Abstract
The integration of emulsion gels in 3D food printing has emerged as a promising strategy to enhance both the structural fidelity and functional performance of printed foods. Emulsion gels, composed of proteins, polysaccharides, lipids, and their complexes, can provide tunable rheological and mechanical properties suitable for extrusion and shape retention. This review explores the formulation strategies, including phase behavior (O/W, W/O, and double emulsions); stabilization methods; and post-printing treatments, such as enzymatic, ionic, and thermal crosslinking. Advanced techniques, including ultrasound and high-pressure homogenization, are highlighted for improving gel network formation and retention of active compounds. Functional applications are addressed, with a focus on meat analogs, bioactive delivery systems, and personalized nutrition. Furthermore, the role of the oil content, interfacial engineering, and protein–polysaccharide interactions in improving print precision and post-processing performance is emphasized. Despite notable advances, challenges remain in scalability, regulatory compliance, and optimization of print parameters. The integration of artificial intelligence can also provide promising advances for smart design, predictive modeling, and automation of the 3D food printing workflow.
1. Introduction
Three-dimensional food printing is an emerging digital technology that enables the creation of complex food structures using layer-by-layer fabrication, also known as additive manufacturing. Three-dimensional printing can be performed with precise control over geometry, texture, and composition, enabling the design of foods that would otherwise be difficult or impossible to produce through conventional methods. Three-dimensional printing can provide several advantages, such as customized shape design, nutritional personalization, and the integration of bioactive compounds [1,2,3]. Three-dimensional printing can be used to fabricate biomimetic scaffolds for cell cultivation. These structures provide a supportive environment for lab-grown meat, facilitating organized cell growth and realistic texture development [4,5,6]. Three-dimensional printing can also be applied to formulate meat analogs using plant-based proteins, enabling control over texture, shape, and nutrient composition [7,8,9]. Moreover, it provides a platform for the encapsulation of bioactives, protecting them from oxidation or degradation during digestion, which is important for functional foods [10,11]. Three-dimensional printing facilitates the incorporation of distinct food-grade components, including proteins, fats, fibers, and micronutrients, into balanced formulations tailored to individual needs. Therefore, it is possible to create personalized diets for specific groups, such as pregnant women, athletes, children, and the elderly. Furthermore, it supports dietary interventions in clinical contexts, such as producing texture-modified foods for dysphagic patients, enhancing both nutritional value and ease of swallowing [12,13,14,15].
Regarding sustainability and resource efficiency, 3D food printing also provides advantages. Traditional food manufacturing often involves multiple preprocessing and post-processing steps, such as cutting, shaping, and molding, which generate material waste and demand considerable time and labor. Nevertheless, 3D printing can consolidate these operations into a single, streamlined process, reducing both processing time and food waste. This efficiency opens pathways to lowering production costs and minimizing the environmental impact. Three-dimensional food printing aligns with the emerging field of digital food science, which integrates artificial intelligence (AI) and other digital tools into food design, preparation, and consumer interaction. These technologies not only enhance creativity and customization but also support the use of alternative, sustainable ingredients, such as plant-based proteins, to reduce dependency on resource-intensive animal products. Such innovations are relevant to addressing global challenges, including hunger, food waste, and nutrition-related diseases, reinforcing the relevance of this technology in modern food systems [16,17,18].
Emulsion gels encompass soft-solid systems formed by dispersing emulsion droplets within a continuous gel matrix. Their hybrid structure combines the stabilizing and delivery benefits of emulsions with the mechanical integrity and viscoelasticity of gels. These systems exhibit rheological, thermal, and structural properties, which can be tailored by adjusting the droplet phase, gel network, or interfacial interactions. One of the most promising applications of emulsion gels in food science is their use as fat replacers. Traditional solid or semi-solid fats, commonly derived from vegetable oils (e.g., palm and coconut) or animal fats, often contain high levels of saturated and trans fatty acids. These lipids are associated with increased risks of cardiovascular and metabolic diseases. Emulsion gels provide a potential solution by mimicking the texture and rheology of solid fats while incorporating healthier lipids, such as polyunsaturated fatty acids [19,20,21,22]. In addition, emulsion gels can act as delivery systems for poorly water-soluble compounds, improving their stability, bioavailability, and controlled release in the gastrointestinal tract. Indeed, amphiphilic biopolymers, such as proteins and polysaccharides, are employed to stabilize the droplet interface and prevent phase separation. These biopolymers are renewable, biodegradable, and nutritionally relevant for the development of sustainable food-grade emulsion gels [23,24].
The rheological and structural properties of emulsion gels are promising for extrusion-based 3D food printing. Their viscoelastic behavior allows them to behave as soft solids at rest while still being deformable under shear, which is an important requirement for printable food materials. During the 3D printing process, three sequential stages, extrusion, recovery, and self-support, are linked to the rheological profile of the printing emulsion gel. In the extrusion stage, the material must exhibit shear-thinning behavior and have appropriate yield stress and viscosity to ensure smooth flow through the nozzle without clogging. In the recovery stage, after extrusion, the material must rapidly recover its mechanical strength, requiring good thixotropy (recovery of viscosity or structure after shear). In the shape retention stage, to maintain the printed structure, the material must have a sufficiently high elastic modulus (G′) to withstand its own weight and maintain layer fidelity. For instance, emulsion gels can be designed to result in the structural and functional properties of 3D printing by adjusting the emulsion type (O/W, W/O, or double emulsions); tuning droplet size; and interfacial stability; selecting appropriate gel matrices (proteins and polysaccharides); and modifying colloidal interactions to balance flowability and rigidity [25,26,27].
Therefore, this review explores the role of the physicochemical properties of emulsion gels in 3D food printing. Special emphasis is placed on rheological behavior, droplet size, interfacial interactions, gelation mechanisms, and matrix design strategies to tailor mechanical stability. In addition, post-printing stabilization approaches, such as enzymatic and ionic crosslinking, thermal setting, cooling-induced gelation, and physical approaches, are discussed. Finally, the review highlights the functional applications of emulsion gels in 3D-printed foods, including their use in meat analogs, bioactive delivery systems, and personalized nutrition, while addressing both current opportunities and the technical challenges associated with their development.
2. Formulation of Emulsion Gels for Printability
Three-dimensional food printing has gained increasing attention within the food industry due to its potential for innovation and its ability to enable the production of personalized foods, not only in terms of shape and texture but also nutritional composition [28]. The materials used in these formulations must exhibit specific characteristics, particularly suitable rheological properties that allow smooth extrusion and rapid structural recovery after deposition. In this context, emulsion gels have emerged as one of the primary matrices used in the formulation of food-grade inks for 3D printing, owing to their structural versatility; physical stability; and capacity to incorporate targeted ingredients, such as bioactive compounds, probiotics, essential oils, hydrolyzed proteins, and other functional additives [29,30,31].
The structural stability of emulsion gels can be attributed to the presence of proteins, polysaccharides, lipids, or protein–polysaccharide conjugates, which play an important role in the formation and maintenance of the gelled matrix’s three-dimensional architecture [23,32,33]. These compounds act as interfacial tension reducers, contribute to high hydrophobicity, serve as natural emulsifiers, and may effectively prevent structural collapse of the gel [23].
For an emulsion gel to be effective in 3D food printing, it must exhibit specific rheological properties, such as yield stress, shear-thinning behavior, and dominant viscoelasticity. These characteristics are directly related to the material’s ability to flow under pressure, as well as to its capacity to retain shape after deposition [23,34,35]. To ensure successful printing, the “ink” must display an adaptive rheological profile, exhibiting low viscosity during extrusion to facilitate smooth flow through the nozzle and recovering higher viscosity upon deposition to ensure structural integrity and shape retention. This dynamic behavior requires the presence of a minimum yield stress to support the stacking of successive layers and prevent collapse [36]. For example, natural matrices, such as vegetable pastes, fruit pulps, and minced meat, are often challenging to print and must be supplemented with functional additives, as they do not inherently exhibit these rheological traits [37].
Table 1 presents examples of natural ingredients used in the formulation of food-grade “inks” for 3D printing, highlighting their technological roles and specific applications. As shown in Table 1, polysaccharides, oils, and proteins are the main components responsible for providing viscosity, elasticity, and gel-forming capability. For instance, Li et al. [38] reported that the combination of biopolymers, specifically proteins and polysaccharides, improved both the viscosity and structural recovery of the emulsion after extrusion, thus achieving an efficient shear-thinning behavior.
Beyond conventional protein- and polysaccharide-based systems, Chao et al. [39], described the development of bigels composed of beeswax-based oleogels and gellan gum hydrogels. The bicontinuous structure of these bigels exhibited ideal printing properties, including thixotropy, appropriate yield stress, and modulation of homogeneous water distribution. Innovative formulations, such as those proposed by Tsai et al. [40], Ma et al. [41], and Kuo et al. [42], further underscore the importance of synergistic interactions between proteins and polysaccharides as fundamental to achieving suitable rheological properties for 3D food printing.

Table 1.
Overview of natural compounds applied in 3D food printing inks.
Table 1.
Overview of natural compounds applied in 3D food printing inks.
| Natural Ingredients | Technological Functions | 3D Printing Applications | Printing Parameters | Ref. |
|---|---|---|---|---|
| Walnut protein/κ-carrageenan | Emulsion stabilization, improved viscosity, and enhanced gelation. | Printable emulsion gels with structural integrity and dietary relevance. | Extrude speed: 5 mm/s; Print speed: 8–15 mm/s; Nozzle diameter: 2–10 mm; | [43] |
| Gelatin/Alginate | Hydrogel formation, biocompatibility, and controlled delivery. | Edible hydrogel scaffolds for personalized nutrition and bioactive compound delivery. | Nozzle diameter: 0.636 mm; Printing speed: 8 mm/s; | [42] |
| Egg albumen/Pea protein, Gellan gum/Sodium alginate/Rice mill | Texturization, nutritional enhancement, and mechanical integrity. | Fabrication of artificial meat (steak-like structure) with plant-animal blend. | Nozzle diameter: 0.636 mm; Printing speed: 8 mm/s; | [40] |
| Cellulose/Methoxyl pectin | Shear-thinning, anisotropic alignment, enhanced print fidelity. | Bio-based inks for precision 3D printing with tunable morphology and mechanical properties. | Nozzle diameter: 0.5 mm; Extrusion speed: 25 mm/s; | [41] |
| Beeswax/Low-acyl gellan gum | Thixotropy, bicontinuous gel networks, and moisture control. | Structurally stable foods for dysphagia patients (Level 5 IDDSI), visually appealing, and safe. | Nozzle diameter: 0.83 mm; nozzle height: 1.0 mm; Injection rate: 15 mm/s | [39] |
2.1. Phase Behavior: W/O, O/W, and Double Emulsions in Gel Matrices
Active systems can be directly categorized according to the type of functionality incorporated into the printed matrix and are typically classified as oil-in-water (O/W), water-in-oil (W/O), or double emulsions. Multiphase gels have been extensively explored for the active delivery of vitamins, antioxidants, and probiotics due to their ability to encapsulate both hydrophilic and lipophilic compounds within a single three-dimensional structure. However, the structural complexity of double emulsions often poses challenges related to interfacial stability, especially during thermal processing, extrusion, and prolonged storage. Such conditions may trigger phenomena, such as droplet coalescence, phase inversion, and premature release of active compounds [44].
Due to these challenges, Guo et al. [45] developed bigels using Spirulina platensis protein nanoparticles combined with sunflower wax-based oleogels and xanthan gum hydrogels. The authors demonstrated that the incorporation of nanoparticles resulted in dual stabilization via Pickering emulsion and bigel network, enhancing the coalescence resistance and preserving the gel morphology after extrusion. Furthermore, by adjusting the oleogel-to-hydrogel ratio, it was possible to control phase inversion, enabling the formation of O/W, bicontinuous, and W/O systems tailored to the specific demands of 3D printing.
When applied strategically, phase inversion can improve the protection and targeted release of bioactive compounds. For instance, W/O systems can facilitate the release of water-soluble compounds upon inversion, whereas O/W emulsions are more suitable for protecting lipophilic compounds sensitive to oxidation, such as unsaturated fatty acids, polyphenols, or carotenoids. Thus, the spatial organization of phases serves as a functional strategy to control fluidity, enhance structural stability during printing, and design gels with tailored release functionalities upon ingestion or heating [36,46].
Therefore, O/W emulsion gels are thermodynamically unstable by nature, which promotes droplet aggregation, flocculation, and phase separation during storage, thereby compromising the shelf life and functionality of food products. To address these limitations, Wang et al. [47] found that incorporating soy hull polysaccharides (SHPs) at concentrations between 0.05% and 0.15% significantly improved the stability of soy protein isolate (SPI)-based emulsions. This improvement was linked to enhanced interfacial pressure and dilatational modulus, which helped delay droplet flocculation and reduce creaming during storage. Additionally, Mao and Meng [48] demonstrated that, by adjusting the ratio of oleogels to hydrogels and using specific emulsifiers, such as PGPR, it was possible to control phase inversion and improve the structural integrity of bigels. These innovations are particularly valuable for 3D food printing applications, where maintaining the shape and stability of the printed product is crucial.
In this context, two main strategies have been adopted for the formation of emulsion gels: the pre-mixing approach, in which proteins and polysaccharides are first blended to allow interaction prior to emulsification, and the two-step emulsification process, where proteins are initially dispersed in the oil phase for primary emulsification, followed by the addition of polysaccharides. In the pre-mixing method, various interactions may occur, including hydrogen bonding, hydrophobic interactions, and, in some cases, covalent conjugation via the Maillard reaction. These interactions contribute to enhanced thermal stability and improved digestibility of the system. In the two-step emulsification process, the resulting structures can exhibit multilayer coatings (layer-by-layer) or increased viscosity in the continuous phase, both of which promote droplet stabilization, protect encapsulated bioactives, and prolong storage stability [49,50].
2.2. Structure and Stabilization of Emulsion Gels
The droplet size strongly influences the stability and texture of emulsion gels within the dispersed phase. Systems with smaller droplets, typically below 10 µm, exhibit a greater interfacial area, which promotes the formation of polymer-stabilized networks. Mwangi et al. [51] demonstrated that increasing the chitosan concentration led to reduced droplet size and enhanced emulsion robustness, highlighting its role as an interfacial stabilizing agent. Such emulsions exhibited improved stacking and resolution in 3D food printing due to their lower tendency for coalescence and deformation during extrusion [49].
To enhance the emulsifying performance of proteins, various physical modification strategies have been employed. For instance, thermal treatment can denature globular protein structures, exposing hydrophobic regions and improving their affinity for oil–water interfaces. Li et al. [52] observed that heat treatment (70–90 °C for 15 min) of soy protein isolate dispersions led to improved emulsion stability and reduced droplet size.
In addition to thermal modifications, non-thermal physical treatments, such as high hydrostatic pressure, have also been employed to improve protein functionality in emulsion stabilization. Falade et al. [53] applied enzymatic hydrolysis followed by high hydrostatic pressure (400 MPa) to sweet potato protein hydrolysates and evaluated the effects of this technique. The authors found that the resulting emulsion had greater physical stability, as well as reduced particle size (239.25 nm). The process resulted in structural changes that favored faster diffusion of molecules to the interface, forming thick layers around the droplets. Additionally, it significantly increased the antioxidant activity of the system, which is an important aspect in preserving the integrity of active compounds during printing.
Besides proteins, functional polysaccharides also play a prominent role in emulsion formation. Pectins from okra and sugar beet, for example, exhibit branched and acetylated structures that favor system stabilization [54]. In general, the mechanisms of stabilization by polysaccharides are divided between interfacial adsorption, where stabilization occurs superficially, and bulk gelation, where the polysaccharides promote the gelation of the continuous phase. When these techniques are combined, they can result in synergistic effects, such as complex stabilization or the formation of dual-layer structures, thus increasing resistance to coalescence and phase separation [55,56].
Another critical factor is the local pH of the food matrix, which directly influences the stability of emulsions and the incorporated active compounds. Meena et al. [57] showed that the stability of anthocyanins is directly related to the pH, as this compound varies in behavior and coloration according to pH changes. The authors indicated that anthocyanins are more stable and colorful at acidic pH (~3) and unstable at neutral pH. This sensitivity also applies to antioxidants, such as vitamin C, which degradation can be accelerated in the presence of metal ions like iron and copper, commonly found in food systems.
3. Post-Printing Stabilization
Most studies on 3D printing of emulsion gels have concentrated on the fabrication stage, with limited attention given to the post-processing steps. A large portion of the research evaluates the mechanical and thermal properties of emulsion gels prior to printing. After printing, stability is typically assessed by photographing the printed structures and measuring their dimensions. To improve post-printing performance, various strategies are often applied during the pre-printing stage. However, some methods can also be implemented after printing to enhance material stability. This section reviews the main stabilization strategies, including enzymatic, ionic crosslinking, and thermal treatments, as well as physical modifications, such as ultrasound and high-pressure homogenization.
Table 2 summarizes recent studies and their key findings related to post-printing stability.

Table 2.
Overview of recent studies on strategies to enhance the post-printing stability of emulsion gels in 3D food printing.
3.1. Chemical Methods
Enzymatic crosslinking uses enzymes, mainly transglutaminase, to form covalent bonds between polymer chains, enhancing gel strength and resistance to deformation after printing. In general, this method offers mild processing conditions, tunable gelation kinetics, and biocompatibility, making it attractive for food and biomedical applications [71,72]. In the study by Luo et al. [58], enzymatic deamidation of the pea protein isolate significantly enhanced the solubility and interfacial activity of the protein, resulting in Pickering emulsion gels with smaller droplet sizes, higher viscosity, and improved mechanical properties. This led to accurate and structurally stable 3D-printed objects, with greater hardness and adhesiveness compared to native protein formulations. Similarly, Du et al. [59] demonstrated that high internal phase emulsions (HIPEs) based on gelatin, when crosslinked enzymatically with transglutaminase, exhibited satisfactory printability and enabled the fabrication of visually appealing multicolored structures. The enzymatic crosslinking contributed to the stability and firmness of the gels after printing. However, enzymatic crosslinking may also alter protein digestibility, which could impact the nutritional quality of the final product [73].
Ionic crosslinking involves the interaction between polyelectrolytes and counter-ions, and the most typical example is the preparation of sodium alginate-based gels. Sodium alginate is a naturally derived anionic polysaccharide composed of two types of sugar residues, which can interact with divalent metal ions to form gels. These ions bind to multiple oxygen atoms along the polymer chains, creating a stable three-dimensional network. Among these, calcium ions (Ca2+) are especially popular for crosslinking due to their biological compatibility and effectiveness. When alginate is crosslinked with calcium ions, it forms negatively charged gel microspheres. These microspheres can be directed and positioned using an electric field, allowing for precise layer-by-layer deposition during 3D printing [74]. Liu et al. [61] immersed high internal phase Pickering emulsions into a calcium ion solution after 3D printing. The resulting printed structures became noticeably firmer and could withstand compression without deforming. This outcome suggests that calcium ions crosslinked with sodium alginate on the emulsion surface, contributing to increased structural hardness. Studies also indicate that the selection of different anionic polysaccharides can modulate emulsion stability. For instance, carrageenan and alginate have been shown to increase the viscosity and structural integrity of multilayer emulsions, suggesting their potential for post-printing reinforcement [75]. It should be noted, however, that excessive Ca2+ crosslinking may lead to over-hardening, which could be undesirable for products intended for dysphagia diets [76].
3.2. Physical Methods
As mentioned previously, thermal treatments contribute to the stabilization of oil-in-water emulsions with proteins, mainly due to protein modification. When proteins are heated, partial denaturation and unfolding occur, leading to the exposure of hydrophobic residues that are normally hidden in the native conformation. This structural rearrangement increases surface hydrophobicity and promotes protein–protein interactions, often resulting in the formation of aggregates stabilized by hydrophobic interactions and disulfide bonds. When heat treatment is applied prior to emulsification, these protein aggregates adsorb easier at the oil–water interface, contributing to the formation of smaller and more stable emulsion droplets. Additionally, the increased interfacial activity of heat-treated proteins can support the rapid development of viscoelastic films around droplets, enhancing both the physical and oxidative stability of emulsions. The extent of these effects is influenced by factors such as protein concentration and heating temperature, which modulate the size and functionality of the resulting aggregates [23,52]. The heat treatment can also promote the formation of high-strength network structures around interfacial layers [60,77].
Another temperature-based gelation mechanism for proteins is cold-setting. This process involves an initial heat treatment under conditions that do not promote gelation (such as high pH, low protein concentration, or low ionic strength) to induce protein denaturation and the formation of soluble aggregates. Gelation is then triggered at lower temperatures by adjusting factors such as pH (bringing it closer to the isoelectric point), increasing the ionic strength, or incorporating hydrocolloids or carbohydrates. As with heat-induced gelation, the cold-set gelation process can be modulated by parameters that affect the aggregation kinetics of protein particles, including protein concentration, pH, ionic strength, and heating conditions. Notably, cold-set gels tend to exhibit greater elasticity compared to those formed through direct heat-induced gelation [60]. Together, both heat-induced and cold-set gelation strategies offer versatile tools for tailoring the mechanical properties and post-printing stability of emulsion gels used in 3D food printing. Uribe-Alvarez et al. [60] evaluated whey protein isolate-based emulsion gels prepared using cold-set or heat-induced gelation. Thermo-irreversible gels showed superior structural recovery after cooling, particularly when formulated with low hardness and low extrusion force.
Other physical methods focus on adjusting the rheological behavior of emulsions through mechanical shearing techniques, such as high-pressure homogenization (HPH) and ultrasound treatment. Meanwhile, other factors, including temperature, pH, and ionic strength, are also manipulated to modulate the emulsion properties. Often, a combination of these approaches is employed to tailor the rheological and viscoelastic characteristics necessary for the optimal direct “ink” writing 3D printing performance. Ultrasonic treatment significantly modifies the protein structure through cavitation, generating shear forces, heat, and free radicals that can induce unfolding, denaturation, or aggregation, depending on the treatment conditions. This process often exposes hydrophobic regions, altering the protein solubility [78]. In peanut protein–guar gum gels, ultrasound pretreatment of proteins prior to printing did not result in a significant improvement in 3D printing accuracy, suggesting that ultrasound alone may not always alter the properties critical for dimensional stability after printing [65]. This indicates that either the ultrasound parameters or the formulation require further optimization to achieve measurable functional effects.
Conversely, the combination of ultrasound treatment with short-term storage in an ovalbumin–gellan gum emulsion gel with peanut oil demonstrated more pronounced benefits. Ultrasound applied for 3.5 min, followed by 4 min of storage, significantly enhanced the viscoelasticity, hardness, and microstructure, enabling accurate printing of fine 3 mm patterns with 91% fidelity [66].
Regarding HPH, it is a non-thermal physical processing technique used to alter the structural and self-assembly characteristics of proteins, as previously mentioned. During HPH, a high-velocity fluid is forced through a narrow homogenizing valve, generating intense shear and impact forces. These mechanical forces reduce particle size and promote a more uniform dispersion of particles, cells, or colloids within the sample. The applied pressure can induce protein unfolding, aggregation, or gelation. HPH application in oil-in-water Pickering emulsion gels stabilized by soybean protein isolate microgel particles showed that increasing the pressure, along with heating, oil content, and particle concentration, strengthened the gel network and improved the viscoelastic properties [67]. These improvements translated into enhanced structural integrity and dimensional stability (97.2%) of the printed materials during storage, particularly at a high oil content (60%). This demonstrates that high-pressure processing is effective in densifying the gel network, supporting the maintenance of the printed shape, especially in oil-rich systems. While these physical methods enhance gel stability and viscoelasticity, they may affect sensitive nutrients or require optimization to avoid compromising the final texture of delicate food products.
3.3. Compositional Effects
Proteins are commonly used to stabilize emulsions due to their amphiphilicity and surface activity. The stability of the emulsion can be reduced due to the low solubility of the protein. Therefore, proteins are often modified to increase emulsion stability. Polysaccharides are also applied as emulsion thickeners, providing stability by changing emulsion viscosity of the aqueous phase. Protein–polysaccharide complexes can be formed through either physical or chemical crosslinking strategies. Physical crosslinking typically involves non-covalent interactions, such as hydrogen bonds, van der Waals forces, electrostatic attractions, and hydrophobic interactions, which facilitate the assembly of protein and polysaccharide molecules into structured networks. These physically crosslinked structures are generally reversible and can be disrupted under suitable environmental conditions.
In contrast, chemical crosslinking relies on covalent bonding formed through specific reactions, including esterification, etherification, sulfhydrylation, and carboxylation. Among these, the Maillard reaction stands out as a key approach, especially in systems where protein–polysaccharide conjugation is induced during processes such as spray drying or freeze drying [79]. Li et al. [80] selected three phase transition polysaccharide hydrocolloids with varying mechanical properties upon cooling: xanthan gum (Xg), low-acyl gellan gum (Gg), and starch (Ss) were each combined with ovalbumin to produce Xg–ovalbumin emulsion gel, Gg–ovalbumin emulsion gel, and Ss–ovalbumin emulsion gel. These polysaccharides were incorporated into a protein-based emulsion gel to create a polysaccharide–protein composite system for 3D-printed food products. The Ss–ovalbumin emulsion gel formulation demonstrated superior extrusion behavior, shape fidelity, print precision, and support for suspended features, successfully reproducing fine details as small as 1.5 mm. According to the researchers, the combination of aggregated ovalbumin and starch (Ss) resulted in a robust and stable network, with emulsified oil droplets embedded within the structure.
In addition, many studies found out that the oil content plays a crucial role in post-printing stability. In general, emulsion gels containing higher levels of oil tend to exhibit superior performance in 3D food printing compared to those with low or moderate oil contents. In general, increasing the oil concentration enhances the mechanical characteristics of the gel, such as storage modulus, viscosity, and overall structural strength [81]. In the study of Liu et al. [82], the authors investigated the use of soy protein isolate complexed with Houttuynia cordata polysaccharide Pickering emulsions stability after printing. The printed structures at optimal oil phase fraction (ϕ = 0.6) exhibited superior shape fidelity, smooth extrusion lines, and high-resolution 3D architectures. Moreover, Lim et al. [83] investigated the impact of emulsion gel incorporation on the dimensional stability of 3D-printed cookie dough during baking. They found that high butter content led to significant spreading and deformation during oven processing, compromising shape retention. In contrast, increasing the concentration of emulsion gel improved thermal stability, with the best results showing up to 99.88% of shape retention. These formulations minimized spread due to the gel-like structure of the emulsions at high temperatures. Formulations with 18% emulsion gels and 6% butter provided the best compromise between print fidelity and post-baking dimensional stability, making it the most suitable formulation for 3D-printed cookies that maintain their designed shape throughout processing.
Likewise, data indicate that Pickering emulsions (PEs) with an oil content of 0.2 v/v achieved the highest structural stability, reaching 97.13% [84]. However, increasing the oil fraction resulted in a slight decline in this parameter. This reduction is likely due to the incomplete emulsification of the oil phase at higher concentrations, leading to phase separation and compromising the integrity of the internal gel network. Such disruption negatively affects the printing performance.
3.4. Stability Evaluation
While mechanical, rheological, and thermal assessments are commonly used to evaluate the performance of various 3D-printed food materials [85], their application to emulsion gels remains limited. In most studies, printing stability is primarily assessed by monitoring dimensional changes in the printed constructs over time. However, few investigations have addressed how extrusion and storage affect the structural integrity and functional properties of printed emulsion gels. Parameters such as hardness, springiness, and cohesiveness, typically measured through TPA, provide insights into gel performance but are rarely reported for emulsion-based systems. Furthermore, factors including biopolymer type, emulsifier selection, droplet size, and particle concentration, which are known to influence gel network formation and shape fidelity, have not been systematically studied in the context of post-printing stability of emulsion gels. This highlights a clear need for a more comprehensive evaluation of these systems beyond simple shape retention.
A frequently used approach for emulsion gel-based printed materials involves measuring the height (or another dimensional parameter) of the printed model immediately after printing and after a certain storage period under ambient conditions. The stability is then calculated using the following equation:
where is the dimension (e.g., height) of the printed structure at 0 h, and is the corresponding value after a certain storage period. A value close to 100% indicates high structural stability, meaning the printed object maintained its shape with minimal deformation or collapse. Lower percentages reflect structural deterioration, often due to weak gel network formation, phase separation, or syneresis during the post-printing period.
4. Functional Applications in 3D-Printed Foods
4.1. Meat Analogs
Meat analogs are food products developed from alternative proteins, mostly from plant origin, such as legumes and cereals, with the aim of mimicking the characteristics of conventional meat, including texture, flavor, appearance, and functionality [86]. The growing demand for these products can be attributed to various contemporary motivations. From an environmental perspective, traditional livestock farming requires large amounts of water and land and contributes significantly to greenhouse gas emissions. From a health perspective, there is a growing trend toward reducing the intake of saturated fats and cholesterol found in animal meat, coinciding with the rise of vegan, vegetarian, and flexitarian diets. Ethical concerns and animal welfare further drive this shift, especially among consumers seeking for alternatives to animal exploitation and slaughter [86,87,88].
From this perspective, 3D printing emerges as a promising technology capable of overcoming limitations associated with conventional methods, such as extrusion and molding. In this context, emulsion gels stand out as structured materials composed of an aqueous phase and an oil phase, playing a crucial role in the formation, stability, and functionality of 3D-printed meat analogs [89]. These gels replicate the intramuscular fat of animal meat, providing marbling, juiciness, and flavor release during cooking. Additionally, they provide the rheological and mechanical properties required for three-dimensional printing, ensuring viscoelasticity, water retention, and cohesion of the food matrix [9,90]. Thus, emulsion gels not only simulate animal fat but also enable the technical and sensory production of meat analogs, making them key components in the advancement of 3D-printed products [91].
The use of emulsion gels in meat analogs has proven to be an effective strategy, both in terms of technological functionality and in visually simulating intramuscular fat, known as marbling. This marbling, characteristic of red meats, consists of fat distributed in small streaks or spots between muscle fibers, giving the meat a marbled appearance [92,93]. Such fat not only enhances the visual appeal but also directly influences attributes such as tenderness, juiciness, and flavor intensity [93,94], being one of the main quality evaluation criteria for meat [95]. In plant-based alternative products, marbling can be mimicked using emulsion gels formulated from mixtures of saturated and unsaturated oils, which form lipid microglobules dispersed in a protein matrix, visually reproducing fat distribution in products, such as conventional burgers or sausages [96].
These gels exhibit physical behavior similar to animal fat and can be structured with proteins, fibers, or food-grade hydrocolloids [97]. When oil droplets are encapsulated within a gelled matrix, they form whitish, opaque, and firm structures that resemble the visible fat in marbled meat. The layer surrounding the droplets consists of proteins or polysaccharides, which interact with the gel network, promoting enhanced stability and mechanical strength [98,99].
The choice of oil directly influences the thermal stability and sensory performance of the final product. Vegetable oils rich in polyunsaturated fatty acids (PUFAs) are widely used to replace animal fats, particularly when structured into emulsion gels [100,101]. These gels enable the encapsulation and stabilization of fine oil-in-water droplets, protecting lipids from oxidation while contributing to the desirable texture and flavor [102,103]. Moreover, the structural integrity of the emulsion gel network is essential for maintaining the product’s shape and stability after printing or cooking. Gel stiffness is influenced by the composition of the continuous phase (protein/polysaccharide), oil concentration, and moisture content; increased rigidity can improve self-support and extrudability, which are critical factors for 3D printing [101,104]. Tan et al. [89] reported that increasing the protein concentration in RuBisCo-based lipid-filled hydrogels resulted in higher water retention capacity, effectively mimicking the juiciness of cooked chicken. Similarly, Ren et al. [101] found that emulsion gels produced juicier textures in meat analogs compared to palm oil and oleogels. Furthermore, stiffer and denser gels tend to retain heat more effectively during cooking, enhancing perceived juiciness by reducing rapid moisture loss [89,104].
Three-dimensional printing of meat analogs using emulsion gels has demonstrated good efficiency when systems achieve a balance between flowability and structural rigidity. According to Yan & Zhang [65], the proportion of oil phase directly affects the print quality: overly high oil fractions lead to structural collapse, while more balanced compositions favor the formation of stable layers. Liu et al. [35] noted that a slightly increased moisture content enhances flowability and definition of printed layers. A study by Shahbazi et al. [9] showed that low-fat gels enriched with surfactant biopolymers exhibited high elastic modulus and strong viscosity recovery, making them ideal for printing stable 3D structures with thermal retention.
The development of a three-dimensional protein network to mimic the fibrous texture and elasticity of meat can be achieved through thermal or chemical crosslinking of plant proteins [101]. Most plant proteins are globular and, when denatured by heat, form thermoset gels with hydrophobic and disulfide bonds, resulting in firm and elastic structures. Increasing the protein concentration tends to strengthen the gel network, which is essential for achieving a meat-like texture [9]. Bohrer [105] emphasized that printing fibrous-structured products requires matrix modifications to enhance extrudability. Accordingly, the creation of three-dimensional networks composed of proteins and polysaccharides has become a key strategy to achieve the desired texture in meat analogs.
Regarding protein–lipid interactions to mimic the microstructure of meat tissues, Chen & Zhang [106] found that peanut proteins modified by ultrasound exhibited increased hardness and yield stress in emulsion gels. This is attributed to enhanced exposure of hydrophobic groups and the formation of disulfide bonds, which strengthen lipid–protein interactions and result in a more cohesive network. However, excessive ultrasound intensity caused protein degradation, weakening the network. Yu et al. [44] reported that the addition of polysaccharides such as xanthan gum contributes to the formation of more viscous and stable gels, improving lipid retention and 3D printing precision. These interactions are essential to prevent coalescence of lipid droplets, thereby preserving the product’s microstructure.
Gel structure directly affects water and fat retention capacity during cooking. Moisture loss in meat analogs may lead to shrinkage and changes in thickness, similar to what occurs in real meat. In studies by Botella-Martinez et al. [107], products made with gelled emulsions as fat sources showed average cooking losses between 14% and 17%, with shrinkage between 3% and 5% and no significant changes in thickness, indicating good thermal performance. Tan et al. [89] also noted that higher protein concentrations in emulsion gels closely reproduced the water retention capacity observed in cooked chicken. It is believed that internal reactions within meat products directly influence the water and fat retention capacity, leading to variations in cooking loss and dimensional changes such as shrinkage and thickness reduction. This behavior is likely transferable to plant-based analogs, as similar structures may exhibit equivalent effects during heating. Dreher et al. [108] emphasized that, in conventional meat, adipose tissue plays a critical role in juiciness, texture, and appearance by providing elastic and plastic properties. Thus, for vegetable oils to replicate these functions, they must be structured into matrices capable of withstanding heat and maintaining sensory attributes similar to those of animal fat [96,101].
Cooking influences the physicochemical properties of 3D-printed meat analogs, especially in terms of texture and the final structure of the product. Heat induces changes in the food matrix that can enhance or compromise product quality, depending on the cooking technique. Combined approaches, such as frying and hot air drying, have shown positive results when applied to these products [109,110]. Wen et al. [111] observed that steam cooking, for instance, significantly alters the hardness and microstructure, indicating that achieving the desired texture requires careful adjustment of both formulation and thermal treatment. According to Bulut & Candoğan [112], convection cooking is considered more efficient due to its uniform heating and better nutrient preservation compared to frying.
Food printing strategies have focused on creating complex and functional food structures, particularly in the development of plant-based meat analogs. One of the key strategies to achieve multilayered and multiphase structures is the use of multi-nozzle printers, which allow the simultaneous deposition of different food materials [110]. By employing nozzles with different diameters, it becomes possible to print with greater precision and detail, either to encapsulate bioactive ingredients, such as lipids within a protein matrix, or to apply visual finishes, such as decorative inscriptions and surface patterns that enhance consumer appeal [103].
The use of coaxial nozzles represents another important advancement in this process. This configuration enables the concentric extrusion of two distinct materials, allowing for the encapsulation of nutrients, flavor compounds, or dietary fibers within a continuous plant protein phase [113]. More recently, this technology has been adapted for the production of meat analogs, supporting the development of textures that more closely resemble traditional meat, with the added benefit of lower energy consumption, as it operates at lower temperatures compared to conventional extrusion methods [103,110]. Additionally, this type of nozzle helps protect compounds sensitive to oxidation and can enhance the product’s stability during processing and storage [9,44].
Looking ahead, further innovative strategies may emerge in the field of food printing, such as 4D printing, which incorporates external stimuli to trigger structural or visual changes over time [103]. Examples include color changes triggered by variations in pH or heat [114], as demonstrated in microwave-responsive edible inks [115]. Meanwhile, 5D printing expands the potential for customization even further by introducing additional angular movements of the print bed and print head, enabling the construction of stacked layers and the creation of curved surfaces and more complex three-dimensional shapes. This advancement requires in-depth knowledge of the rheological properties of food inks, particularly protein-based matrices, which must maintain appropriate consistency and flow during extrusion [103,115].
For plant-based meat analogs, 3D printing enables the development of customized products with adjustable shapes and compositions, offering unique sensory experiences for consumers [9]. The final properties of 3D-printed products, such as moisture retention, chewiness, cohesiveness, hardness, lipid content, cooking loss, and shrinkage, are directly influenced by printing parameters like infill density, as demonstrated in [3,116]. Additionally, the use of vegetable fat analogs, such as those formulated with soybean or coconut oils, has proven effective in mimicking the melting behavior of beef and pork fat, respectively, contributing to the reproduction of meat-like texture and sensory profiles [117].
Visual appearance is a critical factor in product acceptance. The addition of heat-stable colorants, such as beetroot red, can impart a red color to raw products that later transition to brown tones upon cooking via Maillard reactions, simulating the color changes that occur in meat during thermal treatment [118]. While flavor and aroma are also decisive factors in consumer acceptance, the volatile compounds present in plant-based analogs still differ from those found in animal meat. Therefore, ingredients such as additives, natural or artificial flavorings, and spices are incorporated to meet consumer expectations and mask undesirable attributes of plant proteins, such as bitterness and astringency [110,117]. According to Wen et al. [117], further research is needed to map and replicate the aroma profiles of real meat through 3D printing. In this context, microencapsulation emerges as a promising technology, and coaxial nozzles in 3D printers can encapsulate heat-sensitive volatile compounds, protecting flavors, nutrients, and other functional ingredients until the moment of consumption.
From a nutritional perspective, plant-based meat analogs face challenges related to protein quality, since proteins from legumes and grains tend to have limiting amino acids, such as methionine, cysteine, and lysine, which compromise their nutritional equivalence to animal meat [117]. Therefore, it is essential to enrich these products with amino acids; vitamins; and minerals such as iron, zinc, and vitamin B12 to improve their nutritional profile. The incorporation of bioactive compounds, such as curcumin, resveratrol, and anthocyanins, can further enhance the nutritional value while providing antioxidant protection. This is especially important for preventing lipid oxidation and the rancidity of vegetable oils during processing and storage [110,117].
4.2. Delivery Systems for Bioactives in 3D-Printed Foods
The rise of 3D food printing marks a shift from mass production to large-scale personalization, culminating in the additive manufacturing of functional foods. The intrinsic importance of this approach lies in its ability to overcome the inherent limitations of conventional food fortification and processing methods. Bioactive compounds, such as fat-soluble vitamins (e.g., resveratrol and β-carotene), antioxidants (e.g., curcumin and astaxanthin), and volatiles (e.g., limonene from essential oils), are susceptible to degradation by factors such as light; oxygen; extreme pH; and temperature during processing, storage, and gastrointestinal transit, compromising their bioaccessibility and therapeutic effectiveness [11,119,120].
In the food field, the combination of 3D printing and emulsion gel-based “inks” introduces new possibilities for product design and protection of sensitive compounds. This additive manufacturing approach assembles foods layer by layer, enabling precise regulation over both the macrostructure and microenvironment. Such control facilitates the tailored protection and effective separation of different components. When bioactive molecules are encapsulated within emulsion droplets and subsequently immobilized inside a structured gel matrix, they benefit from multiple physical and chemical defense layers against external degrading agents. Advanced processing strategies, such as coaxial co-extrusion, as discussed previously, enable the incorporation of both water-soluble (e.g., anthocyanins) and fat-soluble (e.g., curcumin) bioactives together in the same structured filament. This approach has been shown to promote notable retention rates—up to 90.8% after 15 days of storage—highlighting the exceptional protective nature of these matrices [121]. Beyond complex bioactives, this fine-tuned structuring is also relevant for retaining volatile compounds, such as limonene. In this context, the viscosity of the matrix is particularly important, as it affects the movement and evaporation of aromatic molecules, thereby influencing their preservation [122].
Moreover, customizing both the gel matrix and the spatial arrangement created via 3D printing enables fine-tuning of the release profile of bioactive compounds, directly influencing their absorption within the body. Such systems can be designed to react to specific physiological conditions found throughout the digestive tract, such as changes in pH or the presence of certain enzymes, thereby enabling targeted delivery of active compounds where and when they are most effectively absorbed [123]. For example, amylose-enriched matrices form denser networks that better withstand digestive processes, particularly under simulated gastric conditions. This resistance delays the release of bioactives, such as resveratrol and β-carotene, enhancing their accessibility during the intestinal phase [123].
This level of release control positions 3D food printing as an important strategy for nutritional and therapeutic customization, supporting the design of functional foods with tailored bioactive concentrations for groups such as children, athletes, older adults, and individuals managing chronic diseases. Through these advances, food transcends its traditional role, serving as a platform for personalized health interventions, an emerging concept that will be further discussed in the following section. Thus, integrating 3D printing with emulsion gel science not only enhances the stability and bioaccessibility of bioactive compounds but also paves the way for a new generation of value-added, high-precision functional foods. The encapsulation of bioactive compounds within emulsion gels is a multistep process governed by interfacial dynamics, emulsion thermodynamics, and gel network architecture. The polarity of the target compound influences the selection of dispersed and continuous phases, while the rheological behavior of the matrix, defined by the choice of biopolymers, determines both the printability and post-printing stability [121].
The stabilization of droplets in emulsions is a fundamental prerequisite for the adequate encapsulation of bioactives. The Pickering mechanism, which involves the quasi-irreversible adsorption of solid particles at the oil–water interface, offers superior stability compared to molecular surfactants [120,124]. The hydrophobicity of native starch particles, which are inherently hydrophilic, requires modification to achieve affinity for both oil and water phases [124]. For liposoluble bioactives, such as resveratrol, β-carotene, curcumin, and astaxanthin, dissolution in the oil phase and dispersion in a continuous aqueous phase form the initial framework of oil-in-water (O/W) emulsions. Stabilization of these droplets is governed by the irreversible adsorption of amphiphilic biopolymers at the oil–water interface. Proteins, such as whey protein isolate (WPI) or soy protein isolate (SPI), undergo partial unfolding at the interface, exposing hydrophobic residues to the oil phase and hydrophilic chains to the aqueous phase, drastically reducing the interfacial tension (γi) and forming a protective viscoelastic layer [119]. Polysaccharides, such as modified starches (e.g., octenyl succinate corn starch, OSA starch), also contribute to stabilization through steric hindrance and electrostatic interactions, as demonstrated by the significant increase in Zeta potential after the complexation of rice starch nanoparticles and soy protein isolate with xanthan gum [121]. The wettability of the Pickering particle, quantified by the contact angle (θ), is crucial: particles with θ < 90° (hydrophilic) favor stable O/W emulsions, whereas those with θ > 90° (lipophilic) stabilize W/O emulsions [119,124]. The surface roughness of starch nanoparticles, modified by physical treatments, can increase the available interfacial area, promoting adhesion and emulsion stability [124]. The viscosity of the continuous phase critically influences the retention of volatile bioactive compounds. The viscosity of the continuous phase is a critical factor for the retention of volatile bioactive compounds. Studies on edible films loaded with orange essential oil (rich in limonene) demonstrated that carboxymethylcellulose (CMC), due to its higher apparent viscosity (0.15 ± 0.01 Pa s), resulted in the highest limonene retention (~65%) compared to citrus pectin (CPP) and potato starch (PS). High viscosity restricts the mobility of oil droplets, minimizing volatilization and coalescence during drying, even in the presence of internal cavities [122]. This highlights the importance of rheological engineering of the matrix in ensuring the stability of encapsulated bioactives. For bioactives with disparate polarities or for temporal release modulation, complex systems such as multiple emulsions (e.g., W/O/W) and bigels (mixtures of gelled oleogels and hydrogels) are designed. Bigels, formed by the interpenetration of oleogel and hydrogel networks, enable the selective compartmentalization of lipophilic and hydrophilic bioactives, offering unprecedented spatial control. Modulation of the oleogel/hydrogel ratio and the inter-network interactions (e.g., hydrogen bonding and hydrophobic interactions) influences the rheological properties and release profiles. Okonkwo et al. [125] highlighted that bigels containing polysaccharides (pectin and alginate) and proteins (gelatin and casein) can optimize viscoelasticity, which is essential for printability and post-printing structural stability.
The formation of the gel network represents the final step in bioactive entrapment, providing the structural integrity necessary for 3D printing and functional protection. Therefore, the choice of the aforementioned gelation mechanisms is important not only for the physicochemical properties of 3D-printed foods but also for the protection and delivery of bioactive compounds. In addition to individual gelation mechanisms, biomolecules synergism is a powerful strategy to optimize the functionality of emulsion gels. The strategic combination of proteins and polysaccharides enables the exploration of complex interactions that result in gel matrices with enhanced properties. A notable example is the formation of complexes between starch nanoparticles (SNPs) and soy protein isolate (SPI) with xanthan gum (XG). In this system, starch nanoparticles, when complexed with SPI, alter the surface charge and hydrophobicity of the protein, promoting more effective adsorption at the oil–water interface and, consequently, superior stabilization of droplets. Xanthan gum, in turn, contributes its high pseudoplastic viscosity, helping to stabilize the initial emulsion and form a dense gel network. The combination of these components results in emulsions with reduced droplet size (158 nm for anthocyanins and curcumin), high Zeta potential (≈−38 mV), and a robust interfacial layer that effectively resists droplet aggregation and coalescence, critical elements for the stability of complex bioactive delivery systems in 3D-printed foods [121]. Such improvements in the interfacial and network architecture can influence the retention and controlled release of encapsulated bioactive compounds, enhancing both stability and functionality in food systems, to further clarify the process of gel matrix formation.
4.3. Personalized Nutrition
The growing demand for personalized dietary solutions has made 3D food printing a promising technique for personalized and individualized nutrition, enabling the manufacture of foods tailored to nutritional needs, preferences, and health conditions [126,127]. This approach is particularly relevant for specific populations, such as the elderly, children, athletes, and patients with metabolic disorders or clinical dietary restrictions, who often require precise control over their macronutrient and micronutrient intake [59].
Although 3D printing is a viable technique for additive manufacturing, the US Food and Drug Administration (FDA) regulations currently only apply to medical products. Food products still have open regulations. Uncertainties related include the risk of counterfeiting, allergies, contamination, long-term health risks related to food, and labeling difficulties [126,128].
Figure 1 illustrates the integrated flow of developing personalized foods through 3D printing using emulsified gels as a functional base. To develop personalized foods, it is first necessary to collect individual data (from children, elderly people, athletes, clinical patients, and others) and analyze it using monitoring technologies and digital platforms, such as smartwatches and specialized software. Based on this analysis, a personalized nutritional diagnosis is conducted, which guides the ideal composition of food for each profile. Therefore, the formulation of the emulsified gel is adjusted according to nutritional needs, such as high protein content, low fat content, and addition of omega-3 and bioactive compounds, among others. The 3D-printed formulated gel generates a food in which the desired characteristics, such as high structural fidelity, adequate texture (cohesive and soft), good resilience, and satisfactory aesthetic appearance, are obtained. This approach represents the convergence of food technology, nutritional personalization, and bioengineering, aimed at populations with specific dietary requirements.
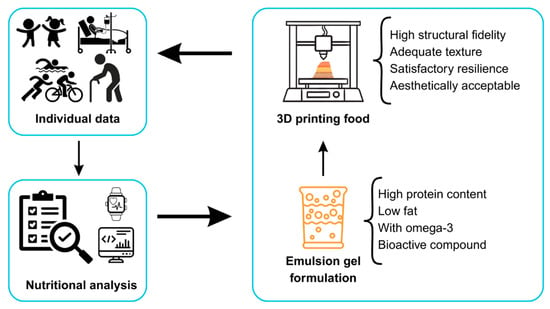
Figure 1.
Three-dimensional printing of foods for personalized nutrition with emulsified gels.
One of the key advantages of 3D emulsion printing for personalized diets is its compatibility with digital health technologies. Integration with systems that process health monitoring data (e.g., wearable sensors, electronic health records, and nutrition tracking apps) enables real-time design of food structures that match individual nutritional profiles. Emerging platforms enable the automated formulation of food “inks” from user-specific data, leading to a convergence between food manufacturing and personalized medicine [129,130]. Future perspectives aim to explore the potential of software-guided emulsion formulation, in which the composition of the oil and water phases will be algorithmically adjusted to match the lipid or protein recommendations for each user [131].
Overall, the use of emulsion gels in 3D-printed foods represents a versatile and scalable platform for advancing the concept of precision nutrition. However, further research is still needed to validate the bioavailability of printed nutrients and their stability under storage and thermal conditions and to ensure regulatory compliance in clinical settings [132]. Table 3 presents recent studies involving the application of emulsions for 3D printing aiming at personalized nutrition.

Table 3.
Studies of 3D-printed emulsions for different personalized nutrition.
The use of proteins for the development of personalized foods is the focus in the development of 3D emulsions. Cod [133], casein [9,134], soy [44,135], pea [24], and peanut [65] proteins are examples of proteins used in the preparation of enriched 3D foods. Some studies, aiming to reduce environmental pollution and reduce the waste of protein sources, are using the protein fraction extracted from industrial waste to produce 3D emulsions. Examples of by-products used as protein sources are those from the tea beverage industry [127,132]. In this way, they provide an appreciation of by-products and the development of 3D foods with functional characteristics.
Associated with proteins, the use of vegetable oils, in addition to promoting improvements in human health, can help lubricate emulsions during printing. Ref. [135] used rosemary, thyme, and basil polyphenols to provide an improved lubrication effect for the construction of a 3D-printed cheese analog. The authors obtained printed functional food with improved lubrication properties, greater creaminess, and mouth-coating characteristics.
The consumption of fatty foods is leading to a significant increase in obesity rates, which leads to a higher risk of cardiovascular disease and mortality. In this sense, personalized nutrition through biogels is a promising alternative for fat replacement [48]. However, as seen in Table 3, studies involving 3D emulsion for fat replacement are still scarce. In this same vein, associated with the growing demand for meat alternatives that mimic the textures of muscle meat, refs. [9,134] used hydrophobically modified microcrystalline cellulose rods (replacing the hydroxyl groups with acetyl groups from olive oil) as a fat substitute and emulsion stabilizer.
Furthermore, the possibility of personalizing food architecture, such as portion size, nutrient layering, and texture modification, improves adherence to therapeutic diets, especially for populations with dysphagia, malabsorption syndromes, or specific caloric needs. Dysphagia is a condition characterized by difficulty swallowing, a common esophageal dysfunction in the elderly or people with specific diseases, such as stroke and Parkinson’s disease [24,136,138,141]. The study of 3D emulsions is important for dietary personalization for dysphagic patients and is currently the focus of 3D emulsion applications.
As seen in Table 3, most personalized 3D emulsion dysphagia foods have focused on macronutrient [24,65] supplementation, with a dearth of micronutrient-supplemented dysphagia foods. In this regard, ref. [140] sensorially evaluated (two trained men and three women, aged between 22 and 37 years) 3D foods developed from egg yolk and carboxymethyl cellulose emulsion gels as a bioaccessibility pathway for essential micronutrients, such as β-carotene. The sensorial judges indicated that, as the carboxymethyl cellulose increased, oral comfort was improved and jaw adhesion decreased, as the formation of a strong gel network increased the lubricity of the sample.
5. Technical Challenges and Future Directions
The application of emulsion gels in 3D food printing presents some technological challenges. As 3D food printing is still an emerging technique, many operational parameters, such as printing speed, resolution, and extrusion behavior, should be carefully optimized based on the properties of the raw materials. Differences in composition, viscosity, and temperature sensitivity can significantly impact printing precision and reproducibility. One major challenge lies in the requirement for specific rheological properties. Printable materials must balance flowability and structural stability, which demands further research on the modification of proteins and polysaccharides, the development of novel gelation mechanisms, and the refinement of post-printing stabilization methods (e.g., enzymatic crosslinking and ionic and thermal treatments). Moreover, these novel formulations must be evaluated for food safety. Strict regulatory guidelines must be followed to ensure that 3D-printed foods are safe, non-toxic, and free of contaminants. All ingredients and materials used in the printing process must be food-grade and approved for human consumption, especially in commercial applications, such as meat analogs or customized nutrition products. In addition to material and safety concerns, scalability remains a barrier. Current 3D food printing technologies face limitations in terms of printing speed, batch size, and continuous production, which makes large-scale manufacturing challenging and economically constrained.
Despite these limitations, there are several opportunities. Emulsion gels can be tailored to develop innovative food products, including meat analogs, bioactive delivery systems, and personalized nutrition. However, further studies are needed to understand how droplet size, interfacial interactions, and gelation mechanisms influence the texture, stability, and nutritional profile of these foods. A promising direction is the integration of artificial intelligence (AI) into the 3D food printing workflow. AI can assist in multiple stages of product development, such as design optimization using deep learning (DL) and machine learning (ML) algorithms to enhance texture, appearance, and nutritional performance; parameter optimization for temperature, print speed, and material flow to increase efficiency and consistency; and real-time monitoring and adaptive control through sensors and intelligent systems to ensure print quality and reduce material waste. For this reason, the studies regarding 3D food printing can support AI development by generating data-rich food models, which can improve training datasets for predictive modeling [143,144,145].
6. Conclusions
Emulsion gels represent a versatile and powerful platform for advancing 3D food printing, enabling the development of customized, nutritious, and structurally robust food products. Their ability to encapsulate both hydrophilic and lipophilic compounds, modulate texture, and provide targeted release mechanisms positions them at the forefront of functional food innovation. Post-printing stabilization strategies, including enzymatic and ionic crosslinking, thermal treatments, and rheological engineering, are key to enhancing shape retention and mechanical integrity during storage and processing. However, critical gaps remain in standardizing rheological evaluation methods, understanding long-term stability, and translating lab-scale processes into industrial applications. Future research should focus on multi-parameter optimization, safety validation, and intelligent automation. Incorporating artificial intelligence into formulation design, print control, and real-time quality monitoring can accelerate the adoption and scalability of this technology. With continued interdisciplinary efforts, emulsion gels could fundamentally transform the way foods are designed, printed, and consumed.
Author Contributions
Conceptualization, B.S.d.F.; methodology, B.S.d.F., L.B.d.C., A.C.R., D.P.J., J.O.G., S.S.F., T.R.S.C.J. and L.A.d.A.P.; resources, B.S.d.F.; data curation, B.S.d.F.; writing—original draft preparation, B.S.d.F., L.B.d.C., A.C.R., D.P.J., J.O.G., S.S.F., T.R.S.C.J. and L.A.d.A.P.; writing—review and editing, B.S.d.F.; visualization, B.S.d.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Brazil-Finance Code 001 and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), grant number 25/2551-0000820-4.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arshad, R.; Saqib, A.; Sharif, H.R.; Liaqat, A.; Xu, B. Recent Advances in 3D Food Printing: Therapeutic Implications, Opportunities, Potential Applications, and Challenges in the Food Industry. Food Res. Int. 2025, 203, 115791. [Google Scholar] [CrossRef]
- Wu, R.; Jiang, J.; An, F.; Ma, X.; Wu, J. Research Progress of 3D Printing Technology in Functional Food, Powering the Future of Food. Trends Food Sci. Technol. 2024, 149, 104545. [Google Scholar] [CrossRef]
- Demei, K.; Zhang, M.; Phuhongsung, P.; Mujumdar, A.S. 3D Food Printing: Controlling Characteristics and Improving Technological Effect during Food Processing. Food Res. Int. 2022, 156, 111120. [Google Scholar] [CrossRef] [PubMed]
- Handral, H.K.; Hua Tay, S.; Wan Chan, W.; Choudhury, D. 3D Printing of Cultured Meat Products. Crit. Rev. Food Sci. Nutr. 2022, 62, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Jing, L.; Zeng, X.; Chen, T.; Liu, H.; Kong, Y.; Wang, X.; Yang, X.; Fu, C.; Sun, J.; et al. 3D-Printed Prolamin Scaffolds for Cell-Based Meat Culture. Adv. Mater. 2023, 35, 2207397. [Google Scholar] [CrossRef]
- Ianovici, I.; Zagury, Y.; Redenski, I.; Lavon, N.; Levenberg, S. 3D-Printable Plant Protein-Enriched Scaffolds for Cultivated Meat Development. Biomaterials 2022, 284, 121487. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.R.; Yeasmen, N.; Ngadi, M. Effect of Food Hydrocolloids on 3D Meat-Analog Printing and Deep-Fat-Frying. Food Hydrocoll. 2025, 159, 110716. [Google Scholar] [CrossRef]
- Ramachandraiah, K. Potential Development of Sustainable 3d-Printed Meat Analogues: A Review. Sustainability 2021, 13, 938. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Chen, J.; Ettelaie, R. Construction of 3D Printed Reduced-Fat Meat Analogue by Emulsion Gels. Part II: Printing Performance, Thermal, Tribological, and Dynamic Sensory Characterization of Printed Objects. Food Hydrocoll. 2021, 121, 107054. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Lenie, M.D.R.; Mirmahdi, R.S.; Ubeyitogullari, A. Designing Future Foods: Harnessing 3D Food Printing Technology to Encapsulate Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2025, 65, 303–319. [Google Scholar] [CrossRef]
- Meigui, H.; Xu, L.; Assadpour, E.; Tan, C.; Jafari, S.M. Application of Nano/Micro-Encapsulated Bioactive Compounds in 3D Printed Foods. Trends Food Sci. Technol. 2025, 158, 104937. [Google Scholar] [CrossRef]
- Pant, A.; Lee, A.Y.; Karyappa, R.; Lee, C.P.; An, J.; Hashimoto, M.; Tan, U.X.; Wong, G.; Chua, C.K.; Zhang, Y. 3D Food Printing of Fresh Vegetables Using Food Hydrocolloids for Dysphagic Patients. Food Hydrocoll. 2021, 114, 106546. [Google Scholar] [CrossRef]
- Eswaran, H.; Ponnuswamy, R.D.; Kannapan, R.P. Perspective Approaches of 3D Printed Stuffs for Personalized Nutrition: A Comprehensive Review. Ann. 3D Print. Med. 2023, 12, 100125. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Trujillo-de Santiago, G.; Álvarez, M.M.; Chuck-Hernández, C. Advances and Prospective Applications of 3D Food Printing for Health Improvement and Personalized Nutrition. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5722–5741. [Google Scholar] [CrossRef]
- Abedini, A.; Sohrabvandi, S.; Sadighara, P.; Hosseini, H.; Farhoodi, M.; Assadpour, E.; Alizadeh Sani, M.; Zhang, F.; Seyyedi-Mansour, S.; Jafari, S.M. Personalized Nutrition with 3D-Printed Foods: A Systematic Review on the Impact of Different Additives. Adv. Colloid. Interface Sci. 2024, 328, 103181. [Google Scholar] [CrossRef] [PubMed]
- Dancausa Millán, M.G.; Millán Vázquez de la Torre, M.G. 3D Food Printing: Technological Advances, Personalization and Future Challenges in the Food Industry. Int. J. Gastron. Food Sci. 2024, 37, 100963. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, M.; Bhandari, B.; Li, C.; Mujumdar, A.S. Utilizing 3D Printing to Create Sustainable Novel Food Products with Innovative Ingredients. Innov. Food Sci. Emerg. Technol. 2025, 99, 103873. [Google Scholar] [CrossRef]
- Baiano, A. 3D Printed Foods: A Comprehensive Review on Technologies, Nutritional Value, Safety, Consumer Attitude, Regulatory Framework, and Economic and Sustainability Issues. Food Rev. Int. 2022, 38, 986–1016. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, H.; Ren, Y.; Sun, M.; Zhang, T.; Li, H.; Liu, X. Functionality and Application of Emulsion Gels in Fat Replacement Strategies for Dairy Products. Trends Food Sci. Technol. 2024, 152, 104673. [Google Scholar] [CrossRef]
- Freire, M.; Cofrades, S.; Pérez-Jiménez, J.; Gómez-Estaca, J.; Jiménez-Colmenero, F.; Bou, R. Emulsion Gels Containing N-3 Fatty Acids and Condensed Tannins Designed as Functional Fat Replacers. Food Res. Int. 2018, 113, 465–473. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Yuan, F.; Gao, Y.; Mao, L. Emulsion Gels with Different Proteins at the Interface: Structures and Delivery Functionality. Food Hydrocoll. 2021, 116, 106637. [Google Scholar] [CrossRef]
- Zhi, L.; Liu, Z.; Wu, C.; Ma, X.; Hu, H.; Liu, H.; Adhikari, B.; Wang, Q.; Shi, A. Advances in Preparation and Application of Food-Grade Emulsion Gels. Food Chem. 2023, 424, 136399. [Google Scholar] [CrossRef]
- Cui, L.; Guo, J.; Meng, Z. A Review on Food-Grade-Polymer-Based O/W Emulsion Gels: Stabilization Mechanism and 3D Printing Application. Food Hydrocoll. 2023, 139, 108588. [Google Scholar] [CrossRef]
- Wang, Y.; Aluko, R.E.; Julian McClements, D.; Yu, Y.; Xu, X.; Sun, Q.; Wang, Q.; Jiao, B.; Dai, L. Emulsion Gel-Based Inks for 3D Printing of Foods for Dysphagia Patients: High Internal Type Emulsion Gel-Biopolymer Systems. Food Hydrocoll. 2024, 156, 110340. [Google Scholar] [CrossRef]
- Wang, C.; Yan, R.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Qiu, C.; et al. Development of Emulsion-Based Edible Inks for 3D Printing Applications: Pickering Emulsion Gels. Food Hydrocoll. 2023, 138, 108482. [Google Scholar] [CrossRef]
- Qiu, R.; Wang, K.; Tian, H.; Liu, X.; Liu, G.; Hu, Z.; Zhao, L. Analysis on the Printability and Rheological Characteristics of Bigel Inks: Potential in 3D Food Printing. Food Hydrocoll. 2022, 129, 107675. [Google Scholar] [CrossRef]
- Tian, H.; Wang, K.; Lan, H.; Wang, Y.; Hu, Z.; Zhao, L. Effect of Hybrid Gelator Systems of Beeswax-Carrageenan-Xanthan on Rheological Properties and Printability of Litchi Inks for 3D Food Printing. Food Hydrocoll. 2021, 113, 106482. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Moses, J.A.; Anukiruthika, T. 3D Printing of Foods. 3D Print. Foods 2022, 1–560. [Google Scholar] [CrossRef]
- Guo, Z.; Arslan, M.; Li, Z.; Cen, S.; Shi, J.; Huang, X.; Xiao, J.; Zou, X. Application of Protein in Extrusion-Based 3D Food Printing: Current Status and Prospectus. Foods 2022, 11, 1902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; McClements, D.J.; Bai, C.; Xu, X.; Sun, Q.; Jiao, B.; Miao, S.; Wang, Q.; Dai, L. Application of Proteins in Edible Inks for 3D Food Printing: A Review. Trends Food Sci. Technol. 2024, 153, 104691. [Google Scholar] [CrossRef]
- Raj, K.V.A.; Biswas, D.; Roy, S. Recent Advances in Protein-Based Edible Films and Coatings for Meat Packaging Applications. Food Bioprocess. Technol. 2025, 1–31. [Google Scholar] [CrossRef]
- Bi, A.Q.; Xu, X.B.; Guo, Y.; Du, M.; Yu, C.P.; Wu, C. Fabrication of Flavour Oil High Internal Phase Emulsions by Casein/Pectin Hybrid Particles: 3D Printing Performance. Food Chem. 2022, 371, 131349. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.; Jäger, H.; Ettelaie, R. Application of Pickering Emulsions in 3D Printing of Personalized Nutrition. Part II: Functional Properties of Reduced-Fat 3D Printed Cheese Analogues. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126760. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, M.; Bhandari, B. Model Building and Slicing in Food 3D Printing Processes: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1052–1069. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D Printing: Printing Precision and Application in Food Sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef]
- Hashemi, B.; Assadpour, E.; Wang, Y.; Jafari, S.M. Application of Oleogels, Hydrogels and Bigels as Novel Edible Inks for 3D/4D Printing of Food Products. Adv. Colloid. Interface Sci. 2025, 343, 103578. [Google Scholar] [CrossRef]
- Tan, C.; Toh, W.Y.; Wong, G.; Li, L. Extrusion-Based 3D Food Printing—Materials and Machines. Int. J. Bioprint 2018, 4, 143. [Google Scholar] [CrossRef]
- Li, X.; Sha, X.-M.; Yang, H.-S.; Ren, Z.-Y.; Tu, Z.-C. Ultrasonic Treatment Regulates the Properties of Gelatin Emulsion to Obtain High-Quality Gelatin Film. Food Chem. X 2023, 18, 100673. [Google Scholar] [CrossRef]
- Chao, E.; Yan, X.; Fan, L. Fabrication of Edible Inks for 3D Printing as a Dysphagia Food: An Emerging Application of Bigels. Food Hydrocoll. 2024, 157, 110463. [Google Scholar] [CrossRef]
- TSAI, C.R.; LIN, Y.K. Artificial Steak: A 3D Printable Hydrogel Composed of Egg Albumen, Pea Protein, Gellan Gum, Sodium Alginate and Rice Mill by-Products. Future Foods 2022, 5, 100121. [Google Scholar] [CrossRef]
- Ma, T.; Lv, L.; Ouyang, C.; Hu, X.; Liao, X.; Song, Y.; Hu, X. Rheological Behavior and Particle Alignment of Cellulose Nanocrystal and Its Composite Hydrogels during 3D Printing. Carbohydr. Polym. 2021, 253, 117217. [Google Scholar] [CrossRef]
- Kuo, C.C.; Qin, H.; Cheng, Y.; Jiang, X.; Shi, X. An Integrated Manufacturing Strategy to Fabricate Delivery System Using Gelatin/Alginate Hybrid Hydrogels: 3D Printing and Freeze-Drying. Food Hydrocoll. 2021, 111, 106262. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Lv, W.; Yang, L.; Xiao, H. Effect of κ-Carrageenan on Physicochemical and 3D Printing Properties of Walnut Protein-Stabilized Emulsion Gel. Food Hydrocoll. 2024, 156, 110288. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.Y.; Li, D.; Wang, L.J.; Wang, Y. Development of Soy Protein Isolate Emulsion Gels as Extrusion-Based 3D Food Printing Inks: Effect of Polysaccharides Incorporation. Food Hydrocoll. 2022, 131, 107824. [Google Scholar] [CrossRef]
- Guo, J.; Gu, X.; Du, L.; Meng, Z. Spirulina Platensis Protein Nanoparticle-Based Bigels: Dual Stabilization, Phase Inversion, and 3D Printing. Food Hydrocoll. 2023, 135, 108160. [Google Scholar] [CrossRef]
- Li, P.; Chen, F.; Yuan, S.; Dai, W.; Yin, L.; Dai, Q.; Li, Z.; Liu, H.; Guo, Q.; Zhu, Q. Development of Novel Lactobacillus Plantarum-Encapsulated Bigel Based on Soy Lecithin-Beeswax Oleogel and Flaxseed Gum Hydrogel for Enhanced Survival during Storage and Gastrointestinal Digestion. Food Hydrocoll. 2025, 163, 111052. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Shao, G.; Qu, D.; Zhao, H.; Yang, L.; Zhu, L.; He, Y.; Liu, H.; Zhu, D. Soy Protein Isolated-Soy Hull Polysaccharides Stabilized O/W Emulsion: Effect of Polysaccharides Concentration on the Storage Stability and Interfacial Rheological Properties. Food Hydrocoll. 2020, 101, 105490. [Google Scholar] [CrossRef]
- Mao, J.; Meng, Z. Fabrication and Characterization of Novel High Internal Phase Bigels with High Mechanical Properties: Phase Inversion and Personalized Edible 3D Food Printing. Food Hydrocoll. 2024, 153, 110019. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Formation, Stability and Properties of Multilayer Emulsions for Application in the Food Industry. Adv. Colloid. Interface Sci. 2006, 128–130, 227–248. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Wandersleben, T.; Marqués, A.M.; Rubilar, M. Multilayer Emulsions Stabilized by Vegetable Proteins and Polysaccharides. Curr. Opin. Colloid. Interface Sci. 2016, 25, 51–57. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Ho, K.W.; Tey, B.T.; Chan, E.S. Effects of Environmental Factors on the Physical Stability of Pickering-Emulsions Stabilized by Chitosan Particles. Food Hydrocoll. 2016, 60, 543–550. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, J.; Ge, G.; Zhao, M.; Sun, W. Impact of Heating Treatments on Physical Stability and Lipid-Protein Co-Oxidation in Oil-in-Water Emulsion Prepared with Soy Protein Isolates. Food Hydrocoll. 2020, 100, 105167. [Google Scholar] [CrossRef]
- Falade, E.O.; Mu, T.H.; Zhang, M. Improvement of Ultrasound Microwave-Assisted Enzymatic Production and High Hydrostatic Pressure on Emulsifying, Rheological and Interfacial Characteristics of Sweet Potato Protein Hydrolysates. Food Hydrocoll. 2021, 117, 106684. [Google Scholar] [CrossRef]
- Alba, K.; Sagis, L.M.C.; Kontogiorgos, V. Engineering of Acidic O/W Emulsions with Pectin. Colloids Surf. B Biointerfaces 2016, 145, 301–308. [Google Scholar] [CrossRef]
- Hu, Z.; Ballinger, S.; Pelton, R.; Cranston, E.D. Surfactant-Enhanced Cellulose Nanocrystal Pickering Emulsions. J. Colloid. Interface Sci. 2015, 439, 139–148. [Google Scholar] [CrossRef]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent Advances in Improving Stability of Food Emulsion by Plant Polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef]
- Meena, L.; Gowda, N.N.; Sunil, C.K.; Rawson, A.; Janghu, S. Effect of Ultrasonication on Food Bioactive Compounds and Their Bio-Accessibility: A Review. J. Food Compos. Anal. 2024, 126, 105899. [Google Scholar] [CrossRef]
- Luo, L.; Li, P.; Deng, Y.; Liu, G.; Zhang, Y.; Tang, X.; Zhou, P.; Zhao, Z.; Zhang, M. Ultrastable High Internal Phase Pickering Emulsions Stabilized by Deamidated Pea Protein: Formation Mechanisms and Applications in 3D Printing. Food Chem. 2025, 485, 144541. [Google Scholar] [CrossRef]
- Du, J.; Dai, H.; Wang, H.; Yu, Y.; Zhu, H.; Fu, Y.; Ma, L.; Peng, L.; Li, L.; Wang, Q.; et al. Preparation of High Thermal Stability Gelatin Emulsion and Its Application in 3D Printing. Food Hydrocoll. 2021, 113, 106536. [Google Scholar] [CrossRef]
- Uribe-Alvarez, R.; Murphy, C.P.; Coleman-Vaughan, C.; O’Shea, N. Evaluation of Ionic Calcium and Protein Concentration on Heat- and Cold-Induced Gelation of Whey Protein Isolate Gels as a Potential Food Formulation for 3D Food Printing. Food Hydrocoll. 2023, 142, 108777. [Google Scholar] [CrossRef]
- Liu, X.; Xie, F.; Zhou, J.; He, J.; Din, Z.; Chen, S.; Cai, J. High Internal Phase Pickering Emulsion Stabilized by Zein-Tannic Acid-Sodium Alginate Complexes: β-Carotene Loading and 3D Printing. Food Hydrocoll. 2023, 142, 108762. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Yang, L.; Lv, W.; Xiao, H. Effect of Salt Valence and Ionic Strength on the Rheology and 3D Printing Performance of Walnut Protein Emulsion Gels. Food Hydrocoll. 2025, 164, 111112. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, S.; Zhou, Q.; Wang, X.; Hu, J.; Zhou, P. HIPE-Gels Performance: Role of Sodium Hyaluronate Conformation and Concentration in Structure and 3D Printing. Food Biosci. 2025, 67, 106361. [Google Scholar] [CrossRef]
- Chakravorty, P.; Badwaik, L.S.; Das, A.B. Impact of Extrusion Cooking on Tree Bean (Parkia Timoriana) Seed Protein Emulsion Gels with Natural Deep Eutectic Solvents and Its Use as an Extrusion-Based 3D Food Printing Ink. J. Food Eng. 2026, 403, 112722. [Google Scholar] [CrossRef]
- Yan, H.Y.; Zhang, S.B. Preparation and Characterization of Ultrasonically Modified Peanut Protein–Guar Gum Composite Emulsion Gels for 3D Printing. Gels 2024, 10, 828. [Google Scholar] [CrossRef]
- Li, R.; Wang, N.; Ma, C.; Wang, J.; Wang, J.; Yang, X. Effect and Mechanism of Ultrasound Time and Storage Time on Physicochemical Properties, 3D Printing Accuracy, and Microstructure of Ovalbumin–Gellan Gum Emulsion Gels. Food Chem. 2025, 477, 143518. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Z.; Hei, X.; Li, S.; Jiao, B.; Ma, X.; Hu, H.; Zhu, J.; Binks, B.P.; Jia, Z.; et al. 3D Printing of Pickering Emulsion Gels of Protein Particles Prepared by High Pressure Homogenization and Heating. LWT 2024, 206, 116568. [Google Scholar] [CrossRef]
- Kamlow, M.A.; Spyropoulos, F.; Mills, T. 3D Printing of Kappa-Carrageenan Emulsion Gels. Food Hydrocoll. Health 2021, 1, 100044. [Google Scholar] [CrossRef]
- Shi, X.; Liu, J.; Liu, Q.; Chen, Q.; Wang, H.; Sun, F.; Kong, B. Influence of Different Carrageenan Contents on the Rheological Properties and 3D Printing Suitability of Whey Isolate Protein-Based Emulsion Gels. Food Hydrocoll. 2025, 161, 110839. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Blennow, A.; Zhao, F.; Wang, N.; Liu, X.; Liu, X.; Zhang, C. 3D Printing of High Internal Phase Emulsions Stabilized by OSA Modified Potato Starch: Effect of Citrus Fiber Addition. Food Hydrocoll. 2025, 163, 111114. [Google Scholar] [CrossRef]
- Shi, Y.; Xia, Y.; Gao, W.; Wang, J.; Shi, B.; Wang, H. Enzymatic Crosslinking of Histidine Side Chains in Peptide Natural Products. Nat. Prod. Rep. 2025, 42, 763–773. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Ghorbani, M.; Roshangar, L.; Soleimani Rad, J. Chemical Gelling of Hydrogels-Based Biological Macromolecules for Tissue Engineering: Photo- and Enzymatic-Crosslinking Methods. Int. J. Biol. Macromol. 2019, 139, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Isaschar-Ovdat, S.; Fishman, A. Crosslinking of Food Proteins Mediated by Oxidative Enzymes—A Review. Trends Food Sci. Technol. 2018, 72, 134–143. [Google Scholar] [CrossRef]
- He, C.; Zhang, M.; Fang, Z. 3D Printing of Food: Pretreatment and Post-Treatment of Materials. Crit. Rev. Food Sci. Nutr. 2020, 60, 2379–2392. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Chang, Y.; Shen, J.; Chen, G.; Xue, C. A Comparative Investigation of Anionic Polysaccharides (Sulfated Fucan, ι-Carrageenan, κ-Carrageenan, and Alginate) on the Fabrication, Stability, Rheology, and Digestion of Multilayer Emulsion. Food Hydrocoll. 2023, 134, 108081. [Google Scholar] [CrossRef]
- Min, C.; Yang, Q.; Pu, H.; Cao, Y.; Ma, W.; Kuang, J.; Huang, J.; Xiong, Y.L. Textural Characterization of Calcium Salts-Induced Mung Bean Starch-Flaxseed Protein Composite Gels as Dysphagia Food. Food Res. Int. 2023, 164, 112355. [Google Scholar] [CrossRef]
- Chen, H.; Sun, Y.; Feng, X.; Ma, L.; Dai, H.; Wang, H.; Zhu, H.; Yu, Y.; Zhang, Y. Thermal-Induced-Stable High Internal Phase Emulsion for Lycopene Encapsulation and Delivery: Pre-Heat Treatment Mediated Facilitation on the Isomerization, Stabilization, and Release of Lycopene. LWT 2023, 187, 115319. [Google Scholar] [CrossRef]
- Gong, P.; Yue, S.; Wang, J.; Xu, K.; Yang, W.; Li, N.; Wang, J.; Zhao, Y.; Chen, F.; Guo, Y. Effect of Ultrasound Synergistic PH Shift Modification Treatment on Hericium Erinaceus Protein Structure and Its Application in 3D Printing. Int. J. Biol. Macromol. 2025, 295, 139562. [Google Scholar] [CrossRef]
- Cen, S.; Li, S.; Meng, Z. Advances of Protein-Based Emulsion Gels as Fat Analogues: Systematic Classification, Formation Mechanism, and Food Application. Food Res. Int. 2024, 191, 114703. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, N.; Ma, C.; Wang, J.; Wang, J.; Yang, X. Construction and Formation Mechanism of Phase-Change Polysaccharide–Protein Composite Emulsion Gels: For Simultaneous Printing of Food Products with Complex Structures and Fine Patterns. Food Hydrocoll. 2025, 160, 110817. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, B.; Lv, W.; Wu, Y.; Lv, Y.; Sheng, S. Recent Research and Applications in Lipid-Based Food and Lipid-Incorporated Bioink for 3D Printing. Food Chem. 2024, 458, 140294. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Pan, Y.; Li, Z.; Tian, J.; Ran, X.; Li, W.; Xing, Y. Investigation of the Stability in Soybean Isolated Protein-Houttuynia Cordata Polysaccharides Complex Stabilized Pickering Emulsions with Construction of Its 3D Printing System. LWT 2025, 225, 117906. [Google Scholar] [CrossRef]
- Lim, W.S.; Lim, N.; Kim, H.W.; Park, H.J. Effect of Emulsion Gel as Butter Substitute on the Dimensional Stability and Nutritional Profile of 3D Printed Cookies. Food Biosci. 2023, 56, 103207. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Wang, L.; Li, L.; Cao, W.; Chen, J.; Law, C.L.; Luo, Z.; Pan, L.; Duan, X.; et al. 3D Printing Based on Pickering Emulsions Stabilized by Oat Insoluble Dietary Fiber-Starch Composite Particles. Int. J. Biol. Macromol. 2025, 313, 144146. [Google Scholar] [CrossRef]
- Sakai, S.; Yamamoto, S.; Hirami, R.; Hidaka, M.; Chamara Manoj Lakmal Elvitigala, K. Enzymatically Gellable Chitosan Inks with Enhanced Printability by Chitosan Nanofibers for 3D Printing of Wound Dressings. Eur. Polym. J. 2024, 210, 112960. [Google Scholar] [CrossRef]
- Feddern, V.; Langone, M.G.S.; da Silva Fortunato, G.; Bonan, C.I.D.G.; Ienczak, J.L.; Feltes, M.M.C. Plant-Based Protein Sources Applied as Ingredients in Meat Analogues Sustainable Production. Braz. J. Food Technol. 2024, 27, e2024001. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Meat Analog as Future Food: A Review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef]
- Baune, M.C.; Terjung, N.; Tülbek, M.Ç.; Boukid, F. Textured Vegetable Proteins (TVP): Future Foods Standing on Their Merits as Meat Alternatives. Future Foods 2022, 6, 100181. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; McClements, D.J. Preparation of Plant-Based Meat Analogs Using Emulsion Gels: Lipid-Filled RuBisCo Protein Hydrogels. Food Res. Int. 2023, 167, 112708. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, Y.; Gu, X.; Meng, Z. Spirulina Platensis Protein-Based Emulsion Gel as Fat Substitute in Meat Analogs: Evaluation Performance across Post-Processing. Food Chem. 2025, 463, 141414. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kaur, L.; Furuhata, Y.; Aoyama, H.; Singh, J. 3D Printing of Textured Soft Hybrid Meat Analogues. Foods 2022, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- ZoBell, D.R.; Holmgren, L.; Whittier, D. Beef Quality and Yield Grading; Utah State University Extension: Logan, UT, USA, 2005. [Google Scholar]
- Ramirez, B.F.D.; De Andrade, T.N.; Britto, B.d.C.; Pontara, J.; Rossini, G.A.; Morais, O.J. Vegetais Análogos à Carnes e o Futuro Da Alimentação: Desafios Da Indústria de Alimentos Frente as Crises Ambientais/Vegetables Analogous to Meat and the Future of Food: Da Challenges of the Food Industry in the Face of Environmental Crises. Braz. J. Health Rev. 2022, 5, 3409–3448. [Google Scholar] [CrossRef]
- Andaya, A.E.; Arboleda, E.R.; Andilab, A.A.; Dellosa, R.M. Meat Marbling Scoring Using Image Processing with Fuzzy Logic Based Classifier. Artic. Int. J. Sci. Technol. Res. 2019, 8, 1442–1445. [Google Scholar]
- Kausar, T.; Hanan, E.; Ayob, O.; Praween, B.; Azad, Z. A Review on Functional Ingredients in Red Meat Products. Bioinformation 2019, 15, 358–363. [Google Scholar] [CrossRef]
- Dreher, J.; König, M.; Herrmann, K.; Terjung, N.; Gibis, M.; Weiss, J. Varying the Amount of Solid Fat in Animal Fat Mimetics for Plant-Based Salami Analogues Influences Texture, Appearance and Sensory Characteristics. LWT-Food Sci. Technol. 2021, 143, 111140. [Google Scholar] [CrossRef]
- Gonçalves, B.N.A.; Silva, K.V.V.; Schmiele, M.; Andrade, M.P.D. Géis de Emulsão Como Substitutos de Gordura Saturada Em Emulsionados Cárneos. Res. Soc. Dev. 2022, 11, e35511629207. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food Gels: Gelling Process and New Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Kothuri, V.; Han, J.H.; Keum, D.H.; Kwon, H.C.; Kim, D.H.; Han, S.G. Utilization of Emulsion Gels in Plant-Based Meat Analog Formulations: A Review. Food Hydrocoll. 2025, 158, 110499. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel Applications of Oil-Structuring Methods as a Strategy to Improve the Fat Content of Meat Products. Trends Food Sci. Technol. 2015, 62, 177–188. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, L.; Zhang, Y.; Li, H.; Zhao, D.; Cao, J.; Liu, X. Application of Emulsion Gels as Fat Substitutes in Meat Products. Foods 2022, 11, 1950. [Google Scholar] [CrossRef] [PubMed]
- Poyato, C.; Astiasarán, I.; Barriuso, B.; Ansorena, D. A New Polyunsaturated Gelled Emulsion as Replacer of Pork Back-Fat in Burger Patties: Effect on Lipid Composition, Oxidative Stability and Sensory Acceptability. LWT-Food Sci. Technol. 2015, 62, 1069–1075. [Google Scholar] [CrossRef]
- Miller, O.; Scarlett, C.J.; Akanbi, T.O. Plant-Based Meat Analogues and Consumer Interest in 3D-Printed Products: A Mini-Review. Foods 2024, 13, 2314. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. Preparation, Structure-Property Relationships and Applications of Different Emulsion Gels: Bulk Emulsion Gels, Emulsion Gel Particles, and Fluid Emulsion Gels. Trends Food Sci. Technol. 2020, 102, 123–137. [Google Scholar] [CrossRef]
- Bohrer, B.M. An Investigation of the Formulation and Nutritional Composition of Modern Meat Analogue Products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.B. Structural and Functional Properties of Self-Assembled Peanut Protein Nanoparticles Prepared by Ultrasonic Treatment: Effects of Ultrasound Intensity and Protein Concentration. Food Chem. 2023, 413, 135626. [Google Scholar] [CrossRef] [PubMed]
- Botella-Martínez, C.; Sayas-Barberá, E.; Pérez-Álvarez, J.Á.; Viuda-Martos, M.; Fernández-López, J. Chia and Hemp Oils-Based Gelled Emulsions as Replacers of Pork Backfat in Burgers: Effect on Lipid Profile, Technological Attributes and Oxidation Stability during Frozen Storage. Int. J. Food Sci. Technol. 2023, 58, 3234–3243. [Google Scholar] [CrossRef]
- Dreher, J.; Blach, C.; Terjung, N.; Gibis, M.; Weiss, J. Formation and Characterization of Plant-Based Emulsified and Crosslinked Fat Crystal Networks to Mimic Animal Fat Tissue. J. Food Sci. 2020, 85, 421–431. [Google Scholar] [CrossRef]
- Dankar, I.; Haddarah, A.; Omar, F.E.L.; Sepulcre, F.; Pujolà, M. 3D Printing Technology: The New Era for Food Customization and Elaboration. Trends Food Sci. Technol. 2018, 75, 231–242. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.R.; Yeasmen, N.; Orsat, V. Plant-Proteins Based 3D Meat Analog Printing: A Review. Food Chem. 2025, 482, 144157. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Kim, H.W.; Park, H.J. Effects of Transglutaminase and Cooking Method on the Physicochemical Characteristics of 3D-Printable Meat Analogs. Innov. Food Sci. Emerg. Technol. 2022, 81, 103114. [Google Scholar] [CrossRef]
- Bulut, E.G.; Candoğan, K. Development and Characterization of a 3D Printed Functional Chicken Meat Based Snack: Optimization of Process Parameters and Gelatin Level. LWT 2022, 154, 112768. [Google Scholar] [CrossRef]
- Ko, H.J.; Wen, Y.; Choi, J.H.; Park, B.R.; Kim, H.W.; Park, H.J. Meat Analog Production through Artificial Muscle Fiber Insertion Using Coaxial Nozzle-Assisted Three-Dimensional Food Printing. Food Hydrocoll. 2021, 120, 106898. [Google Scholar] [CrossRef]
- Ghazal, A.F.; Zhang, M.; Liu, Z. Spontaneous Color Change of 3D Printed Healthy Food Product over Time after Printing as a Novel Application for 4D Food Printing. Food Bioproc Tech. 2019, 12, 1627–1645. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Mujumdar, A.S.; Phuhongsunge, P. 4D Printing Induced by Microwave and Ultrasound for Mushroom Mixtures: Efficient Conversion of Ergosterol into Vitamin D2. Food Chem. 2022, 387, 132840. [Google Scholar] [CrossRef]
- Wilson, A.; Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Customized Shapes for Chicken Meat-Based Products: Feasibility Study on 3D-Printed Nuggets. Food Bioproc Tech. 2020, 13, 1968–1983. [Google Scholar] [CrossRef]
- Wen, Y.; Chao, C.; Che, Q.T.; Kim, H.W.; Park, H.J. Development of Plant-Based Meat Analogs Using 3D Printing: Status and Opportunities. Trends Food Sci. Technol. 2023, 132, 76–92. [Google Scholar] [CrossRef]
- Wen, Y.; Kim, H.W.; Park, H.J. Effect of Xylose on Rheological, Printing, Color, Texture, and Microstructure Characteristics of 3D-Printable Colorant-Containing Meat Analogs Based on Mung Bean Protein. Food Res. Int. 2022, 160, 111704. [Google Scholar] [CrossRef]
- Funami, T.; Ishihara, S.; Maeda, K.; Nakauma, M. Review Paper: Recent Development in Pickering Emulsion Gel Technology for Food and Beverage Applications. Food Hydrocoll. 2025, 162, 110901. [Google Scholar] [CrossRef]
- Ramos, G.V.C.; Ramírez-López, S.; de Pinho, S.C.; Ditchfield, C.; Moraes, I.C.F. Starch-Based Pickering Emulsions for Bioactive Compound Encapsulation: Production, Properties, and Applications. Processes 2025, 13, 342. [Google Scholar] [CrossRef]
- Niu, R.; Zhao, R.; Hu, H.; Yu, X.; Huang, Z.; Cheng, H.; Yin, J.; Zhou, J.; Xu, E.; Liu, D. Co-Encapsulation of Hydrophilic and Hydrophobic Bioactives Stabilized in Nanostarch-Assisted Emulsion for Inner Core Gel of Coaxial 3D Printing. Carbohydr. Polym. 2024, 343, 122499. [Google Scholar] [CrossRef] [PubMed]
- Nahas, E.O.; Furtado, G.F.; Lopes, M.S.; Silva, E.K. From Emulsions to Films: The Role of Polysaccharide Matrices in Essential Oil Retention Within Active Packaging Films. Foods 2025, 14, 1501. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, D.; Wang, L.; Wang, Y. Tailoring 3D-Printed High Internal Phase Emulsion-Rice Starch Gels: Role of Amylose in Rheology and Bioactive Stability. Carbohydr. Polym. 2024, 331, 121891. [Google Scholar] [CrossRef]
- Ramos, G.V.C.; Rabelo, M.E.A.; de Pinho, S.C.; Valencia, G.A.; Sobral, P.J.d.A.; Moraes, I.C.F. Dual Modification of Cassava Starch Using Physical Treatments for Production of Pickering Stabilizers. Foods 2024, 13, 327. [Google Scholar] [CrossRef]
- Okonkwo, C.E.; Ofoedu, C.E.; Hussain, S.Z.; Adeyanju, A.A.; Naseer, B.; Inyinbor, A.A.; Olaniran, A.F.; Kamal-Eldin, A. Application of Biogels for Bioactives Delivery: Recent Developments and Future Research Insights. Appl. Food Res. 2022, 2, 100238. [Google Scholar] [CrossRef]
- Samota, M.K.; Kaur, M.; Selvan, S.S.; Kaur, R.; Varinda; Ahlawat, A.; Kaur, M. Shaping the Future of Food: 3D-Printed Personalized Nutrition and Sustainable Production Pathways. Food Humanit. 2025, 4, 100625. [Google Scholar] [CrossRef]
- An, Z.; Liu, Z.; Mo, H.; Hu, L.; Li, H.; Xu, D.; Chitrakar, B. Preparation of Pickering Emulsion Gel Stabilized by Tea Residue Protein/Xanthan Gum Particles and Its Application in 3D Printing. J. Food Eng. 2023, 343, 111378. [Google Scholar] [CrossRef]
- Bedoya, M.G.; Montoya, D.R.; Tabilo-Munizaga, G.; Pérez-Won, M.; Lemus-Mondaca, R. Promising Perspectives on Novel Protein Food Sources Combining Artificial Intelligence and 3D Food Printing for Food Industry. Trends. Food Sci. Technol. 2022, 128, 38–52. [Google Scholar] [CrossRef]
- Theodore Armand, T.P.; Kim, H.C.; Kim, J.I. Digital Anti-Aging Healthcare: An Overview of the Applications of Digital Technologies in Diet Management. J. Pers. Med. 2024, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandey, V.K.; Tripathi, A.; Singh, R.; Net, T.S.S.; Ramniwas, S.; Pandiselvam, R. 4D Food Printing Technology: Structural Changes to Culinary Art and Beyond. J. Food Process Eng. 2024, 47, 14535. [Google Scholar] [CrossRef]
- Kok Wah, J.N. AI-Driven 3D and 4D Food Printing: Innovations for Sustainability, Personalization, and Global Applications. Food Rev. Int. 2025, 1–29. [Google Scholar] [CrossRef]
- Xu, D.; Liu, Z.; An, Z.; Hu, L.; Li, H.; Mo, H.; Hati, S. Incorporation of Probiotics into 3D Printed Pickering Emulsion Gel Stabilized by Tea Protein/Xanthan Gum. Food Chem. 2023, 409, 135289. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Song, L.; Bi, A.; Wu, C.; Ma, Y.; Du, M.; Zhu, B. High Internal Phase Emulsion for Food-Grade 3D Printing Materials. ACS Appl. Mater. Interfaces 2020, 12, 45493–45503. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ettelaie, R. Application of Pickering Emulsions in 3D Printing of Personalized Nutrition. Part I: Development of Reduced-Fat Printable Casein-Based Ink. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126641. [Google Scholar] [CrossRef]
- Mohammadi, A.; Kashi, P.A.; Kashiri, M.; Bagheri, A.; Chen, J.; Ettelaie, R.; Jäger, H.; Shahbazi, M. Self-Assembly of Plant Polyphenols-Grafted Soy Proteins to Manufacture a Highly Stable Antioxidative Pickering Emulsion Gel for Direct-Ink-Write 3D Printing. Food Hydrocoll. 2023, 142, 108851. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, Y.; Zhang, P.; Wang, H.; Tan, M. Development and Characterization of Emulsion Gels Prepared via Gliadin-Based Colloidal Particles and Gellan Gum with Tunable Rheological Properties for 3D Printed Dysphagia Diet. Int. J. Biol. Macromol. 2023, 253, 126839. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Lavados, C.; Tabilo-Munizaga, G.; Rivera-Tobar, D.; Carvajal-Mena, N.; Palma-Acevedo, A.; Moreno-Osorio, L.; Pérez-Won, M. Development of Bean-Based Emulgels for 3D Printing Applications: Feasibility for Dysphagia Diets. J. Food Eng. 2023, 358, 111687. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, F.; Deng, Y.; Tang, X.; Li, P.; Zhao, Z.; Zhang, M.; Liu, G. Texture Characterization of 3D Printed Fibrous Whey Protein-Starch Composite Emulsion Gels as Dysphagia Food: A Comparative Study on Starch Type. Food Chem. 2024, 458, 140302. [Google Scholar] [CrossRef]
- Liang, W.; Wang, Y.; Li, C.; Wang, P.; Rong, J.; Liu, R.; Xiong, S.; Hu, Y. Development of Easy-to-Swallow and Lipid-Enhanced 3D Printed Surimi Based on High Internal Phase Emulsions. Food Hydrocoll. 2024, 155, 110207. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, Z.; Wang, J.; Xu, L.; Zhang, H.; Li, H.; Yu, X.; Xia, N.; Ma, Y.; Rayan, A.M.; et al. Micronutrient Supplemented Dysphagia Food: Rheology and β-Carotene Delivery of 3D Printing Egg Yolk-Carboxymethyl Cellulose Emulsion Gels. Food Res. Int. 2025, 208, 116213. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, M.; Liu, Y.; Chuang, R.; Zhang, H.; Zheng, L.; Li, H.; Xia, N.; Ma, Y.; Rayan, A.M.; et al. Strategy to Kill Two Birds with One Stone: High Internal Phase Pickering Emulsions to Modulate 3D Printed Pork Texture as a Dysphagia Diet. Food Chem. 2025, 463, 141319. [Google Scholar] [CrossRef]
- Zhao, P.; Kou, D.; Qiu, R.; Li, S.; Awais, M.; Tong, L.; Wang, L.; Fan, B.; Wang, F.; Liu, L. Development of Soy Protein Emulsion Gels-Based 3D Printed Dysphagia Foods: Effects of the Egg White Protein Supplementation. Food Hydrocoll. 2025, 160, 110737. [Google Scholar] [CrossRef]
- Rahmani Dabbagh, S.; Ozcan, O.; Tasoglu, S. Machine Learning-Enabled Optimization of Extrusion-Based 3D Printing. Methods 2022, 206, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Luo, Q.; Liu, K.; Li, Y.; Hou, Y.; Long, W. Research Status and Prospect of Machine Learning in Construction 3D Printing. Case Stud. Constr. Mater. 2023, 18, e01952. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, M.; Mujumdar, A.S.; Li, J. AI-Based Additive Manufacturing for Future Food: Potential Applications, Challenges and Possible Solutions. Innov. Food Sci. Emerg. Technol. 2024, 92, 103599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).