Drying and Film Formation Processes of Graphene Oxide Suspension on Nonwoven Fibrous Membranes with Varying Wettability
Abstract
1. Introduction
2. Materials and Methods
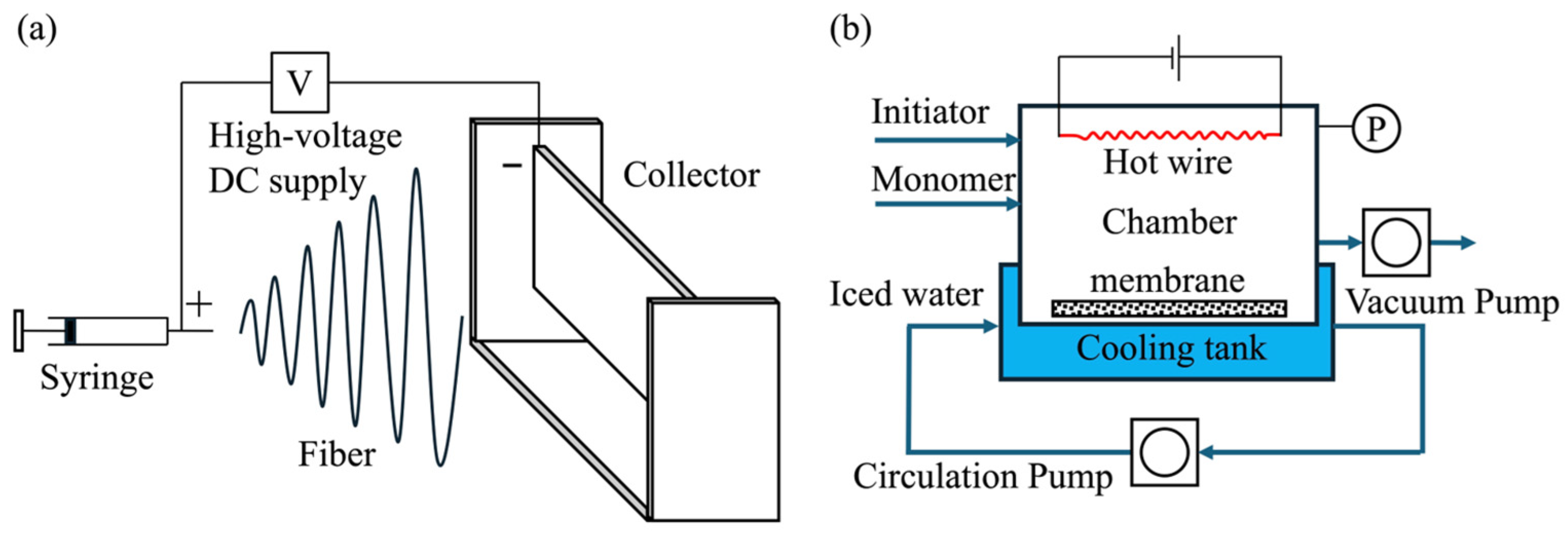
2.1. Electrospinning
2.2. Surface Fluorination Treatment
2.3. Contact Angle
2.4. GO Film Preparation
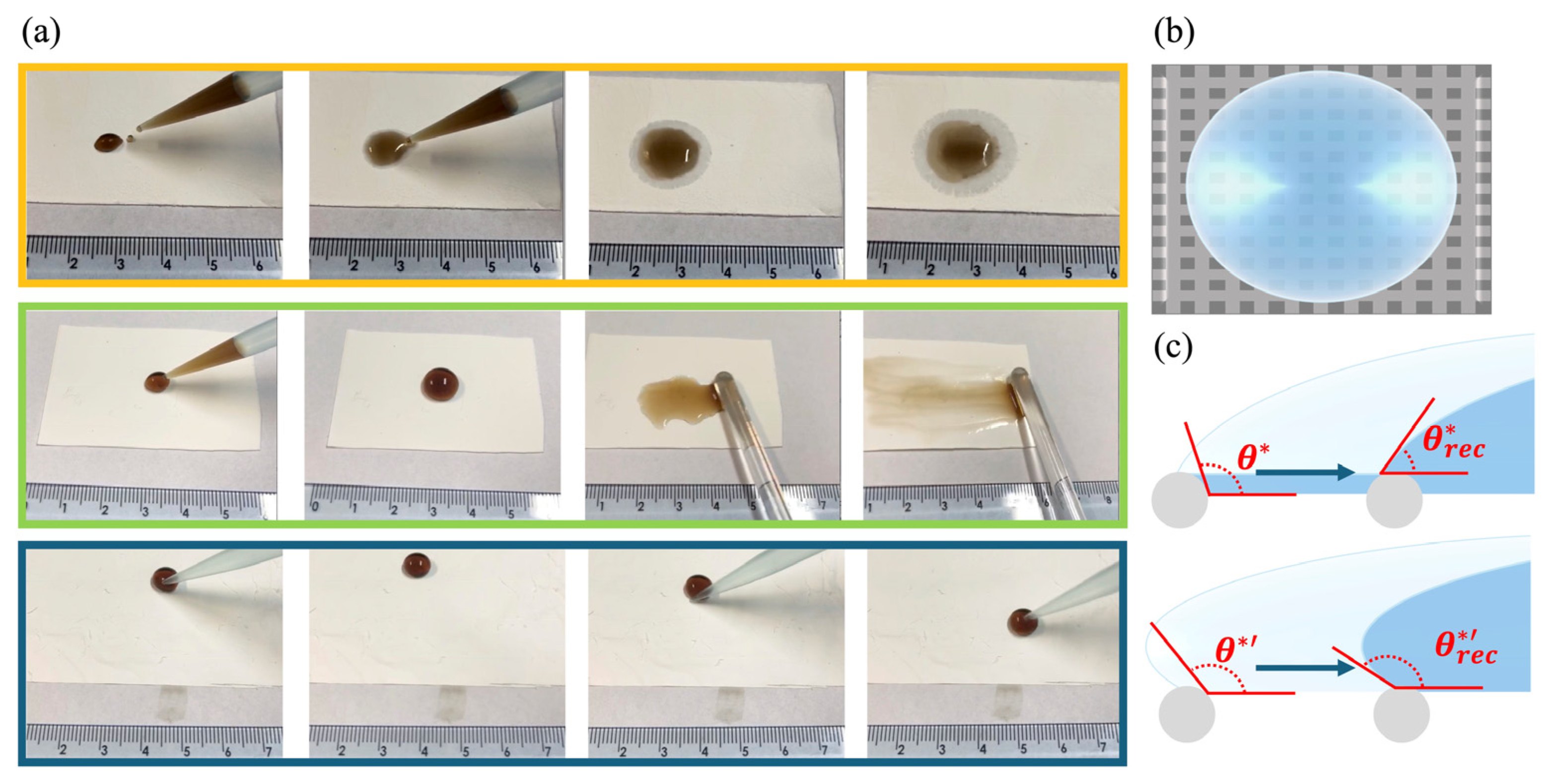
2.4.1. Spreading Behaviors of GO Droplets on Fibrous Membranes with Varying Wettability
2.4.2. GO Film Preparation on Fibrous Membranes with Varying Wettability
3. Results and Discussion
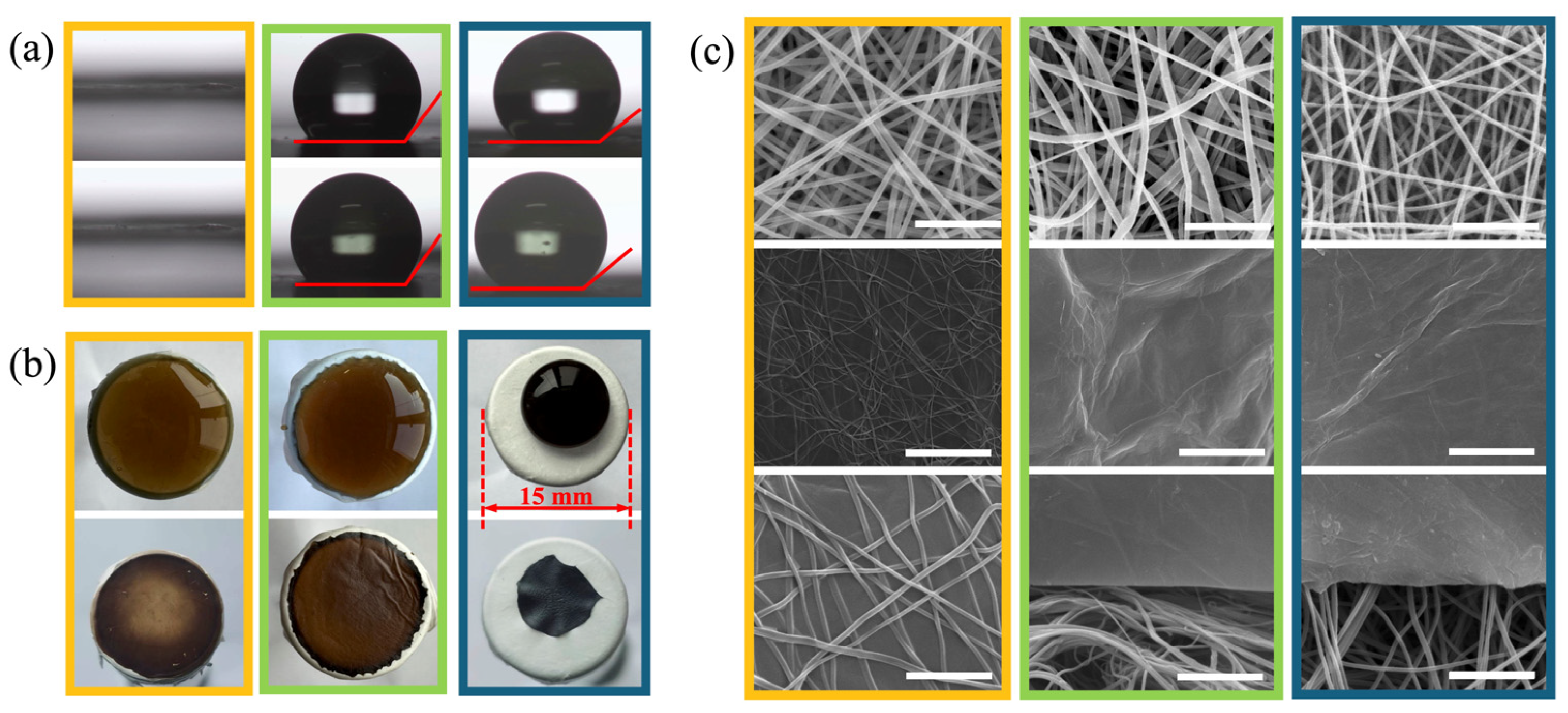
3.1. Preparation of Fibrous Membranes and GO Films
3.2. Spreading Behaviors of GO Droplets on Fibrous Membranes with Varying Wettability
3.3. Morphologies of GO Films
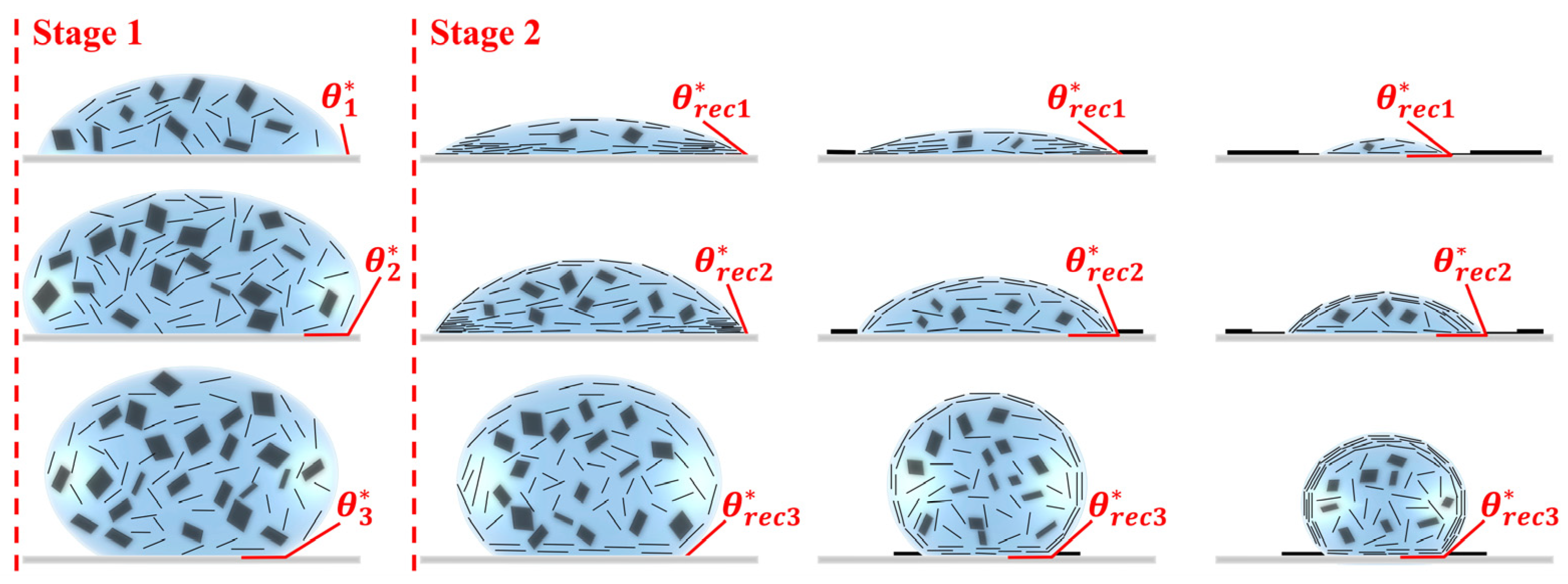
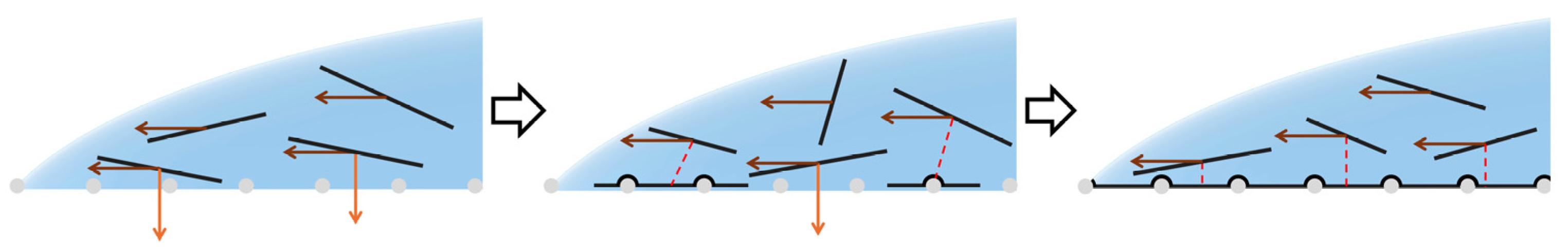
3.4. Mechanisms of GO Film Formation
3.4.1. Hydrophilic Fibrous Membrane
3.4.2. Hydrophobic Fibrous Membrane
3.4.3. Superhydrophobic Fibrous Membrane
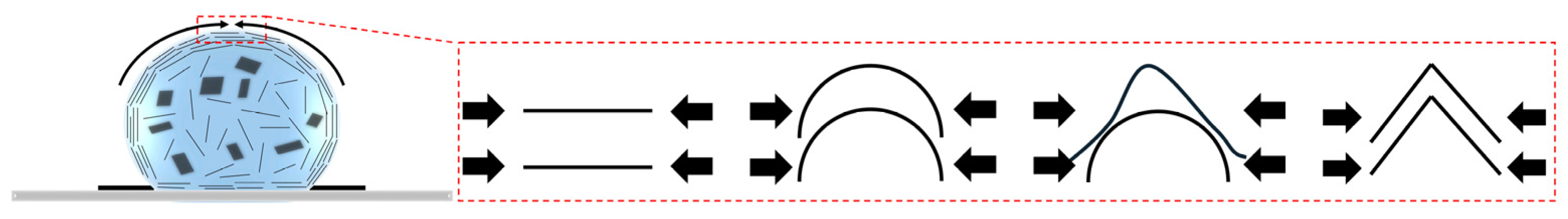
3.5. Wrinkling
3.6. Suppression of Wrinkling
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary Flow as the Cause of Ring Stains from Dried Liquid Drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical Properties of Monolayer Graphene Oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Asghar, F.; Shakoor, B.; Fatima, S.; Munir, S.; Razzaq, H.; Naheed, S.; Butler, I.S. Fabrication and Prospective Applications of Graphene Oxide-Modified Nanocomposites for Wastewater Remediation. RSC Adv. 2022, 12, 11750–11768. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, X.; Wang, Q.; Liu, Y.; Liu, G.; Wang, K. Enhancing Helium Separation by Ionic-Liquid-Modulated Nanoporous Graphene Oxide Membrane. J. Membr. Sci. 2025, 722, 123861. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-Quality Graphene via Microwave Reduction of Solution-Exfoliated Graphene Oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef]
- Liu, Z.-B.; Xu, Y.-F.; Zhang, X.-Y.; Zhang, X.-L.; Chen, Y.-S.; Tian, J.-G. Porphyrin and Fullerene Covalently Functionalized Graphene Hybrid Materials with Large Nonlinear Optical Properties. J. Phys. Chem. B 2009, 113, 9681–9686. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Centeno, A.; Zurutuza, A.; Cocemasov, A.I.; Nika, D.L.; Balandin, A.A. Strongly Anisotropic Thermal Conductivity of Free-Standing Reduced Graphene Oxide Films Annealed at High Temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, J.; Mu, X.; Qiu, C.; Kang, M.; Zhang, Z.; Gao, J.; Liu, J.; Miao, L. Photo-Fenton Catalyst Embedded in Photothermal Aerogel for Efficient Solar Interfacial Water Evaporation and Purification. Green Carbon 2024, S2950155524000843, in press. [Google Scholar] [CrossRef]
- Wei, W.; Wei, Z.; Li, R.; Yuan, H.; Chen, J.; Lv, J.; Ouyang, S. Fine-Tuning N-Doped Species of C Catalysts for 98% Current Efficiency of Electrocatalytic Decarboxylation into Hindered Ether. Green Carbon 2024, 2, 291–299. [Google Scholar] [CrossRef]
- He, P.; Derby, B. Controlling Coffee Ring Formation during Drying of Inkjet Printed 2D Inks. Adv. Mater. Interfaces 2017, 4, 1700944. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, X.; Thebo, K.H.; Cheng, H.-M.; Ren, W. Controlling Reduction Degree of Graphene Oxide Membranes for Improved Water Permeance. Sci. Bull. 2018, 63, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huang, S.; Xin, W.; He, X.; Wan, S.; Yang, C.; Zhao, J.; Shi, L.; Yan, H.; Zhou, T.; et al. High-Performance Graphene Oxide / Sodium Alginate Composite Membrane for Marine Osmotic Energy Conversion. J. Membr. Sci. 2025, 724, 123987. [Google Scholar] [CrossRef]
- Xing, C.; Liu, C.-Y.; Lai, C.; Zhang, S.-Q. Tuning D-Spacing of Graphene Oxide Nanofiltration Membrane for Effective Dye/Salt Separation. Rare Met. 2023, 42, 418–429. [Google Scholar] [CrossRef]
- Mazlan, N.A.; Lewis, A.; Chen, Z.; Butt, F.S.; Han, J.; Radacsi, N.; Yang, S.; Huang, Y. Photocatalytic Self-Cleaning Graphene Oxide/ZnO Hybrid Membrane for Ultrafast Cyclic Small Organic Molecule Separation. J. Membr. Sci. 2024, 697, 122539. [Google Scholar] [CrossRef]
- Kim, J.P.; Go, C.Y.; Kang, J.; Choi, Y.; Kim, J.Y.; Kim, J.; Kwon, O.; Kim, K.C.; Kim, D.W. Nanoporous Multilayer Graphene Oxide Membrane for Forward Osmosis Metal Ion Recovery from Spent Li-Ion Batteries. J. Membr. Sci. 2023, 676, 121590. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Liang, B.; Liu, Y.; Xu, T.; Wang, L.; Cao, B.; Pan, K. Graphene Oxide as an Effective Barrier on a Porous Nanofibrous Membrane for Water Treatment. ACS Appl. Mater. Interfaces 2016, 8, 6211–6218. [Google Scholar] [CrossRef]
- Guan, K.; Shen, J.; Liu, G.; Zhao, J.; Zhou, H.; Jin, W. Spray-Evaporation Assembled Graphene Oxide Membranes for Selective Hydrogen Transport. Sep. Purif. Technol. 2017, 174, 126–135. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Zhang, N.; Bao, J.; Zhang, X.; He, G.; Chen, C.; Song, Y. Ultra-Thin Size-Sieving Gas Separation Membrane by Precisely Contra-Diffusion-Match Growth of ZIF-67 among GO Laminates. J. Membr. Sci. 2024, 694, 122428. [Google Scholar] [CrossRef]
- Chong, J.; Wang, B.; Li, K. Graphene Oxide Membranes in Fluid Separations. Curr. Opin. Chem. Eng. 2016, 12, 98–105. [Google Scholar] [CrossRef]
- Kong, F.; Liu, Q.; Dong, L.; Zhang, T.; Wei, Y.; Chen, J.; Wang, Y.; Guo, C. Rejection of Pharmaceuticals by Graphene Oxide Membranes: Role of Crosslinker and Rejection Mechanism. J. Membr. Sci. 2020, 612, 118338. [Google Scholar] [CrossRef]
- Wu, D.; He, Y.; Wu, J.; He, Y.; Peng, W. Photocatalytic Self-Cleaning Holey Graphene Oxide Membranes by a Synergistic Strategy of Etching and Intercalation for Efficient Dye Separation. J. Membr. Sci. 2025, 716, 123514. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, H.; Cui, H.; Xiao, H.; Jiang, X.; Dai, Y.; Zhang, X.; Bao, J.; He, G. Bio-Inspired Molecular Bridge Anchoring GO Laminates onto PAN Substrate for Molecular Separation. Adv. Membr. 2022, 2, 100034. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; He, X.; Yi, C.; Xie, Z. Super-Crosslinked Graphene Oxide Membranes with Enhanced Stability and Selectivity for Efficient Alcohol/Water Separation via Pervaporation. J. Membr. Sci. 2025, 720, 123776. [Google Scholar] [CrossRef]
- Xu, Z.; Yan, X.; Du, Z.; Li, J.; Cheng, F. Effect of Oxygenic Groups on Desalination Performance Improvement of Graphene Oxide-Based Membrane in Membrane Distillation. Sep. Purif. Technol. 2020, 251, 117304. [Google Scholar] [CrossRef]
- Zeynali, R.; Ghasemzadeh, K.; Sarand, A.B.; Kheiri, F.; Basile, A. Performance Evaluation of Graphene Oxide (GO) Nanocomposite Membrane for Hydrogen Separation: Effect of Dip Coating Sol Concentration. Sep. Purif. Technol. 2018, 200, 169–176. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Layer-by-Layer Assembly of Graphene Oxide Membranes via Electrostatic Interaction. J. Membr. Sci. 2014, 469, 80–87. [Google Scholar] [CrossRef]
- Tsou, C.-H.; An, Q.-F.; Lo, S.-C.; De Guzman, M.; Hung, W.-S.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Effect of Microstructure of Graphene Oxide Fabricated through Different Self-Assembly Techniques on 1-Butanol Dehydration. J. Membr. Sci. 2015, 477, 93–100. [Google Scholar] [CrossRef]
- Chong, W.S.; Gan, S.X.; Al-Tuwirit, H.M.; Chong, W.Y.; Lim, C.S.; Ahmad, H. Nanolitre Solution Drop-Casting for Selective Area Graphene Oxide Coating on Planar Surfaces. Mater. Chem. Phys. 2020, 249, 122970. [Google Scholar] [CrossRef]
- Zhao, C.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Sai, H.; Li, Z.; Li, F. Formation of Uniform Reduced Graphene Oxide Films on Modified PET Substrates Using Drop-Casting Method. Particuology 2014, 17, 66–73. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, J.; Mao, Y.; Liu, G.; Jin, W. Effect of Substrate on Formation and Nanofiltration Performance of Graphene Oxide Membranes. J. Membr. Sci. 2019, 574, 196–204. [Google Scholar] [CrossRef]
- Kim, T.; Cho, H.; Choi, S.T.; Nam, W.; Lee, S.; Liang, H.; Kim, S. Controlled Deposition of Graphene Oxide on an Anodic Aluminum Oxide Substrate via Coffee-Ring Effect. J. Alloys Compd. 2023, 958, 170464. [Google Scholar] [CrossRef]
- Burton, N.D.; Zarshenas, K.; Somvanshi, C.; Chekini, M.; Grishkewich, N.; Ghanbari, S.; Abdala, A.; Gostick, J.T.; Pope, M.A. Unleashing the Potential of Scalable, High-Water Vapor Permeance Graphene Oxide Membranes Using Electrospun Supports. J. Membr. Sci. 2024, 690, 122142. [Google Scholar] [CrossRef]
- Gopal, R.; Kaur, S.; Feng, C.Y.; Chan, C.; Ramakrishna, S.; Tabe, S.; Matsuura, T. Electrospun Nanofibrous Polysulfone Membranes as Pre-Filters: Particulate Removal. J. Membr. Sci. 2007, 289, 210–219. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.C.; Matsuura, T.; Tabe, S.; Ismail, A.F. Preparation and Characterization of Electro-Spun Nanofiber Membranes and Their Possible Applications in Water Treatment. Sep. Purif. Technol. 2013, 102, 118–135. [Google Scholar] [CrossRef]
- Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous Materials and Their Applications. Annu. Rev. Mater. Res. 2006, 36, 333–368. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. Functional Nanofibers for Environmental Applications. J. Mater. Chem. 2008, 18, 5326. [Google Scholar] [CrossRef]
- Rashid, T.U.; Gorga, R.E.; Krause, W.E. Mechanical Properties of Electrospun Fibers—A Critical Review. Adv. Eng. Mater. 2021, 23, 2100153. [Google Scholar] [CrossRef]
- Wu, W.; Yu, L.; Li, L.; Li, Z.; Kang, J.; Pu, S.; Chen, D.; Ma, R.; An, K.; Liu, G.; et al. Electrospun Nanofiber Based Forward Osmosis Membrane Using Graphene Oxide as Substrate Modifier for Enhanced Water Flux and Rejection Performance. Desalination 2021, 518, 115283. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, L.; Yu, X.; Yang, Y.; Li, X.; Wang, X. Robust Construction of a Graphene Oxide Barrier Layer on a Nanofibrous Substrate Assisted by the Flexible Poly(Vinylalcohol) for Efficient Pervaporation Desalination. J. Mater. Chem. A 2017, 5, 3558–3568. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Butt, F.S.; Mazlan, N.A.; Chen, S.; Radacsi, N.; Yang, S.; Yoon, Y.; Huang, Y. Tuning the Interlayer Spacing of Graphene Oxide Membrane via Surfactant Intercalation for Ultrafast Nanofiltration. J. Membr. Sci. 2024, 706, 122942. [Google Scholar] [CrossRef]
- Lowery, J.L.; Datta, N.; Rutledge, G.C. Effect of Fiber Diameter, Pore Size and Seeding Method on Growth of Human Dermal Fibroblasts in Electrospun Poly(ɛ-Caprolactone) Fibrous Mats. Biomaterials 2010, 31, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Tuteja, A.; Mabry, J.M.; Cohen, R.E.; McKinley, G.H. A Modified Cassie–Baxter Relationship to Explain Contact Angle Hysteresis and Anisotropy on Non-Wetting Textured Surfaces. J. Colloid Interface Sci. 2009, 339, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and Characterization of Graphene Oxide Paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, X.; Zhao, Y.; Guo, F. Membrane Desalination Using Surface Fluorination Treated Electrospun Polyacrylonitrile Membranes with Nonwoven Structure and Quasi-Parallel Fibrous Structure. Desalination 2018, 429, 70–75. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene Oxide Sheets at Interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef]
- Soltman, D.; Subramanian, V. Inkjet-Printed Line Morphologies and Temperature Control of the Coffee Ring Effect. Langmuir 2008, 24, 2224–2231. [Google Scholar] [CrossRef]
- Hong, S.-H.; Shen, T.-Z.; Song, J.-K. Water Front Recession and the Formation of Various Types of Wrinkles in Dried Graphene Oxide Droplets. Carbon 2016, 105, 297–304. [Google Scholar] [CrossRef]
- Guo, F.; Kim, F.; Han, T.H.; Shenoy, V.B.; Huang, J.; Hurt, R.H. Hydration-Responsive Folding and Unfolding in Graphene Oxide Liquid Crystal Phases. ACS Nano 2011, 5, 8019–8025. [Google Scholar] [CrossRef]
- McBride, S.A.; Atis, S.; Pahlavan, A.A.; Varanasi, K.K. Crystal Patterning from Aqueous Solutions via Solutal Instabilities. ACS Appl. Mater. Interfaces 2024, 16, 70980–70990. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Contact Line Deposits in an Evaporating Drop. Phys. Rev. E 2000, 62, 756–765. [Google Scholar] [CrossRef]
- Shen, X.; Lin, X.; Yousefi, N.; Jia, J.; Kim, J.-K. Wrinkling in Graphene Sheets and Graphene Oxide Papers. Carbon 2014, 66, 84–92. [Google Scholar] [CrossRef]
- Luo, Y.; Braggin, G.A.; Olson, G.T.; Stevenson, A.R.; Ruan, W.L.; Zhang, S. Nematic Order Drives Macroscopic Patterns of Graphene Oxide in Drying Drops. Langmuir 2014, 30, 14631–14637. [Google Scholar] [CrossRef]
| Material | Concentration (wt%) | Temperature (°C) | Humidity (%) | Distance (cm) | Flow Rate (mL/min) | Positive Voltage (kV) | Negative Voltage (kV) |
|---|---|---|---|---|---|---|---|
| PAN | 13 | 25 | 20 | 20 | 0.015 | 15 | 4 |
| PVDF | 16 | 25 | 20 | 20 | 0.015 | 15 | 4 |
| Material | Fiber Distribution (nm) | Amount | Average (nm) |
|---|---|---|---|
| PAN | 200–300 | 14 | 300 |
| 300–400 | 16 | ||
| PVDF | 100–200 | 320 | |
| 200–300 | |||
| 300–400 | |||
| 400–500 | |||
| 500–600 | |||
| Surface fluorinated PAN | 200–300 | 20 | 290 |
| 300–400 | 8 | ||
| 400–500 | 2 |
| Material | Contact Angle (°) | Wettability |
|---|---|---|
| PAN | Unmeasured | Hydrophilic |
| PVDF | 120–144 (DI water) | Hydrophobic |
| 129–143 (GO suspension) | ||
| Surface-fluorinated PAN | 152–157 (DI water) | Superhydrophobic |
| 151–154 (GO suspension) |
| Wettability | Pattern | Fiber Encapsulation | Wrinkle | Mechanism |
|---|---|---|---|---|
| Hydrophilic | Belt | More than the upper half | Negligible | Suppressed coffee-ring effect |
| Hydrophobic | Ring | Approximately the upper half | Pronounced | Coffee-ring effect + interfacial GO layer deposition |
| Superhydrophobic | Circular | The top portion | Pronounced | Interfacial GO layer deposition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Fan, J.; Xue, J.; Guo, F. Drying and Film Formation Processes of Graphene Oxide Suspension on Nonwoven Fibrous Membranes with Varying Wettability. Surfaces 2025, 8, 39. https://doi.org/10.3390/surfaces8020039
Liu Z, Fan J, Xue J, Guo F. Drying and Film Formation Processes of Graphene Oxide Suspension on Nonwoven Fibrous Membranes with Varying Wettability. Surfaces. 2025; 8(2):39. https://doi.org/10.3390/surfaces8020039
Chicago/Turabian StyleLiu, Zeman, Jiaxing Fan, Jian Xue, and Fei Guo. 2025. "Drying and Film Formation Processes of Graphene Oxide Suspension on Nonwoven Fibrous Membranes with Varying Wettability" Surfaces 8, no. 2: 39. https://doi.org/10.3390/surfaces8020039
APA StyleLiu, Z., Fan, J., Xue, J., & Guo, F. (2025). Drying and Film Formation Processes of Graphene Oxide Suspension on Nonwoven Fibrous Membranes with Varying Wettability. Surfaces, 8(2), 39. https://doi.org/10.3390/surfaces8020039








