The Effect of Temperature and Treatment Regime on the Physical, Chemical, and Biological Properties of Poultry Litter Biochar †
Abstract
1. Introduction
2. Procedure
2.1. Poultry Litter Samples Collection
2.2. Poultry Litter Samples Preparation
2.2.1. Biochar Samples Obtained by Torrefaction
2.2.2. Biochar Samples Obtained by Using a Tube Furnace
2.2.3. Biochar Samples Obtained by Hydrothermal Carbonisation
2.3. Characterisation of Biochar Samples
2.3.1. Total Digestion Method
2.3.2. pH
2.3.3. X-ray Diffraction (XRD)
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Flame Photometry (FP)
2.3.6. Atomic Absorption Spectroscopy (AAS)
2.3.7. Inductively Coupled Plasma–Mass Spectrometry (ICP-MS)
2.3.8. Germination
2.3.9. Thermogravimetric Analysis (TGA)
2.3.10. Statistics and Calculations
3. Results and Discussion
3.1. Prepared Samples and Characterisation Methods
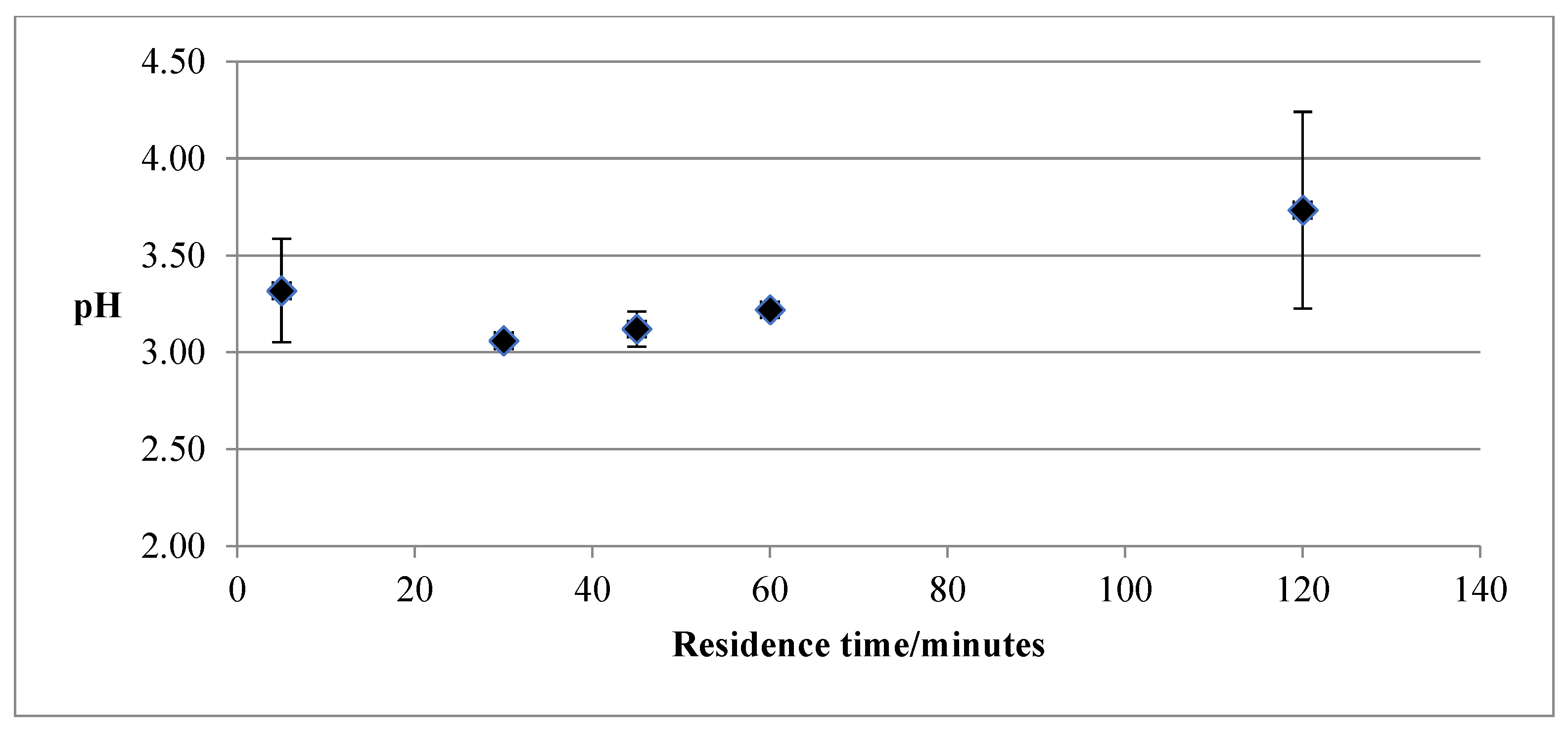
3.2. Effect of Production Method and Temperature on PL Biochars’ pH
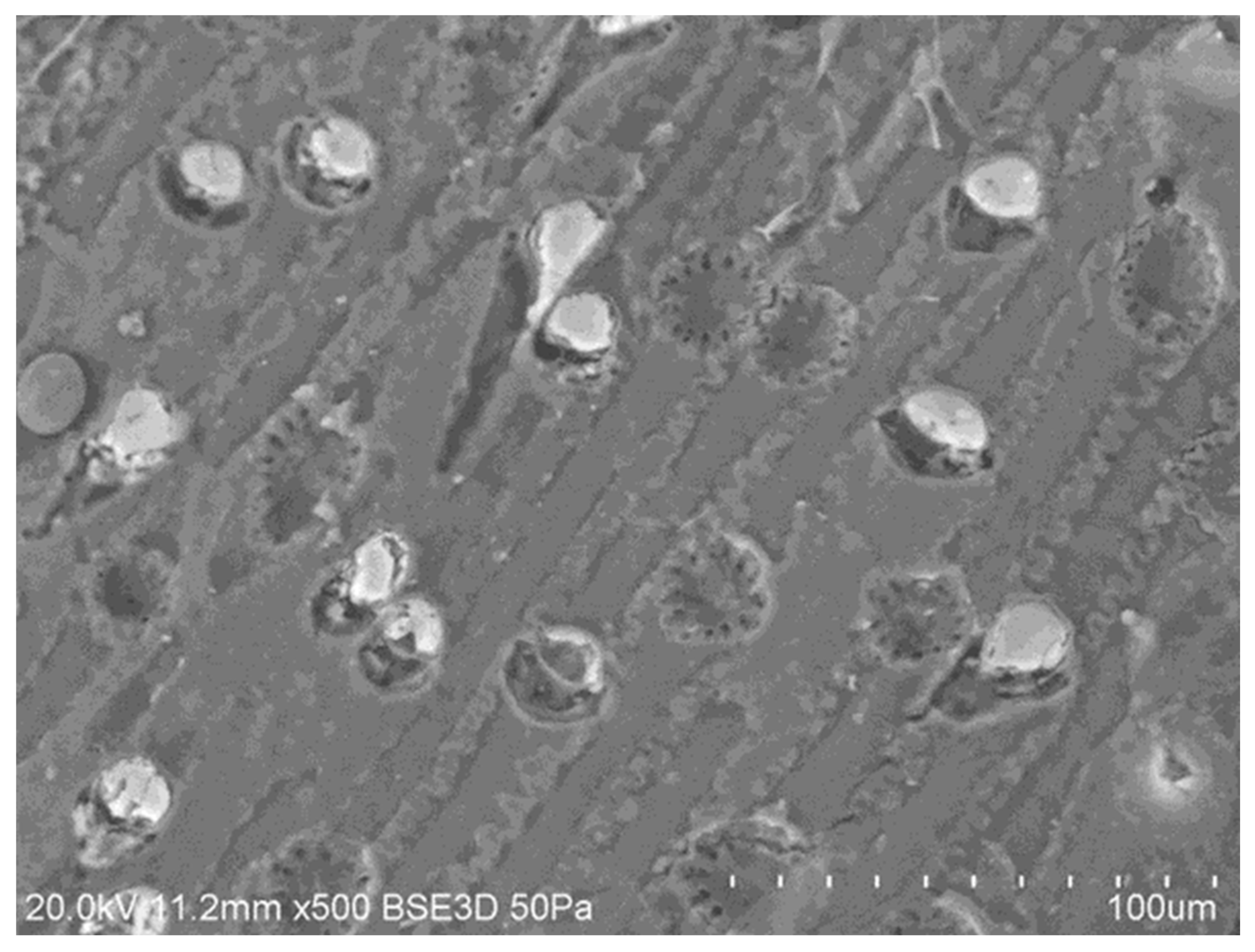
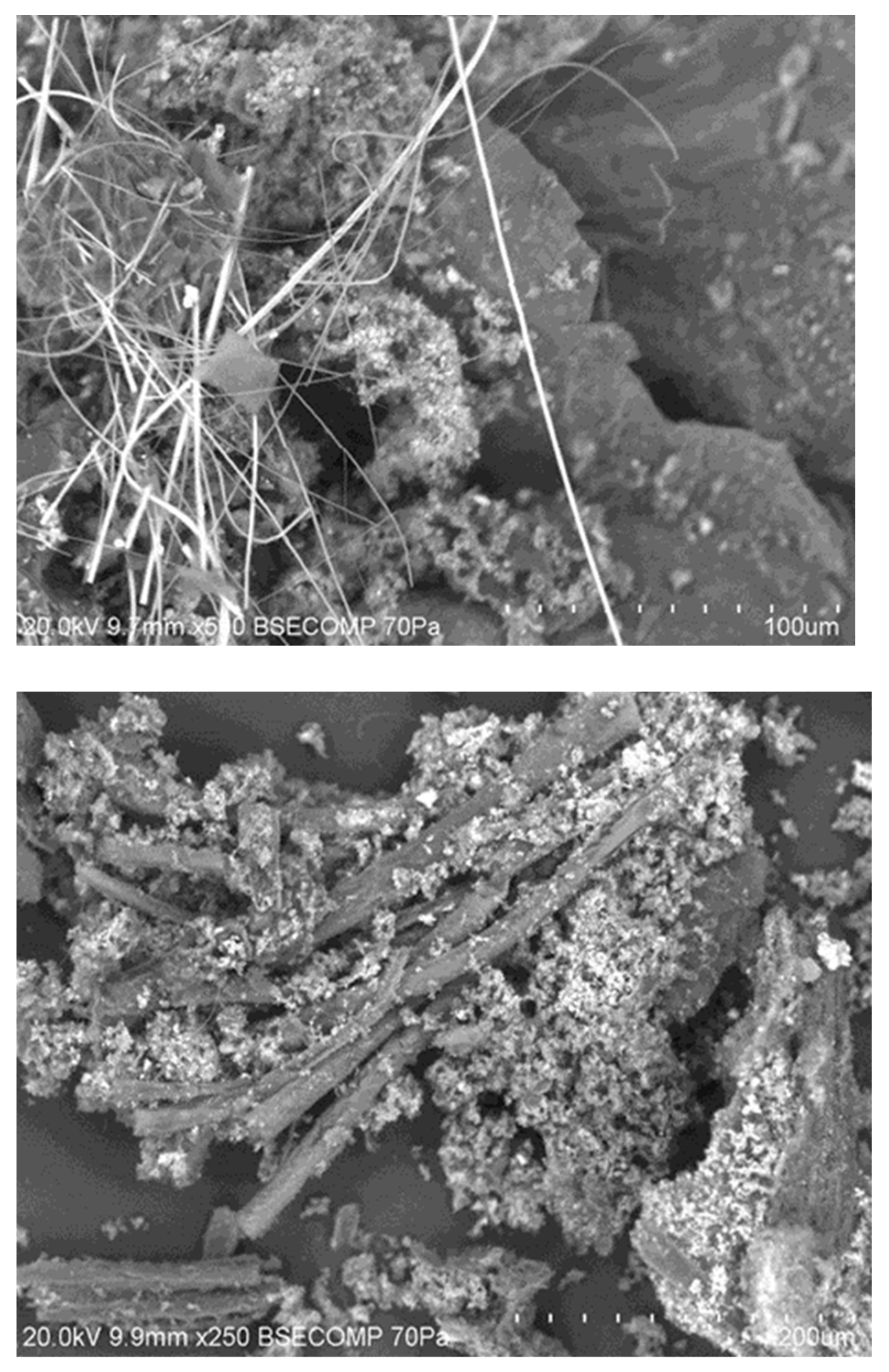
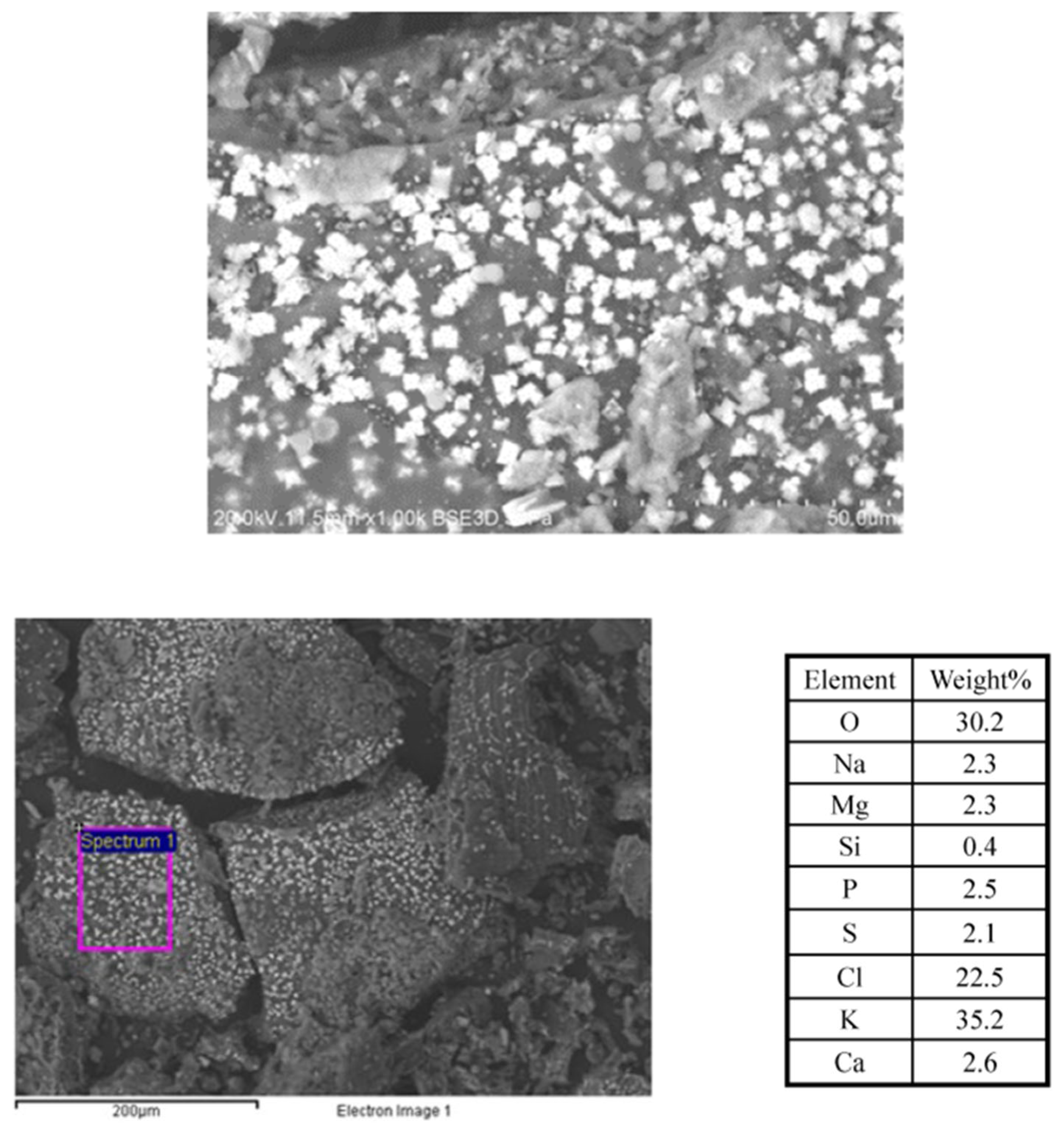
3.3. Effect of Production Method and Temperature on Samples’ Morphology, Composition, and Structure
3.3.1. Amorphous Structures
3.3.2. Crystalline Structures
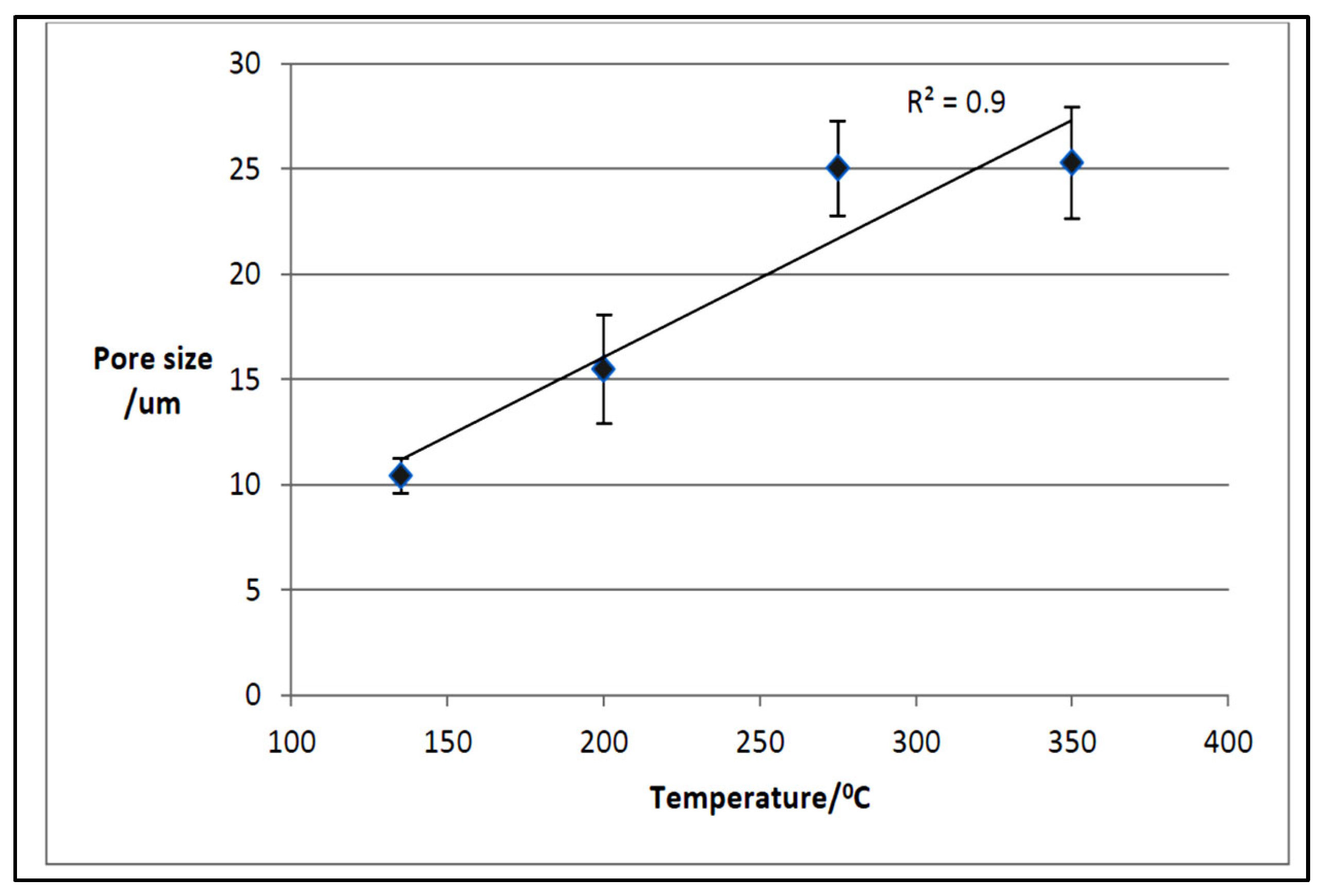
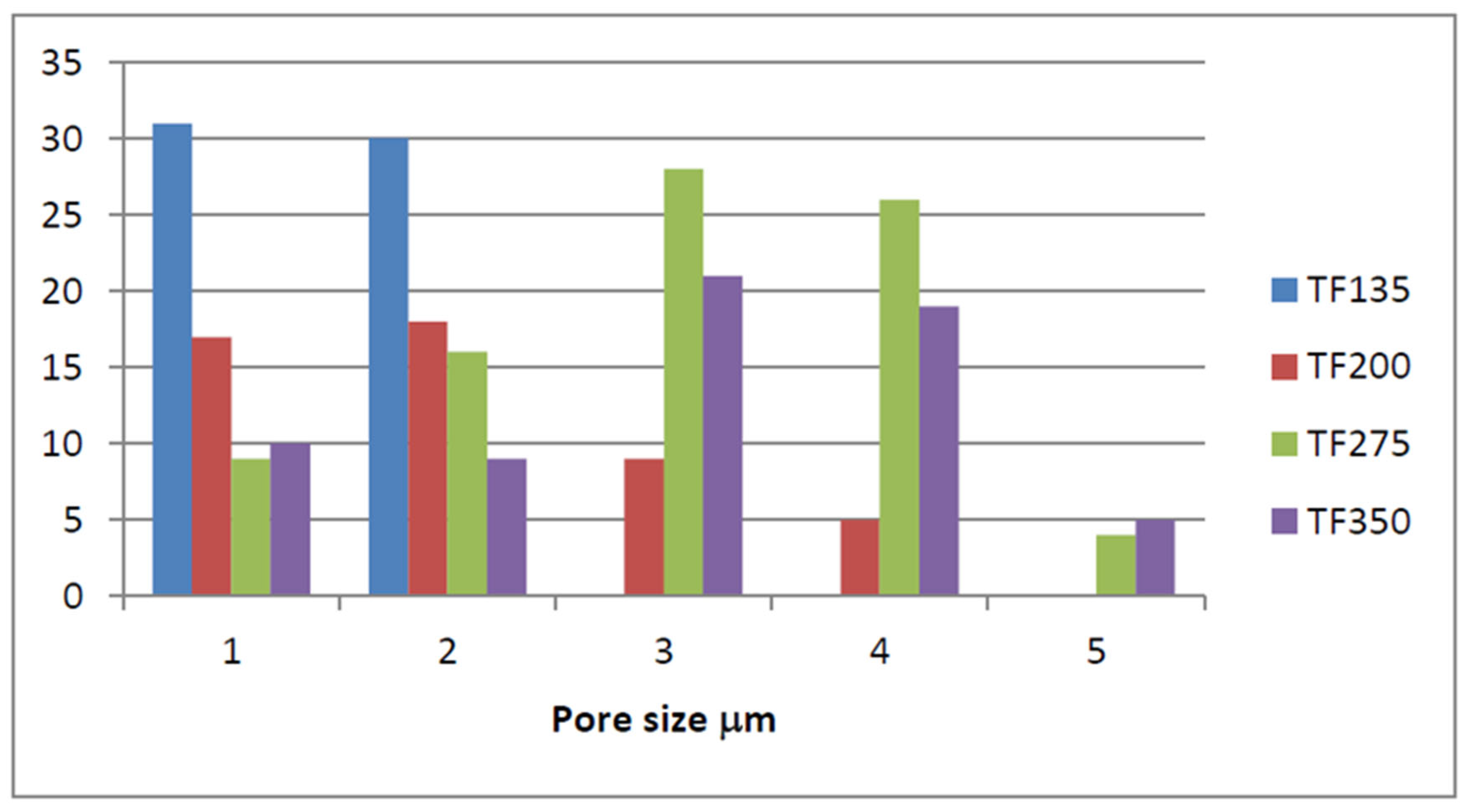
3.3.3. Macroporosity and Elemental Composition (Surface, Subsurface, and Bulk) of Biochar Samples
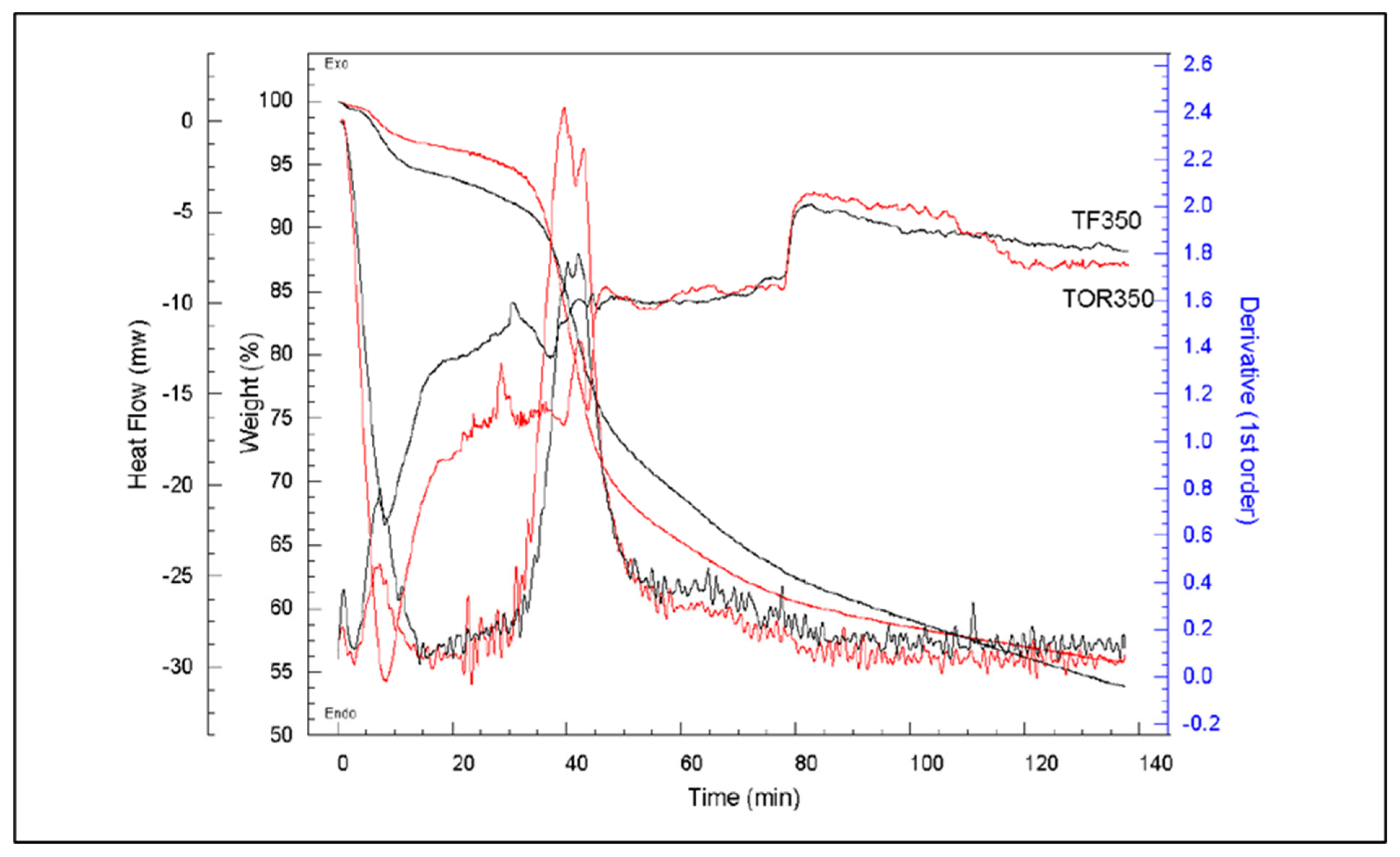
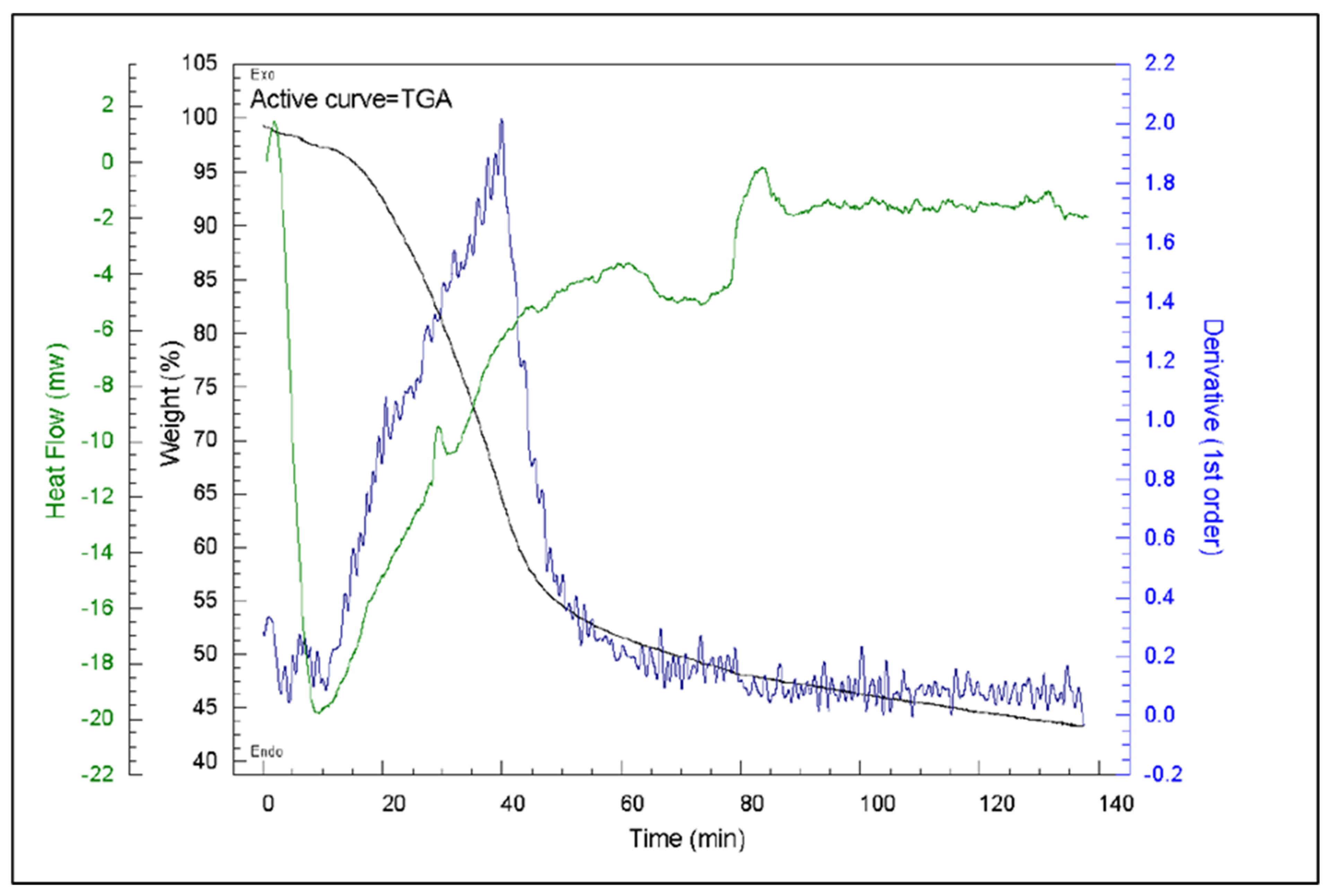
3.4. Effect of Production Method and Temperature on Samples’ Thermal Stability
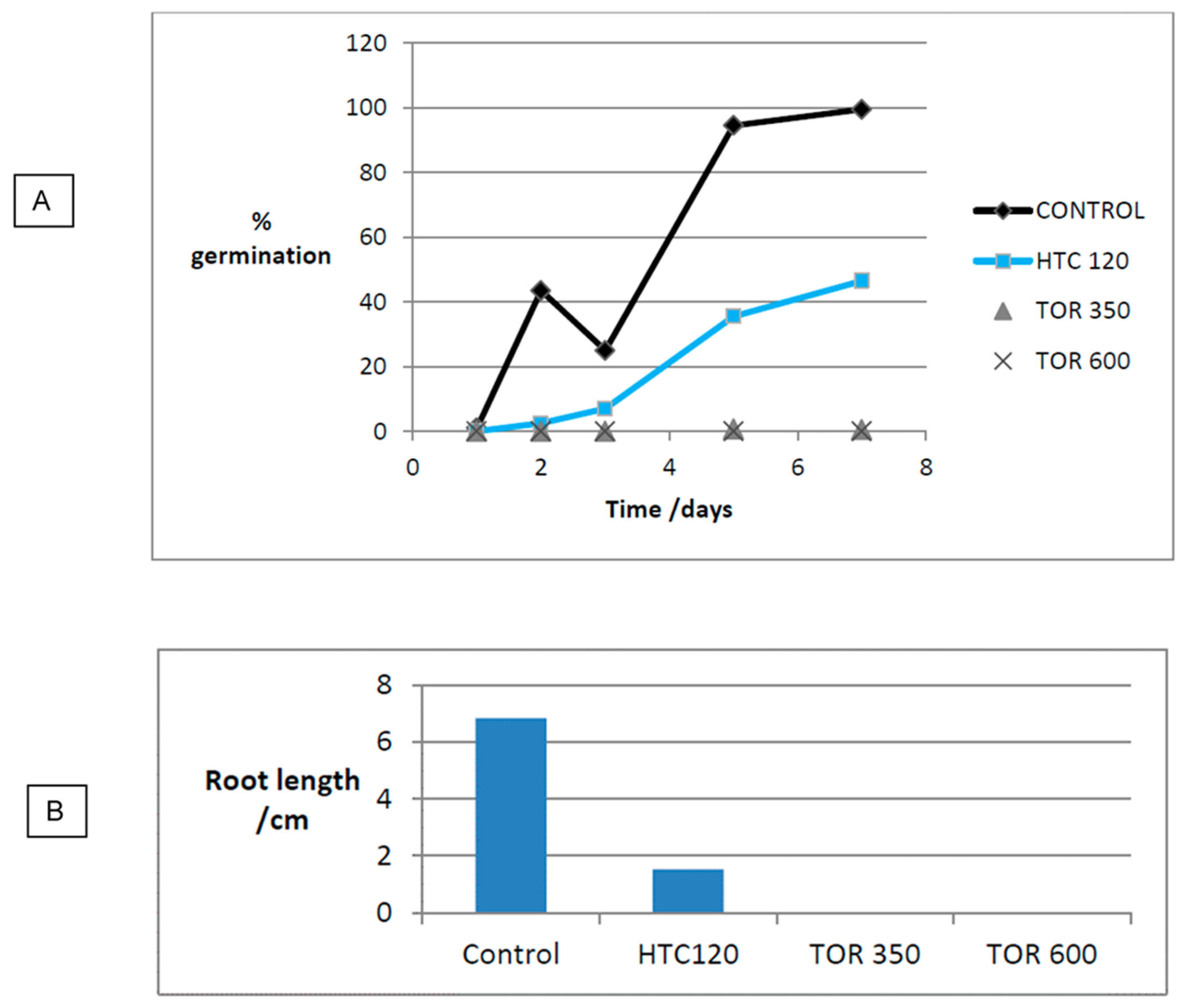
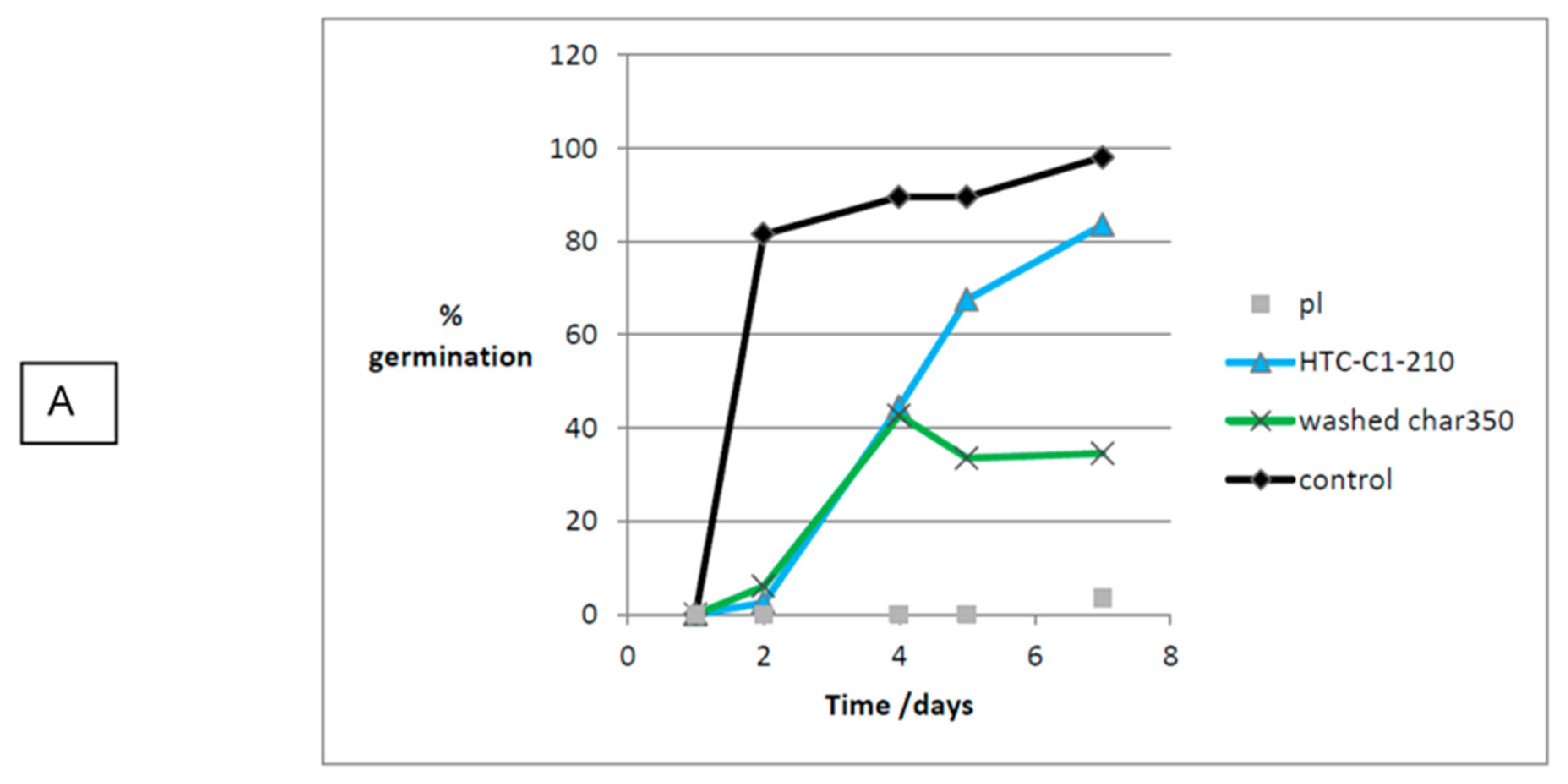
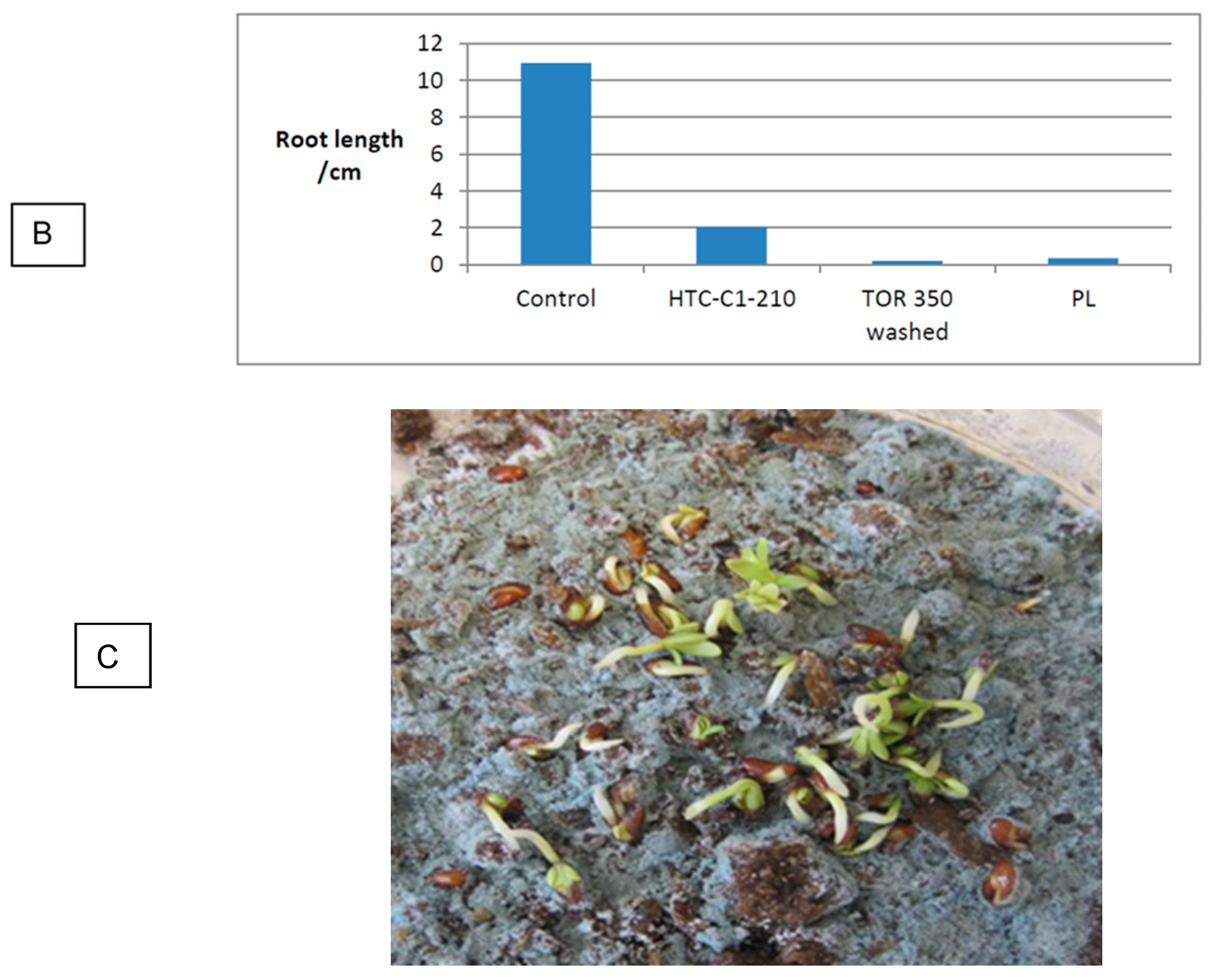
3.5. Effect of Production Method and Temperature on Germination
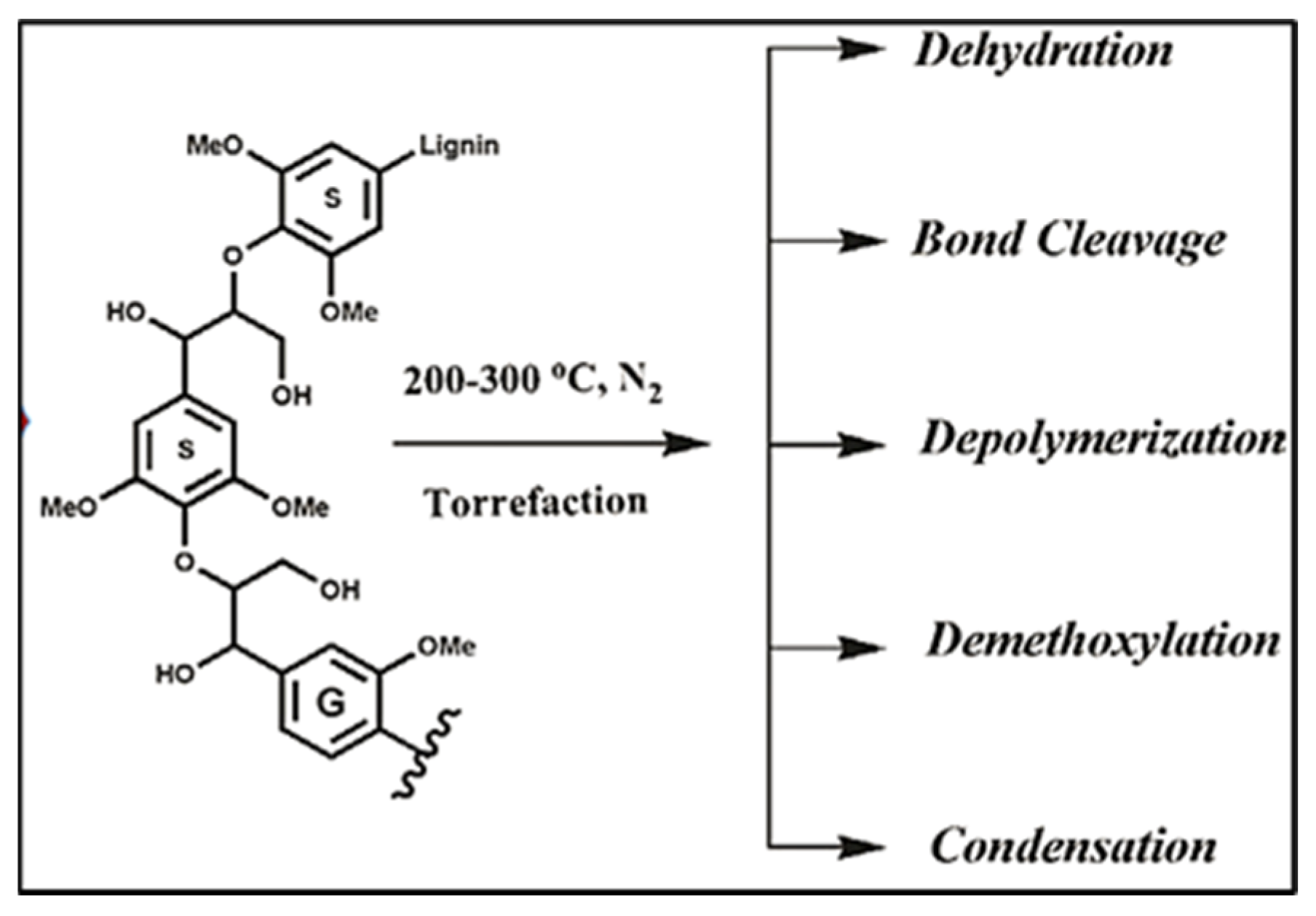
4. Preliminary Kinetic Analysis of Poultry Litter’s Thermochemical Conversion via Torrefaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glaser, B.; Birk, J.J. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de Índio). Geochim. Cosmochim. Acta 2012, 82, 39–51. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management Science and Technology; Routledge: London, UK, 2009. [Google Scholar]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, C.C.; Chagas, J.K.M.; da Silva, J.; Paz-Ferreiro, J. Short-term effects of a sewage sludge biochar amendment on total and available heavy metal content of a tropical soil. Geoderma 2019, 344, 31–39. [Google Scholar] [CrossRef]
- Dhungana, A.; Dutta, A.; Basu, P. Torrefaction of non-lignocellulose biomass waste. Can. J. Chem. Eng. 2012, 90, 186–195. [Google Scholar] [CrossRef]
- Méndez, A.; Terradillos, M.; Gascó, G. Physicochemical and agronomic properties of biochar from sewage sludge pyrolysed at different temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 124–130. [Google Scholar] [CrossRef]
- Novak, J.M.; Johnson, M.G.; Spokas, K.A. Concentration and Release of Phosphorus and Potassium From Lignocellulosic- and Manure-Based Biochars for Fertilizer Reuse. Front. Sustain. Food Syst. 2018, 2, 54. [Google Scholar] [CrossRef]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; van der Velde, M.; Diafas, I. Biochar Application to Soils. 2010. Available online: http://eusoils.jrc.ec.europa.eu/esdb_archive/eusoils_docs/other/eur24099.pdf (accessed on 29 August 2019).
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of Low-Temperature Pyrolysis Conditions on Biochar for Agricultural Use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2011, 94, 138–145. [Google Scholar] [CrossRef]
- van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Shalini, S.S.; Kurian, J. Nitrogen management in landfill leachate: Application of SHASRON, ANAMMOX and combined SHARON_ANAMMOX process. Waste Manag. 2012, 32, 2385–2400. [Google Scholar] [CrossRef]
- Prakongkep, N.; Gilkes, R.; Wiriyakitnateekul, W.; Duangchan, A.; Darunsontaya, T. The Effects of Pyrolysis Conditions on the Chemical and Physical Properties of Rice Husk Biochar. Intern. J. Mater. Sci. 2013, 3, 97–103. [Google Scholar]
- Wang, A.; Zou, D.; Zeng, X.; Chen, B.; Zheng, X.; Li, L.; Zhang, L.; Xiao, Z.; Wang, H. Speciation and environmental risk of heavy metals in biochars produced by pyrolysis of chicken manure and water-washed swine manure. Sci. Rep. 2021, 11, 11994. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Z.; Fu, S.; Méndez, A.; Gascó, G.; Paz-Ferreiro, J. Effect of Biochar in Cadmium Availability and Soil Biological Activity in an Anthrosol Following Acid Rain Deposition and Aging. Water Air Soil Pollut. 2015, 226, 164. [Google Scholar] [CrossRef]
- Liang, C.; Gascó, G.; Fu, S.; Méndez, A.; Paz-Ferreiro, J. Biochar from pruning residues as a soil amendment: Effects of pyrolysis temperature and particle size. Soil Tillage Res. 2015, 164, 3–10. [Google Scholar] [CrossRef]
- Adhikari, S.; Gascó, G.; Méndez, A.; Surapaneni, A.; Jegatheesan, V.; Shah, K.; Paz-Ferreiro, J. Influence of pyrolysis parameters on phosphorus fractions of biosolids derived biochar. Sci. Total. Environ. 2019, 695, 133846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification—A critical review. Sci. Total Environ. 2017, 581–582, 601–611. [Google Scholar] [CrossRef]
- Gascó, G.; Paz-Ferreiro, J.; Álvarez, M.; Saa, A.; Méndez, A. Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag. 2018, 79, 395–403. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Shackley, S.; Sohi, S. An Assessment of the Benefits and Issues Associated with the Application of Biochar to Soil. 2011. Available online: http://www.geos.ed.ac.uk/homes/sshackle/SP0576_final_report.pdf (accessed on 20 August 2019).
- Van Zwieten, L.; Kimber, S.; Morris, S.; Singh, B.; Grace, P.; Scheer, C.; Rust, J.; Downie, A.; Cowie, A. Pyrolysing poultry litter reduces N2O and CO2 fluxes. Sci. Total Environ. 2013, 465, 279–287. [Google Scholar] [CrossRef]
- Gong, P.; Wilke, B.-M.; Strozzi, E.; Fleischmann, S. Evaluation and refinement of a continuous seed germination and early seedling growth test for the use in the ecotoxicological assessment of soils. Chemosphere 2001, 44, 491–500. [Google Scholar] [CrossRef] [PubMed]
- International Biochar Initiative. Technical Bulletin 101. 2010. Available online: http://www.biochar-international.org/sites/default/files/Technical_bulletin_101_english.pdf (accessed on 10 March 2019).
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and Biochar Effects on Germination of Spring Barley. J. Agron. Crop Sci. 2013, 199, 360–373. [Google Scholar] [CrossRef]
- Tiquia, S. Reduction of compost phytotoxicity during the process of decomposition. Chemosphere 2010, 79, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of Pyrolysis Temperature on Biochar Property and Function as a Heavy Metal Sorbent in Soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef]
- Cely, P.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. Agronomic properties of biochars from different manure wastes. J. Anal. Appl. Pyrolysis 2014, 111, 173–182. [Google Scholar] [CrossRef]
- Catalkopru, A.K.; Kantarli, I.C.; Yanik, J. Effects of spent liquor recirculation in hydrothermal carbonization. Bioresour. Technol. 2017, 226, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 89–124. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787–789. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, B.; Chen, L.; Li, Y.; Li, W.; Luo, Z. Characteristics of biochar produced from yak manure at different pyrolysis temperatures and its effects on the yield and growth of highland barley. Chem. Speciat. Bioavailab. 2018, 30, 57–67. [Google Scholar] [CrossRef]
- Jenkins, E. Phytolith taphonomy: A comparison of dry ashing and acid extraction on the breakdown of conjoined phytoliths formed in Triticum durum. J. Archaeol. Sci. 2009, 36, 2402–2407. [Google Scholar] [CrossRef]
- Cennatek. Feasibility of Improving Biomass Combustion by Extraction of Nutrients. 2011. Available online: https://ofa.on.ca/wp-content/uploads/2017/11/Improving_Biomass_Combustion_-utrient_Extraction-2_RAAC.pdf (accessed on 24 September 2019).
- Misra, M.; Kar, P.; Priyadarshan, G.; Licata, C.; Maxim, L.L.C. Keratin Protein Nano-fiber for Removal of Heavy Metals and Contaminants. MRS Proc. 2001, 702, 1–7. [Google Scholar] [CrossRef]
- Kuncaka, A.; Rambe, M.R.; Islam, H.P.; Suherman; Suratman, A. Muslem Preparation and Characterization of Composite from Poly(vinyl chloride) Hydrochar and Hydrolyzate of Keratin from Chicken Feather by Hydrothermal Carbonization. Asian J. Chem. 2021, 33, 2483–2488. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Thygesen, L.G.; Felby, C.; Jorgensen, H.; Elder, T. Cell wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 2008, 1, 5. [Google Scholar] [CrossRef]
- Royal Society of Chemistry. ChemSpider. 2019. Available online: http://www.chemspider.com/Chemical-Structure.4707.html (accessed on 24 September 2019).
- Busch, D.; Stark, A.; Kammann, C.I.; Glaser, B. Genotoxic and phytotoxic risk assessment of fresh and treated hydrochar from hydrothermal carbonization compared to biochar from pyrolysis. Ecotoxicol. Environ. Saf. 2013, 97, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Joseph, S.; Munroe, P.; Lin, Y.; Downie, A.; Hook, J.; Shasha, A.; van Zweiten, L.; Kimber, S.; Cowie, A.L.; Singh, B.P.; et al. Towards an Understanding of the Properties and Structure of High Mineral Ash Biochars. 2008. Available online: http://www.biochar-international.org/images/Joseph_IBI_poster_PMb.pdf (accessed on 14 September 2019).
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Dutrow, B.L.; Clark, C.M. X-ray Powder Diffraction. 2013. Available online: http://serc.carleton.edu/research_education/geochemsheets/techniques/XRD.html (accessed on 4 October 2019).
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Pandey, D.S.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of treatment temperature and residence time on yields and chemical properties of hydrochars. Bioresour. Technol. 2016, 216, 373–380. [Google Scholar] [CrossRef]
- Farrell, M.; Rangott, G.; Krull, E. Difficulties in using soil-based methods to assess plant availability of potentially toxic elements in biochars and their feedstocks. J. Hazard. Mater. 2013, 250–251, 29–36. [Google Scholar] [CrossRef]
- Agblevor, F.A.; Grysko, D.; Revelle, K. Pyrolysis Technology: Environmentally Friendly Solution to Nutrient Management in the Chesapeake Bay. 2010. Available online: http://www.chesapeake.org/stac/presentations/63_agblevor.pdf (accessed on 5 October 2019).
- Carneiro, J.S.d.S.; Filho, J.F.L.; Nardis, B.O.; Ribeiro-Soares, J.; Zinn, Y.L.; Melo, L.C.A. Carbon Stability of Engineered Biochar-Based Phosphate Fertilizers. ACS Sustain. Chem. Eng. 2018, 6, 14203–14212. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, S.-M.; Lee, H.-W.; Lee, J.-W. Physical and chemical characteristics of products from the torrefaction of yellow poplar (Liriodendron tulipifera). Bioresour. Technol. 2012, 116, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Kuo, P.-C. Isothermal torrefaction kinetics of hemicellulose, cellulose, lignin and xylan using thermogravimetric analysis. Energy 2011, 36, 6451–6460. [Google Scholar] [CrossRef]
- Florin, N.; Maddocks, A.; Wood, S.; Harris, A. High-temperature thermal destruction of poultry derived wastes for energy recovery in Australia. Waste Manag. 2009, 29, 1399–1408. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Understanding the chemical and structural transformations of lignin macromolecule during torrefaction. Appl. Energy 2014, 121, 1–9. [Google Scholar] [CrossRef]
- Cai, J.; Bi, L. Precision of the Coats and Redfern Method for the Determination of the Activation Energy without Neglecting the Low-Temperature End of the Temperature Integral. Energy Fuels 2008, 22, 2172–2174. [Google Scholar] [CrossRef]
- Xiao, H.-M.; Ma, X.-Q.; Lai, Z.-Y. Isoconversional kinetic analysis of co-combustion of sewage sludge with straw and coal. Appl. Energy 2009, 86, 1741–1745. [Google Scholar] [CrossRef]
- Zikhali, V.N.; Mpofu, C.; Nyama, D.; Nyoni, B.; Mushonga, K. Kinetic and Thermodynamic Analysis of Chicken Manure Pyrolysis for Sustainable Waste Management in the Poultry Industry. Sch. Int. J. Chem. Mater. Sci. 2023, 6, 135–140. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O. Mobile organic compounds in biochar–A potential source of contamination–Phytotoxic effects on cress seed (Lepidium sativum) germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef] [PubMed]









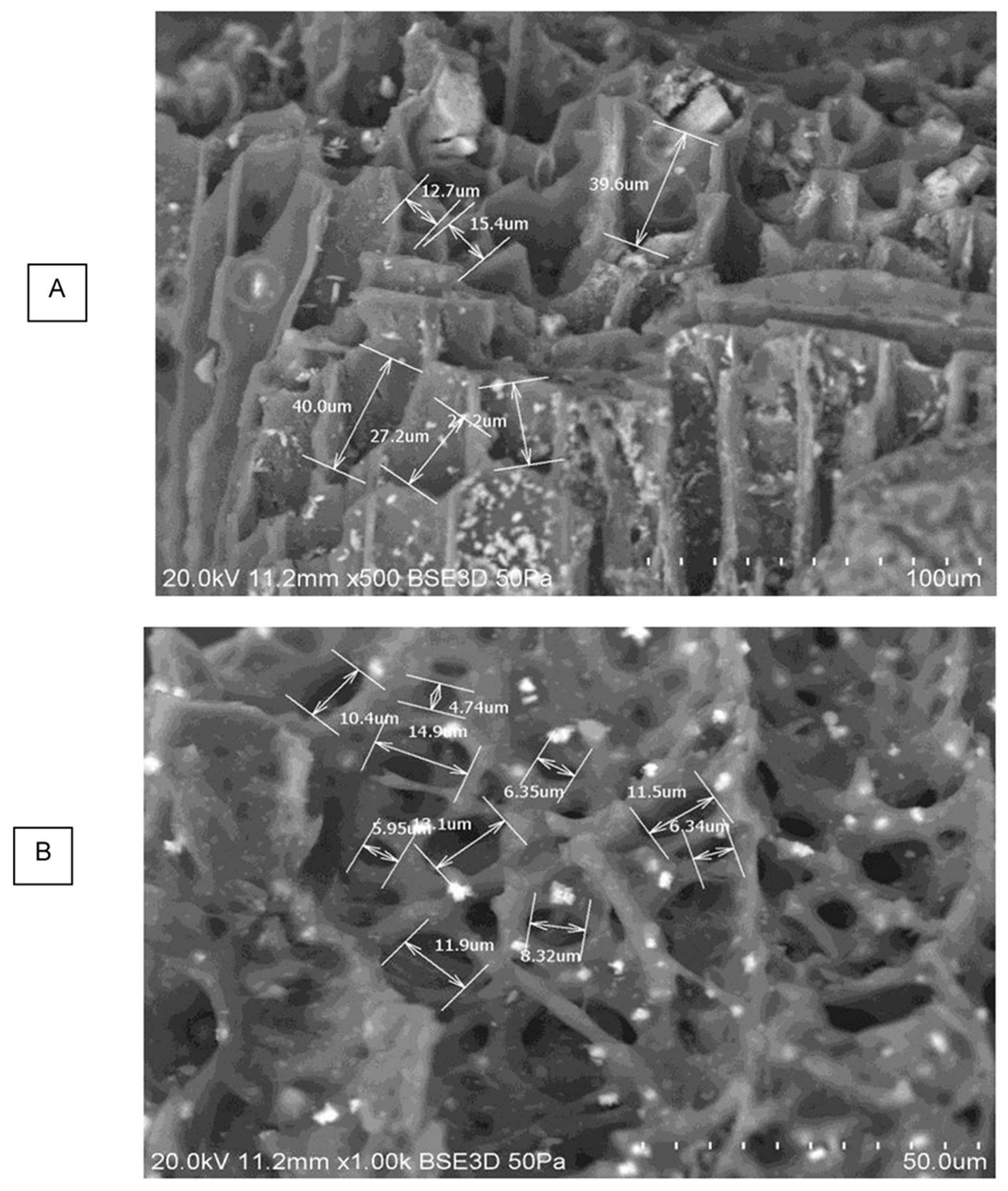
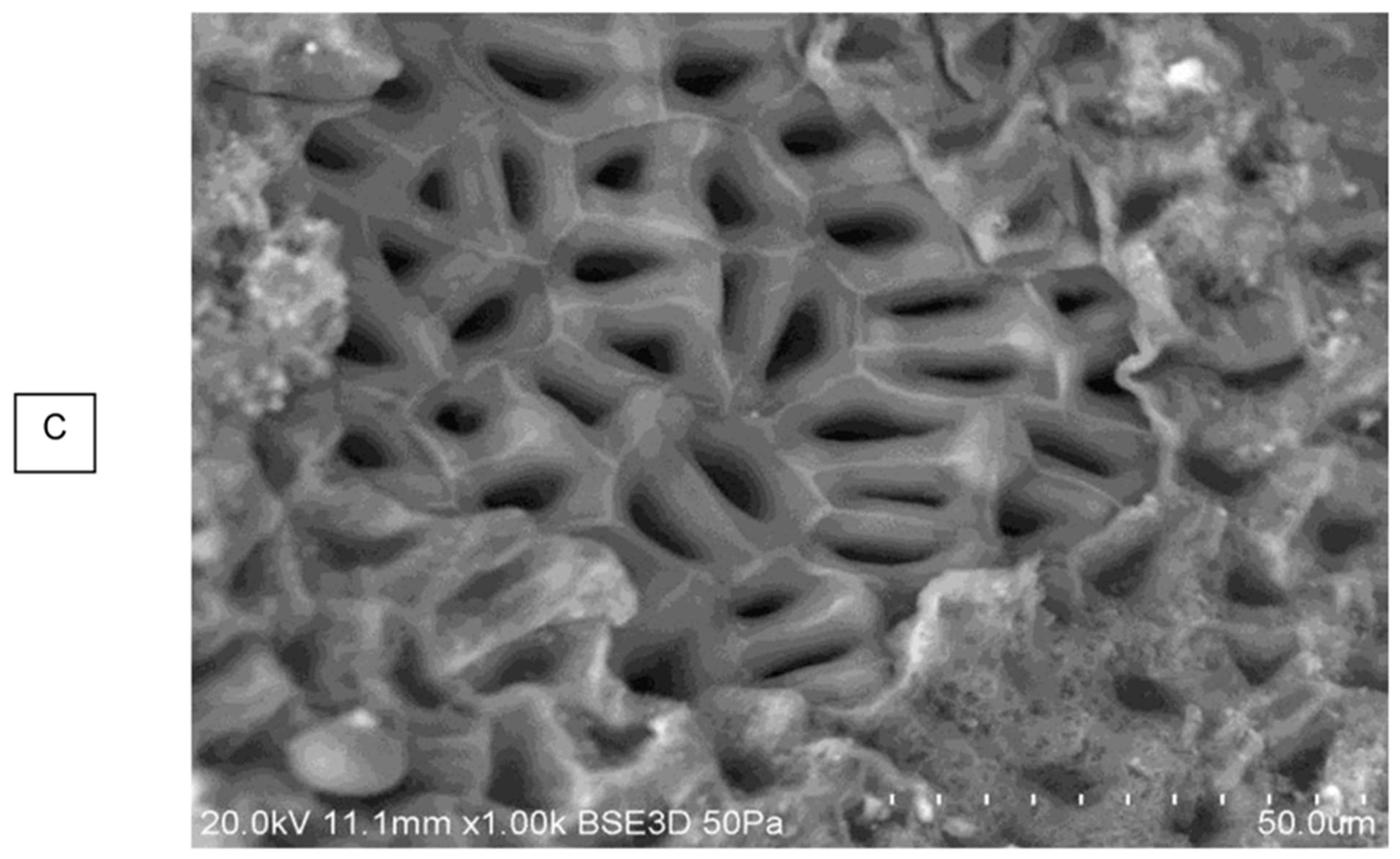
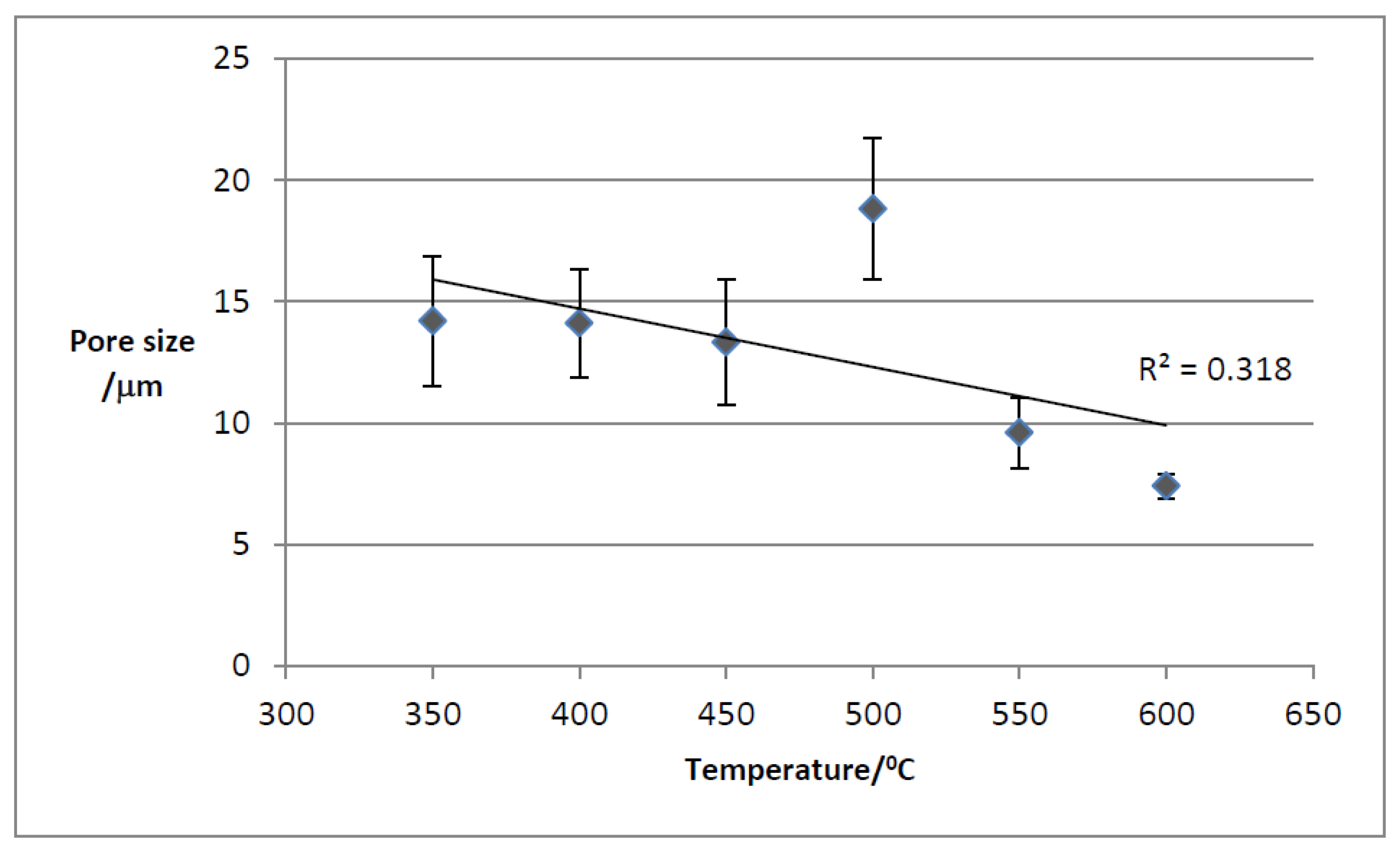
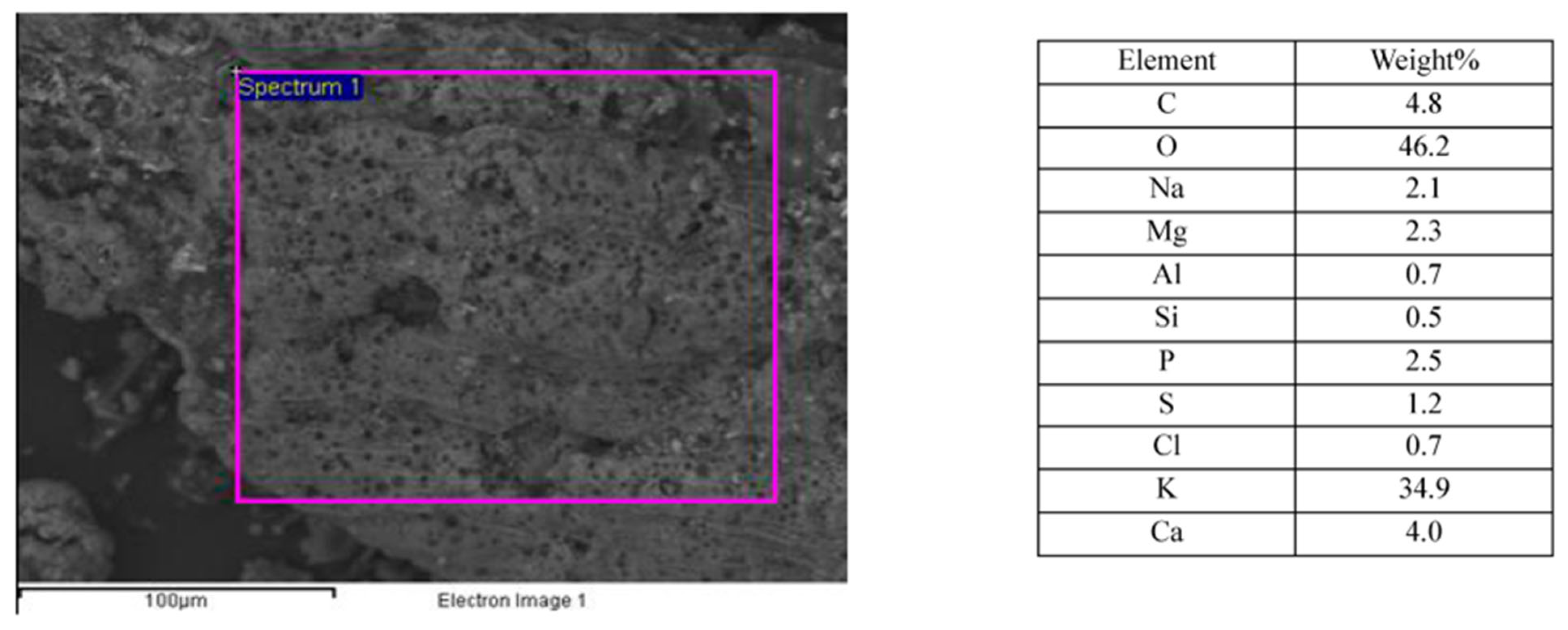




| Sample | pH | ICP-MS | AAS | TGA | Pore Size | EDX | XRD | LOI * and Ash | FP | Yield | Germination |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | ✓✓ | ✓ | ✓ | ||||||||
| TOR 350 | ✓✓ | ✓ | ✓ | ✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓✓ | ✓ | ✓ | |
| TOR 400 | ✓✓✓ | ✓ | ✓✓✓ | ✓✓✓ | |||||||
| TOR 450 | ✓✓ | ✓ | ✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓✓✓✓ | ||||
| TOR 500 | ✓ | ✓✓✓ | ✓✓✓ | ✓ | |||||||
| TOR 550 | ✓✓ | ✓ | ✓✓✓ | ✓✓✓ | ✓✓ | ✓ | |||||
| TOR 600 | ✓✓ | ✓ | ✓ | ✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓✓ | ✓ | ✓ | |
| TF 135 | ✓✓✓ | ✓✓✓ | ✓ | ||||||||
| TF 200 | ✓✓ | ✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓ | ✓ | ||||
| TF 275 | ✓✓ | ✓ | ✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓ | ✓ | |||
| TF 350 | ✓ | ✓ | ✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓ | ||||
| TF 400 | ✓ | ||||||||||
| TF 450 | ✓ | ✓ | |||||||||
| TF 500 | ✓ | ✓ | |||||||||
| HTC 80 | ✓ | ✓ | ✓✓✓ | ✓ | ✓ | ||||||
| HTC 95 | ✓ | ✓ | ✓✓✓ | ✓ | ✓ | ||||||
| HTC 120 | ✓ | ✓ | ✓✓✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| HTC-C1-210 | ✓ | ✓ | ✓ | ||||||||
| HTC 250 | ✓ | ✓ | ✓ | ✓ |
| Sample | Pore Size | EDX | Structures and Crystals |
|---|---|---|---|
| TOR 350 strip | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| TOR 600 strip | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| TOR 350 ash | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| TOR 400 ash | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| TOR 450 ash | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| TOR 500 ash | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| TOR 550 ash | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| TOR 600 ash | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| TOR 550 ash strip | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| TOR 600 ash strip | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| Feather | ✓✓✓ | ✓✓✓ | |
| Wheat | ✓✓✓ | ✓✓✓ | |
| Barley | ✓✓✓ | ✓✓✓ | |
| Egg shell | ✓✓✓ | ✓✓✓ | ✓✓✓ |
| KCl crystals | ✓✓✓ | ✓✓✓ |
| TOR 350 | TOR 450 | TOR 550 | ||||
|---|---|---|---|---|---|---|
| 2θ | Intensity (Counts) | 2θ | Intensity (Counts) | 2θ | Intensity (Counts) | Identification |
| 28.6 | 514 | 28.44 | 390 | 28.44 | 504 | Sylvite |
| 29.6 | 266 | 29.48 | 278 | Calcite | ||
| 30.46 | 178 | 30.92 | 244 | Pyrocoproite (K2MgP2O7) | ||
| 36.46 | 178 | Quartz (SiO2) | ||||
| 40.74 | 290 | 40.64 | 239 | 40.62 | 291 | Sylvite |
| 43.4 | 148 | Quartz (SiO2) | ||||
| 50.52 | 142 | 50.28 | 137 | Sylvite | ||
| 58.7 | 96 | Sylvite | ||||
| 60.56 | 110 | 59.86 | 84 | Quartz (SiO2) | ||
| 66.34 | 93 | Sylvite | ||||
| 73.84 | 98 | 73.74 | 90 | Sylvite (Anon, 2008) | ||
| 83.56 | 70 | Unidentified | ||||
| 86.62 | 74 | Unidentified | ||||
| Element | Mean (Weight %) | |||
|---|---|---|---|---|
| Production Temperature (°C) | ||||
| 135 | 200 | 275 | 350 | |
| C | 48.3 | 44.2 | 48.4 | 48.9 |
| O | 42.4 | 44.3 | 35.5 | 31.4 |
| Na | 0.4 | 0.5 | 0.7 | 0.8 |
| Mg | 0.8 | 1.1 | 1.4 | 1.8 |
| P | 1.2 | 1.6 | 2.0 | 2.6 |
| S | 0.8 | 0.6 | 0.9 | 0.9 |
| Cl | 0.7 | 0.8 | 1.4 | 1.4 |
| K | 3.8 | 4.5 | 6.8 | 9.0 |
| Ca | 1.3 | 2.0 | 2.5 | 2.9 |
| Si | 0.2 | 0.3 | 0.3 | 0.3 |
| Mn | ND | ND | ND | 0.1 |
| Element | Mean (Weight %) | ||
|---|---|---|---|
| Production Temperature (°C) | |||
| 80 | 120 | 221 | |
| C | 58.2 | 49.8 | 51.9 |
| O | 39.7 | 46.0 | 44.3 |
| Na | 0.0 | 0.0 | 0.0 |
| Mg | 0.1 | 0.2 | 0.2 |
| P | 0.3 | 0.4 | 0.4 |
| S | 0.4 | 0.6 | 0.5 |
| Cl | 0.1 | 0.2 | ND |
| K | 0.4 | 1.0 | 0.9 |
| Ca | 0.4 | 1.8 | 1.4 |
| Si | 0.1 | 0.3 | 0.2 |
| Fe | ND | ND | ND |
| Mn | ND | ND | 0.1 |
| Y | ND | ND | ND |
| Cu | ND | ND | ND |
| Al | ND | ND | ND |
| Br | 0.1 | ND | ND |
| Chars | Concentration Minus Blank Sample’s Concentration (ppb) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | K | Ca | Mn | Cu * | Zn * | Sr | Ba | |
| TOR 350 | 61.3 | 95.0 | 3.1 | 20.5 | 106.6 | 7.0 | 1.6 | 7.9 | 0.4 | 0.2 |
| TOR 450 | 24.3 | 131.8 | 4.9 | 0.0 | 157.5 | 9.6 | 2.7 | 6.5 | 0.7 | 0.5 |
| TOR 550 | 69.5 | 146.0 | 4.4 | 0.0 | 155.5 | 12.1 | 1.3 | 5.2 | 0.7 | 0.5 |
| TOR 600 | 77.9 | 138.5 | 3.8 | 0.0 | 177.9 | 10.6 | 0.9 | 3.1 | 0.7 | 0.5 |
| TF 200 | 0.0 | 45.5 | 0.0 | 57.4 | 0.0 | 3.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| TF 275 | 33.1 | 98.1 | 0.0 | 71.5 | 121.0 | 8.0 | 0.6 | 3.0 | 0.3 | 0.2 |
| TF 350 | 44.3 | 136.2 | 2.9 | 0.0 | 129.0 | 10.3 | 1.6 | 4.7 | 0.6 | 0.3 |
| PL | - | 83.0 | 4.3 | - | - | 4.4 | 1.8 | 9.0 | 0.9 | 0.6 |
| Sample | Weight Remaining at 800 °C (Weight %) | Weight Remaining after 60 Min Run at 800 °C (Weight %) | Loss Over 60 Min Residence Time at 800 °C (Weight %) | Residual Ash (Weight %) after 60 Min Run at 800 °C | Loss of Volatiles at 400 °C (Weight %) |
|---|---|---|---|---|---|
| PL | 26.0 | 22.0 | 4.0 | 22.0 | 55.0 |
| TOR 350 | 62.0 | 56.0 | 6.0 | 56.0 | 10.0 |
| TOR 450 | 76.0 | 69.0 | 7.0 | 69.0 | 8.0 |
| TOR 600 | 87.5 | 82.5 | 5.0 | 82.5 | 8.0 |
| TF 275 | 45.0 | 37.5 | 7.5 | 37.5 | 30.0 |
| TF 350 | 64.0 | 55.0 | 9.0 | 55.0 | 10.0 |
| HTC 216 | 50.0 | 37.5 | 7.5 | 37.5 | 28.0 |
| Substrate | Germination (%) | Germination (% of Control) | Mean Root Length (cm) | Mean Root Length (% of Control) | GI |
|---|---|---|---|---|---|
| Control 1 | 99.5 | 6.8 | |||
| Poultry litter | 3.5 | 3.5 | 0.3 | 3.2 | 0.1 |
| TOR 350 | 0.5 | 0.1 | 0 | 7.3 | 0.01 |
| TOR 600 | 0 | 0 | 0 | 0 | 0 |
| Control 2 | 98.0 | 11.0 | |||
| Washed TOR 350 | 34.5 | 32.2 | 0.2 | 1.8 | 0.6 |
| HTC 120 | 46.5 | 46.7 | 1.5 | 22.4 | 10.5 |
| HTC-C1-210 | 83.5 | 85.2 | 0.3 | 18.6 | 15.8 |
| Extract | |||||
| KCl | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Washed TOR 350 | 99.3 | 101.4 | 3.7 | 33.8 | 34.3 |
| HTC 250 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| HTC 87 | 89.0 | 90.8 | 0.3 | 2.6 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, J.; Olea, M. The Effect of Temperature and Treatment Regime on the Physical, Chemical, and Biological Properties of Poultry Litter Biochar. Reactions 2024, 5, 379-418. https://doi.org/10.3390/reactions5030020
Clarke J, Olea M. The Effect of Temperature and Treatment Regime on the Physical, Chemical, and Biological Properties of Poultry Litter Biochar. Reactions. 2024; 5(3):379-418. https://doi.org/10.3390/reactions5030020
Chicago/Turabian StyleClarke, Joyce, and Maria Olea. 2024. "The Effect of Temperature and Treatment Regime on the Physical, Chemical, and Biological Properties of Poultry Litter Biochar" Reactions 5, no. 3: 379-418. https://doi.org/10.3390/reactions5030020
APA StyleClarke, J., & Olea, M. (2024). The Effect of Temperature and Treatment Regime on the Physical, Chemical, and Biological Properties of Poultry Litter Biochar. Reactions, 5(3), 379-418. https://doi.org/10.3390/reactions5030020







