Hydrophobic Boron Nitride Nanoflower Coatings on Mild Steel Surfaces
Abstract
1. Introduction
2. Experimental
2.1. Materials
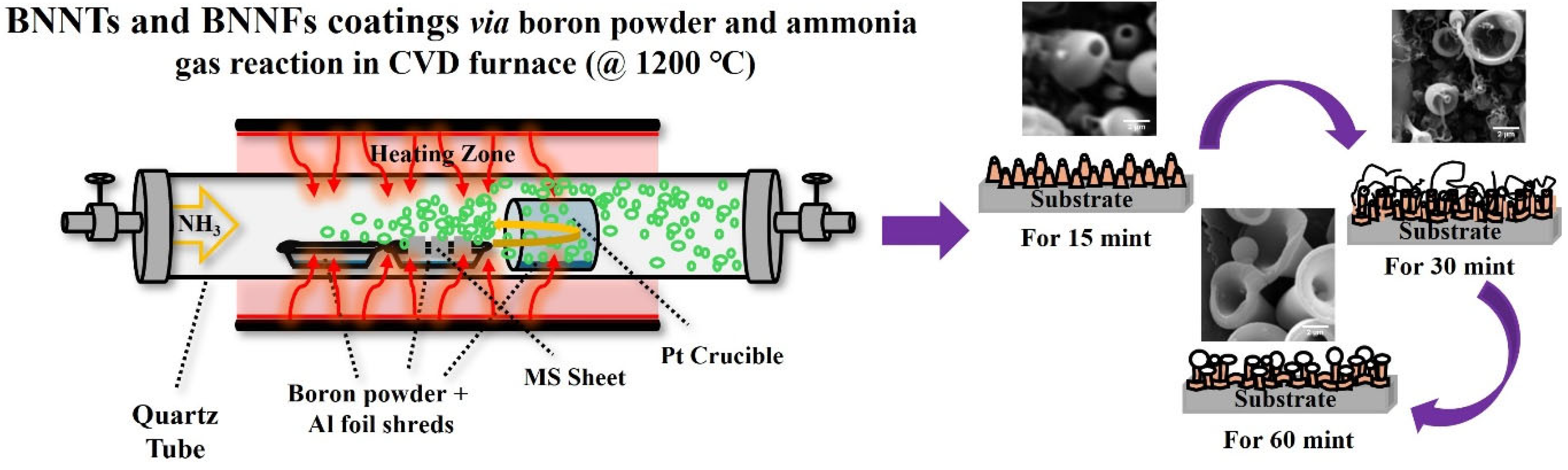
2.2. Coating Procedure
2.3. Characterization
3. Results and Discussion
3.1. XRD and FTIR
3.2. SEM and EDX Analysis
3.3. Growth Mechanism
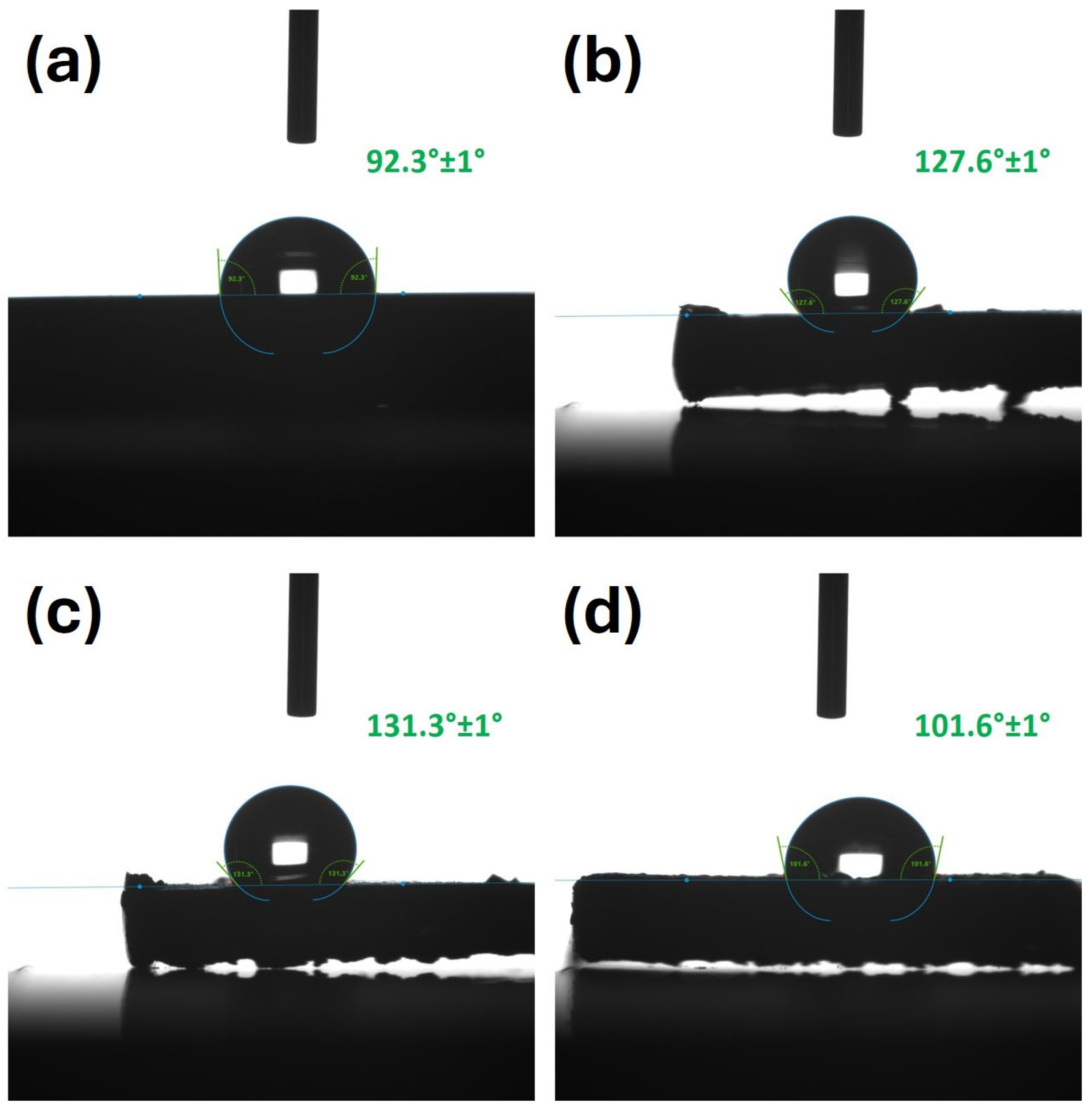
3.4. Water Contact Angle (WCA)
3.5. Electrochemical Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sastri, V.S. Challenges in Corrosion: Costs, Causes, Consequences, and Control; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Bender, R.; Féron, D.; Mills, D.; Ritter, S.; Bäßler, R.; Bettge, D.; De Graeve, I.; Dugstad, A.; Grassini, S.; Hack, T. Corrosion challenges towards a sustainable society. Mater. Corros. 2022, 73, 1730–1751. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Raza, M.A.; Ghauri, F.A.; Rehman, Z.U.; Ilyas, M.T. Corrosion study of graphene oxide coatings on AZ31B magnesium alloy. J. Coat. Technol. Res. 2020, 17, 1321–1329. [Google Scholar] [CrossRef]
- Abbas, M.; Shafiee, M. An overview of maintenance management strategies for corroded steel structures in extreme marine environments. Mar. Struct. 2020, 71, 102718. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Isahak, W.N.R.W.; Al-Azzawi, W.K. Sustainable corrosion Inhibitors: A key step towards environmentally responsible corrosion control. Ain Shams Eng. J. 2024, 15, 102672. [Google Scholar] [CrossRef]
- Fayomi, O.S. Metallurgical Advances in Coatings and Corrosion; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Ashwathareddy, A.; Rao, S.; Subramaniyam, S.S.; Krishna, P.G.; Rama, K.M.; Kodange, S. Corrosion mitigation of mild steel in acidic medium using liquid crystals: A comprehensive review. Inorg. Chem. Commun. 2024, 169, 113071. [Google Scholar] [CrossRef]
- Kadhim, A.; Betti, N.; Al-Bahrani, H.; Al-Ghezi, M.; Gaaz, T.; Kadhum, A.; Alamiery, A. A mini review on corrosion, inhibitors and mechanism types of mild steel inhibition in an acidic environment. Int. J. Corros. Scale Inhib. 2021, 10, 861–884. [Google Scholar]
- Aslam, R.; Mobin, M.; Zehra, S.; Aslam, J. A comprehensive review of corrosion inhibitors employed to mitigate stainless steel corrosion in different environments. J. Mol. Liq. 2022, 364, 119992. [Google Scholar] [CrossRef]
- Odio, B.; Chinwuko, E.; Chukwuneke, J.; Sinebe, J. Investigation of the effect of corrosion on mild steel in five different environments. Int. J. Sci. Technol. Res. 2014, 3, 306–310. [Google Scholar]
- Maqsood, M.F.; Raza, M.A.; Rehman, Z.U.; Abid, M.; Inam, A.; Iqbal, S. Corrosion study of zinc rich epoxy ester paints for cold galvanizing of mild steel. Surf. Rev. Lett. 2021, 28, 2150064. [Google Scholar] [CrossRef]
- Caldona, E.B.; Wipf, D.O.; Smith, D.W., Jr. Characterization of a tetrafunctional epoxy-amine coating for corrosion protection of mild steel. Prog. Org. Coat. 2021, 151, 106045. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Molaei, M.; Babaei, K.; Fattah-alhosseini, A. New promising ceramic coatings for corrosion and wear protection of steels: A review. Surf. Interfaces 2021, 23, 100997. [Google Scholar] [CrossRef]
- Randis, R.; Darmadi, D.B.; Gapsari, F.; Sonief, A.A.A.; Akpan, E.D.; Ebenso, E.E. The potential of nanocomposite-based coatings for corrosion protection of metals: A review. J. Mol. Liq. 2023, 390, 123067. [Google Scholar] [CrossRef]
- Farh, H.M.H.; Seghier, M.E.A.B.; Zayed, T. A comprehensive review of corrosion protection and control techniques for metallic pipelines. Eng. Fail. Anal. 2023, 143, 106885. [Google Scholar] [CrossRef]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Nazdracheva, T. Anti-corrosion coatings for protection of steel railway structures exposed to atmospheric environments: A review. Constr. Build. Mater. 2021, 288, 123115. [Google Scholar] [CrossRef]
- Nadeem, A.; Maqsood, M.F.; Raza, M.A.; Karim, M.R.; Ghafoor, F.; Lee, Y.; Ali, S.; Rehman, M.A.; Khan, M.F. Thermally stable and anti-corrosive polydimethyl siloxane composite coatings based on nanoforms of boron nitride. Inorg. Chem. Commun. 2024, 168, 112989. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Sun, Y.; Zhao, W.; Wang, L. 2D nanomaterials reinforced organic coatings for marine corrosion protection: State of the art, challenges, and future prospectives. Adv. Mater. 2024, 36, 2312460. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Zubair, M.A.A.; Raza, M.A.; Mehdi, S.M.Z.; Lee, N.; Rehman, Z.U.; Park, K.; Bhatti, M.U.; Latif, U.; Tawakkal, A. Fabrication and characterization of graphene oxide and glass fiber-based hybrid epoxy composites. Polym. Compos. 2022, 43, 8072–8083. [Google Scholar] [CrossRef]
- Raza, M.A.; Maqsood, M.F.; Rehman, Z.U.; Westwood, A.; Inam, A.; Sattar, M.M.S.; Ghauri, F.A.; Ilyas, M.T. Thermally Reduced Graphene Oxide-Reinforced Acrylonitrile Butadiene Styrene Composites Developed by Combined Solution and Melt Mixing Method. Arab. J. Sci. Eng. 2020, 45, 9559–9568. [Google Scholar] [CrossRef]
- Naclerio, A.E.; Kidambi, P.R. A review of scalable hexagonal boron nitride (h-BN) synthesis for present and future applications. Adv. Mater. 2023, 35, 2207374. [Google Scholar] [CrossRef]
- Nadeem, A.; Raza, M.A.; Maqsood, M.F.; Ilyas, M.T.; Westwood, A.; Rehman, Z.U. Characterization of boron nitride nanosheets synthesized by boron-ammonia reaction. Ceram. Int. 2020, 46, 20415–20422. [Google Scholar] [CrossRef]
- Nadeem, A.; Maqsood, M.F.; Raza, M.A.; Ilyas, M.T.; Iqbal, M.J.; Rehman, Z.U. Binder free boron nitride-based coatings deposited on mild steel by chemical vapour deposition: Anti-corrosion performance analysis. Phys. B Condens. Matter 2021, 602, 412600. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Qiu, F.; Chen, C.; Liu, L.; Dai, G. Effect of h-BN Orientation on the Corrosion Resistance of Bioinspired Layered Phosphate Composite Coatings. ACS Appl. Nano Mater. 2024, 7, 20733–20744. [Google Scholar] [CrossRef]
- Luo, X.; Li, X.; Li, M.; Ding, D.; Tong, L. A bi-layer superhydrophobic coating with long-term anti-corrosion enabled by functionalized hexagonal boron nitride and PDMS-based polyurethane on Mg alloy. Polymer 2024, 315, 127816. [Google Scholar] [CrossRef]
- Yu, R.; Yuan, X. Rising of boron nitride: A review on boron nitride nanosheets enhanced anti-corrosion coatings. Prog. Org. Coat. 2024, 186, 107990. [Google Scholar] [CrossRef]
- Verma, C.; Dubey, S.; Barsoum, I.; Alfantazi, A.; Ebenso, E.E.; Quraishi, M. Hexagonal boron nitride as a cutting-edge 2D material for additive application in anticorrosive coatings: Recent progress, challenges and opportunities. Mater. Today Commun. 2023, 35, 106367. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Mehdi, S.M.Z.; Ashraf, A.; Azhar, U.; Abbas, N.; Raza, M.A.; Amer, M. Binder-Free Hexagonal Boron Nitride Nanosheets (BNNSs) as Protective Coatings for Copper, Steel, and Wood: A Review. Crystals 2025, 15, 99. [Google Scholar] [CrossRef]
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Babu, G.; Susarla, S.; Saju, S.K.; Tran, M.K. Structure, properties and applications of two-dimensional hexagonal boron nitride. Adv. Mater. 2021, 33, 2101589. [Google Scholar] [CrossRef]
- Li, M.; Huang, G.; Chen, X.; Yin, J.; Zhang, P.; Yao, Y.; Shen, J.; Wu, Y.; Huang, J. Perspectives on environmental applications of hexagonal boron nitride nanomaterials. Nano Today 2022, 44, 101486. [Google Scholar] [CrossRef]
- Mehdi, S.M.Z.; Maqsood, M.F.; Dahshan, A.; Ahmad, S.; Ur Rehman, M.; Lee, N.; Rehman, M.A.; Khan, M.F. Emerging boron nitride nanosheets: A review on synthesis, corrosion resistance coatings, and their impacts on the environment and health. Rev. Adv. Mater. Sci. 2024, 63, 20240075. [Google Scholar] [CrossRef]
- Mane, S.K.B.; Shaishta, N.; Manjunatha, G. Biocompatibility, toxicity evaluations, environmental and health impact of hexagonal boron nitride. In Hexagonal Boron Nitride; Elsevier: Amsterdam, The Netherlands, 2024; pp. 613–636. [Google Scholar]
- Jiang, X.-F. Recent progress on fabrications and applications of boron nitride nanomaterials: A review. J. Mater. Sci. Technol. 2015, 31, 589–598. [Google Scholar] [CrossRef]
- Seo, J.-W.; Pophali, A.; An, S.; Liang, C.S.L.; Li, S.; Liu, H.; Kim, J.; An, K.; Kim, J.; Kim, T. Fundamental structural study of hexagonal boron nitride (h-BN) and boron nitride nanotube (BNNT) at low and high temperatures. J. Mol. Struct. 2025, 1319, 139545. [Google Scholar] [CrossRef]
- Han, N.; Wang, S.; Rana, A.K.; Asif, S.; Klemeš, J.J.; Bokhari, A.; Long, J.; Thakur, V.K.; Zhao, X. Rational design of boron nitride with different dimensionalities for sustainable applications. Renew. Sustain. Energy Rev. 2022, 170, 112910. [Google Scholar] [CrossRef]
- Wadhwa, G.K.; Late, D.J.; Charhate, S.; Sankhyan, S.B. 1D and 2D Boron Nitride Nano Structures: A Critical Analysis for Emerging Applications in the Field of Nanocomposites. ACS Omega 2024, 9, 26737–26761. [Google Scholar] [CrossRef]
- Mapleback, B.J.; Brack, N.; Thomson, L.; Spencer, M.J.; Osborne, D.A.; Doshi, S.; Thostenson, E.T.; Rider, A.N. Development of stable boron nitride nanotube and hexagonal boron nitride dispersions for electrophoretic deposition. Langmuir 2020, 36, 3425–3438. [Google Scholar] [CrossRef]
- Sukanya, R.; Barwa, T.N.; Luo, Y.; Dempsey, E.; Breslin, C.B. Emerging layered materials and their applications in the corrosion protection of metals and alloys. Sustainability 2022, 14, 4079. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Xiao, X.; Wang, N.; Meng, G.; Gu, L. Graphene-like two-dimensional nanosheets-based anticorrosive coatings: A review. J. Mater. Sci. Technol. 2022, 129, 139–162. [Google Scholar] [CrossRef]
- Shang, C.; Sang, D.; Li, C.; Zou, L.; Wu, J.; Wang, Q. 2D materials for marine corrosion protection: A review. APL Mater. 2024, 12, 060601. [Google Scholar] [CrossRef]
- Liu, H.; Yan, M.; Jing, W.; Zeng, G.; Gengxin, X.; Pu, X.; Fu, Y.; Peng, X.; Wang, H.; Lai, C. Hexagonal boron nitride for extreme environment application. Diam. Relat. Mater. 2024, 148, 111410. [Google Scholar] [CrossRef]
- Tang, X.; Wang, H.; Liu, C.; Zhu, X.; Gao, W.; Yin, H. Direct growth of hexagonal boron nitride nanofilms on stainless steel for corrosion protection. ACS Appl. Nano Mater. 2021, 4, 12024–12033. [Google Scholar] [CrossRef]
- Deshmukh, K.; Pandey, M.; Hussain, C.M. Hexagonal Boron Nitride: Synthesis, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Farhan, M.; Subhan, M.; Naseem, S.; Riaz, S.; Awan, R.; Mujahid, A.; Afzal, A. Polycrystalline boron nitride microflowers for non-invasive vitamin B3 (nicotinamide) sensing. Diam. Relat. Mater. 2025, 155, 112296. [Google Scholar] [CrossRef]
- Liu, Z.; Rhimi, B.; Liu, Z.; Mao, L.; Zhou, M.; Jiang, Z.; Jiang, Z. Modulating Defect Concentration of Boron Nitride Flowers for CO2 Photoreduction. Inorg. Chem. 2025, 64, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, A.; Zhi, C.; Bando, Y.; Nakayama, T.; Golberg, D. Boron Nitride Nanosheet Coatings with Controllable Water Repellency. ACS Nano 2011, 5, 6507–6515. [Google Scholar] [CrossRef]
- Kumar Verma, A.; Govind Rajan, A. Surface roughness explains the observed water contact angle and slip length on 2D hexagonal boron nitride. Langmuir 2022, 38, 9210–9220. [Google Scholar] [CrossRef]
- Yang, F.; McQuain, A.D.; Kumari, A.; Gundurao, D.; Liu, H.; Li, L. Understanding the intrinsic water wettability of hexagonal boron nitride. Langmuir 2024, 40, 6445–6452. [Google Scholar] [CrossRef]
- McLean, B.; Eveleens, C.A.; Mitchell, I.; Webber, G.B.; Page, A.J. Catalytic CVD synthesis of boron nitride and carbon nanomaterials–synergies between experiment and theory. Phys. Chem. Chem. Phys. 2017, 19, 26466–26494. [Google Scholar] [CrossRef]
- Ma, R.; Bando, Y.; Sato, T.; Kurashima, K. Growth, morphology, and structure of boron nitride nanotubes. Chem. Mater. 2001, 13, 2965–2971. [Google Scholar] [CrossRef]
- Pakdel, A.; Zhi, C.; Bando, Y.; Nakayama, T.; Golberg, D. A comprehensive analysis of the CVD growth of boron nitride nanotubes. Nanotechnology 2012, 23, 215601. [Google Scholar] [CrossRef]
- Gohier, A.; Ewels, C.; Minea, T.; Djouadi, M. Carbon nanotube growth mechanism switches from tip-to base-growth with decreasing catalyst particle size. Carbon 2008, 46, 1331–1338. [Google Scholar] [CrossRef]
- Yoshida, H.; Takeda, S.; Uchiyama, T.; Kohno, H.; Homma, Y. Atomic-scale in-situ observation of carbon nanotube growth from solid state iron carbide nanoparticles. Nano Lett. 2008, 8, 2082–2086. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J. Diameter controlled growth of single-walled carbon nanotubes from SiO2 nanoparticles. Carbon 2011, 49, 3316–3324. [Google Scholar] [CrossRef]
- Lee, C.H.; Xie, M.; Kayastha, V.; Wang, J.; Yap, Y.K. Patterned growth of boron nitride nanotubes by catalytic chemical vapor deposition. Chem. Mater. 2010, 22, 1782–1787. [Google Scholar] [CrossRef]
- Ma, R.; Bando, Y.; Sato, T. Coaxial nanocables: Fe nanowires encapsulated in BN nanotubes with intermediate C layers. Chem. Phys. Lett. 2001, 350, 1–5. [Google Scholar] [CrossRef]
- Lourie, O.R.; Jones, C.R.; Bartlett, B.M.; Gibbons, P.C.; Ruoff, R.S.; Buhro, W.E. CVD growth of boron nitride nanotubes. Chem. Mater. 2000, 12, 1808–1810. [Google Scholar] [CrossRef]
- Caneva, S.; Weatherup, R.S.; Bayer, B.C.; Blume, R.; Cabrero-Vilatela, A.; Braeuninger-Weimer, P.; Martin, M.-B.; Wang, R.; Baehtz, C.; Schloegl, R. Controlling catalyst bulk reservoir effects for monolayer hexagonal boron nitride CVD. Nano Lett. 2016, 16, 1250–1261. [Google Scholar] [CrossRef]
- Jana, M.; Singh, R.N. Progress in CVD synthesis of layered hexagonal boron nitride with tunable properties and their applications. Int. Mater. Rev. 2018, 63, 162–203. [Google Scholar] [CrossRef]
- Caneva, S.; Weatherup, R.S.; Bayer, B.C.; Brennan, B.; Spencer, S.J.; Mingard, K.; Cabrero-Vilatela, A.; Baehtz, C.; Pollard, A.J.; Hofmann, S. Nucleation control for large, single crystalline domains of monolayer hexagonal boron nitride via Si-doped Fe catalysts. Nano Lett. 2015, 15, 1867–1875. [Google Scholar] [CrossRef]
- McLean, B.; Webber, G.B.; Page, A.J. Energy and Charge Transfer at the Boron Nitride Nanotube—Catalyst Growth Interface. J. Phys. Chem. C 2020, 124, 11662–11668. [Google Scholar] [CrossRef]
- McLean, B.; Webber, G.B.; Page, A.J. Boron nitride nanotube nucleation via network fusion during catalytic chemical vapor deposition. J. Am. Chem. Soc. 2019, 141, 13385–13393. [Google Scholar] [CrossRef]
- McLean, B. Growth Mechanisms of Boron Nitride Nanotubes during Chemical Vapour Deposition. Ph.D Thesis, University of Newcastle, New South Wales, Australia, 2019. [Google Scholar]
- Lee, C.H.; Drelich, J.; Yap, Y.K. Superhydrophobicity of boron nitride nanotubes grown on silicon substrates. Langmuir 2009, 25, 4853–4860. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Mitropoulos, A.C. Advanced Low-Cost Separation Techniques in Interface Science; Academic Press: Cambridge, MA, USA, 2019; Volume 30. [Google Scholar]
- Fillion, R.; Riahi, A.; Edrisy, A. A review of icing prevention in photovoltaic devices by surface engineering. Renew. Sustain. Energy Rev. 2014, 32, 797–809. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, H.; Li, C. Composite hollow fiber membrane dehumidification: A review on membrane module, moisture permeability and self-cleaning performance. Int. J. Heat Mass Transf. 2021, 181, 121832. [Google Scholar] [CrossRef]
- Wang, Z.; Paul, S.; Stein, L.H.; Salemi, A.; Mitra, S. Recent developments in blood-compatible superhydrophobic surfaces. Polymers 2022, 14, 1075. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Raza, M.A.; Rehman, Z.U.; Tayyeb, A.; Makhdoom, M.A.; Ghafoor, F.; Latif, U.; Khan, M.F. Role of Solvent Used in Development of Graphene Oxide Coating on AZ31B Magnesium Alloy: Corrosion Behavior and Biocompatibility Analysis. Nanomaterials 2022, 12, 3745. [Google Scholar] [CrossRef]




| Sample | βa (mV/Decade) | βc (mV/Decade) | Icorr (μA/cm2) | Εcorr (mV) | Rp (Ω·cm2) | Avg. Corrosion Rate (mpy) |
|---|---|---|---|---|---|---|
| Bare MS | 73.30 | 325.7 | 9.450 ± 0.75 | 18.50 | 2.749 | 4.316 |
| BN-15 | 80.10 | 348.7 | 5.170 ± 0.082 | −21.70 | 5.471 | 2.363 |
| BN-30 | 69.00 | 371.4 | 0.974 ± 0.023 | −33.80 | 25.94 | 0.445 |
| BN-60 | 66.90 | 468.6 | 4.080 ± 0.047 | −21.90 | 6.230 | 1.867 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadeem, A.; Maqsood, M.F.; Raza, M.A.; Mehdi, S.M.Z.; Ahmad, S. Hydrophobic Boron Nitride Nanoflower Coatings on Mild Steel Surfaces. Surfaces 2025, 8, 42. https://doi.org/10.3390/surfaces8030042
Nadeem A, Maqsood MF, Raza MA, Mehdi SMZ, Ahmad S. Hydrophobic Boron Nitride Nanoflower Coatings on Mild Steel Surfaces. Surfaces. 2025; 8(3):42. https://doi.org/10.3390/surfaces8030042
Chicago/Turabian StyleNadeem, Aamir, Muhammad Faheem Maqsood, Mohsin Ali Raza, Syed Muhammad Zain Mehdi, and Shahbaz Ahmad. 2025. "Hydrophobic Boron Nitride Nanoflower Coatings on Mild Steel Surfaces" Surfaces 8, no. 3: 42. https://doi.org/10.3390/surfaces8030042
APA StyleNadeem, A., Maqsood, M. F., Raza, M. A., Mehdi, S. M. Z., & Ahmad, S. (2025). Hydrophobic Boron Nitride Nanoflower Coatings on Mild Steel Surfaces. Surfaces, 8(3), 42. https://doi.org/10.3390/surfaces8030042










