Valiev, R.Z.; Akiba, E.; Tanaka, I.; Horita, Z. Ultra-Severe Plastic Deformation: Evolution of Microstructure, Phase Transformation and Hardness in Immiscible Magnesium-Based Systems. Mater. Sci. Eng. A 2017, 701, 158–166. [Google Scholar] [CrossRef]
- Castro, M.M.; Sabbaghianrad, S.; Pereira, P.H.R.; Mazzer, E.M.; Isaac, A.; Langdon, T.G.; Figueiredo, R.B. A Magnesium-Aluminium Composite Produced by High-Pressure Torsion. J. Alloys Compd. 2019, 804, 421–426. [Google Scholar] [CrossRef]
- Cubero-Sesin, J.M.; Horita, Z. Powder Consolidation of Al-10wt% Fe Alloy by High-Pressure Torsion. Mater. Sci. Eng. A 2012, 558, 462–471. [Google Scholar] [CrossRef]
- Ibrahim, N.; Peterlechner, M.; Emeis, F.; Wegner, M.; Divinski, S.V.; Wilde, G. Mechanical Alloying via High-Pressure Torsion of the Immiscible Cu50Ta50 System. Mater. Sci. Eng. A 2017, 685, 19–30. [Google Scholar] [CrossRef]
- Edalati, K.; Daio, T.; Lee, S.; Horita, Z.; Nishizaki, T.; Akune, T.; Nojima, T.; Sasaki, T. High Strength and Superconductivity in Nanostructured Niobium-Titanium Alloy by High-Pressure Torsion and Annealing: Significance of Elemental Decomposition and Supersaturation. Acta Mater. 2014, 80, 149–158. [Google Scholar] [CrossRef]
- Campos-Quirós, A.; Cubero-Sesín, J.M.; Edalati, K. Synthesis of Nanostructured Biomaterials by High-Pressure Torsion: Effect of Niobium Content on Microstructure and Mechanical Properties of Ti-Nb Alloys. Mater. Sci. Eng. A 2020, 795, 139972. [Google Scholar] [CrossRef]
- Gajdics, M.; Calizzi, M.; Pasquini, L.; Schafler, E.; Révész, Á. Characterization of a Nanocrystalline Mg–Ni Alloy Processed by High-Pressure Torsion during Hydrogenation and Dehydrogenation. Int. J. Hydrogen Energy 2016, 41, 9803–9809. [Google Scholar] [CrossRef]
- Révész, A.; Kánya, Z.; Verebélyi, T.; Szabó, P.J.; Zhilyaev, A.P.; Spassov, T. The Effect of High-Pressure Torsion on the Microstructure and Hydrogen Absorption Kinetics of Ball-Milled Mg70Ni30. J. Alloys Compd. 2010, 504, 83–88. [Google Scholar] [CrossRef]
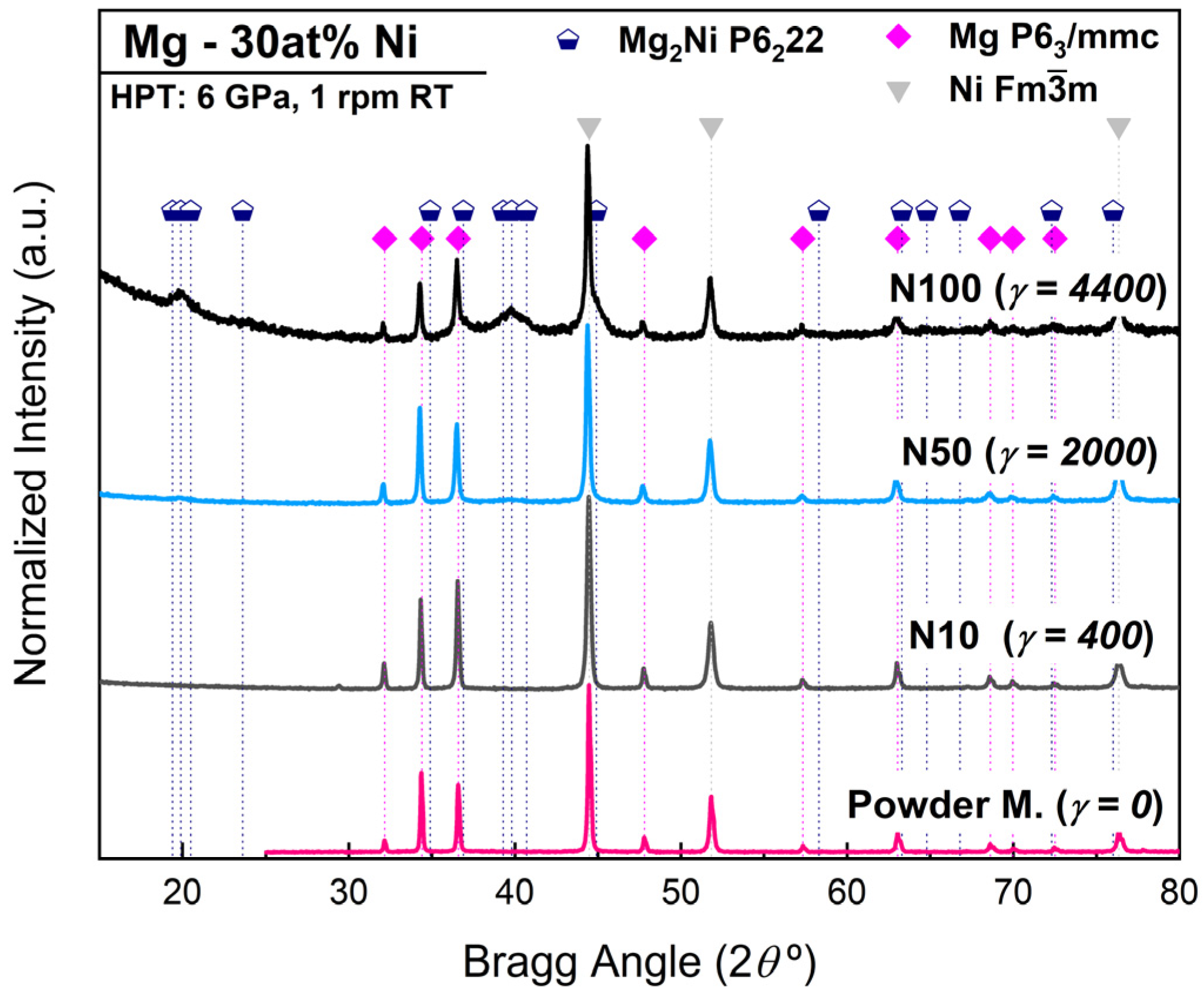





| Condition | Phase | wt% | a (Å) | c (Å) | Crystallite Size (nm) |
|---|---|---|---|---|---|
| Powder Mixture | Mg hcp | 46 (3) | 3.2102 (3) | 5.2118 (1) | - |
| Ni fcc | 54 (5) | 3.5251 (2) | - | - | |
| HPT N10 γ = 400 | Mg hcp | 44 (3) | 3.2104 (1) | 5.2125 (2) | 163 (7) |
| Ni fcc | 56 (3) | 3.5252 (1) | - | 82 (2) | |
| HPT N50 γ = 2000 | Mg hcp | 42 (2) | 3.2101 (2) | 5.2110 (3) | 168 (2) |
| Ni fcc | 47 (3) | 3.5246 (1) | 80 (2) | ||
| Mg2Ni hcp | 11 (2) | 5.27 (1) | 13.35 (6) | 6 (1) | |
| HPT N100 γ = 4400 | Mg hcp | 18 (2) | 3.2099 (4) | 5.2129 (6) | 70 (3) |
| Ni fcc | 24 (3) | 3.5249 (3) | - | 57 (1) | |
| Mg2Ni hcp | 59 (3) | 5.230 (2) | 13.30 (1) | 6 (1) |
| Condition | Phase | wt% | a (Å) | c (Å) | Crystallite Size (nm) |
|---|---|---|---|---|---|
| HPT N100 | Mg hcp | 34 (2) | 3.2124 (2) | 5.2148 (3) | 156 (11) |
| γ = 0–3100 | Ni fcc | 44 (3) | 3.5267 (1) | - | 89 (2) |
| Upper surface | Mg2Ni hcp | 21 (2) | 5.252 (5) | 13.35 (3) | 6 (3) |
| HPT N100 | Mg hcp | 31 (2) | 3.2108 (2) | 5.2119 (3) | 121 (7) |
| γ = 0–3100 | Ni fcc | 39 (2) | 3.5258 (2) | - | 76 (6) |
| Lower surface | Mg2Ni hcp | 30 (2) | 5.248 (6) | 13.39 (3) | 5 (2) |
| Condition | Phase | wt% | a (Å) | c (Å) | Crystallite Size (nm) |
|---|---|---|---|---|---|
| HPT N10 | Mg hcp | 33 (2) | 3.2110 (2) | 5.2106 (2) | - |
| γ = 400 | Ni fcc | 33 2) | 3.5251 (1) | - | 146 (7) |
| HT 350 °C | Mg2Ni hcp | 34 (2) | 5.2196 (3) | 13.2713 (18) | 85 (5) |
| Sample + Condition | Synthesis Process | SPD Process | Activation | Ref. |
|---|---|---|---|---|
| Mg2Ni/annealing | Casting | - | 2.2 wt%, 20 h | [58] |
| Mg2Ni/annealing + HPT | Casting | HPT, 6 GPa, N10 | 3.3 wt%, 20 h | |
| Mg2Ni/annealing + HPT + annealing | Casting | HPT, 6 GPa, N10 | 3.3 wt%, 20 h | |
| Mg-25 at% Ni/HEBM+ HPT | HEBM | HPT, 2 GPa, N5 | 1.6 wt%, 5 h | [73] |
| Mg-25 at% Ni/HEBM+ CR4 | HEBM 10h | Cold rolling | 2.4 wt%, 0.5 h | [44] |
| Mg-25 at% Ni/HEBM+ CR10 | HEBM 10h | Cold rolling | 2.4 wt%, 1 h | |
| Mg-25 at% Ni/HEBM+ ECAP 2x | HEBM 10h | ECAP | 1.5 wt%, 0.5 h | |
| Mg-25 at% Ni/HEBM+ ECAP 6x | HEBM 10h | ECAP | 1.5 wt%, 0.5 h | |
| Mg-30 at% Ni/HEBM | HEBM 1h | - | 2 wt%, 0.5 h | [74] |
| Mg-30 at% Ni/HEBM + HPT | HEBM 1h | HPT, 6 GPa, N5 | 3 wt%, 1.7 h | |
| Mg-30 at% Ni/HEBM | HEBM 10h | - | 2.4 wt, 0.5 h | |
| Mg-30 at% Ni/HEBM + HPT | HEBM 10h | HPT, 6 GPa, N5 | 3 wt%, 1.7 h | |
| Mg-30 at% Ni/HPT (+60 days in Air) | HPT: 6 GPa, 1 rpm, N= 3–100 | 3.8 wt%, 18 h | This work | |
| Condition | Phase | wt% | a (Å) | c (Å) | Crystallite Size (nm) |
|---|---|---|---|---|---|
| HPT | Ni fcc | 25 (1) | 3.5252 (1) | - | 103 (4) |
| N50, γ = 2000 | MgH2 | 23 (1) | 4.5181 (3) | 3.0224 (3) | 127 (18) |
| Activated | MgO | 22 (2) | 4.218 (3) | - | 5 (1) |
| Mg2NiH4 | 31 (1) | 14.614 (4) | b = 6.415 (2), c= 6.494 (3), beta = 115.7 (1) | 24 (1) | |
| HPT | Ni fcc | 22.5 (9) | 3.5251 (14) | - | 91 (3) |
| N100, γ = 4400 | MgH2 | 17.0 (8) | 4.5184 (3) | 3.0225 (3) | 162 (12) |
| Activated | MgO | 23 (2) | 4.216 (4) | - | 3.2 (3) |
| Mg2NiH4 | 38 (2) | 14.6163 (18) | b = 6.426 (4), c = 6.489 (1), beta = 115.9º (1) | 40 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Gómez, E.I.; Gonzalez, J.; Cubero-Sesin, J.M.; Huot, J. Synthesis of Nanostructured Mg2Ni for Hydrogen Storage by Mechanical Alloying via High-Pressure Torsion. Reactions 2024, 5, 651-663. https://doi.org/10.3390/reactions5040033
López Gómez EI, Gonzalez J, Cubero-Sesin JM, Huot J. Synthesis of Nanostructured Mg2Ni for Hydrogen Storage by Mechanical Alloying via High-Pressure Torsion. Reactions. 2024; 5(4):651-663. https://doi.org/10.3390/reactions5040033
Chicago/Turabian StyleLópez Gómez, Edgar Ignacio, Joaquín Gonzalez, Jorge M. Cubero-Sesin, and Jacques Huot. 2024. "Synthesis of Nanostructured Mg2Ni for Hydrogen Storage by Mechanical Alloying via High-Pressure Torsion" Reactions 5, no. 4: 651-663. https://doi.org/10.3390/reactions5040033
APA StyleLópez Gómez, E. I., Gonzalez, J., Cubero-Sesin, J. M., & Huot, J. (2024). Synthesis of Nanostructured Mg2Ni for Hydrogen Storage by Mechanical Alloying via High-Pressure Torsion. Reactions, 5(4), 651-663. https://doi.org/10.3390/reactions5040033









