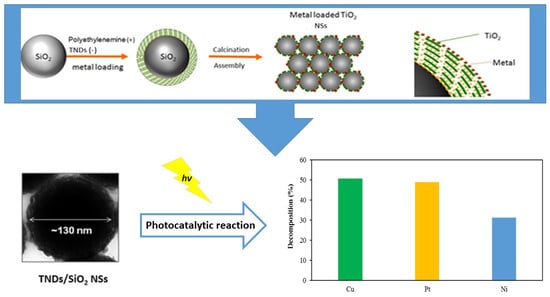
Metal-Supported TiO2/SiO2 Core-Shell Nanosphere Photocatalyst for Efficient Sunlight-Driven Methanol Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material Synthesis
2.2.1. Synthesis of TND
2.2.2. Synthesis of SiO2 NSs
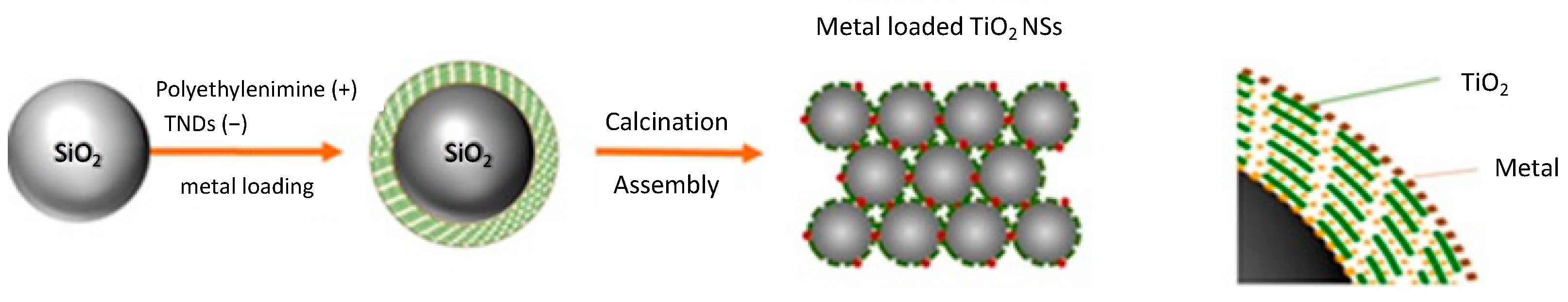
2.2.3. Depositing TNDs on SiO2 NSs
2.2.4. Depositing Metal on TNDs/SiO2 NSs
2.2.5. H2S Treatment
2.3. Material Characterization
2.4. Photocatalytic Experiments
3. Results
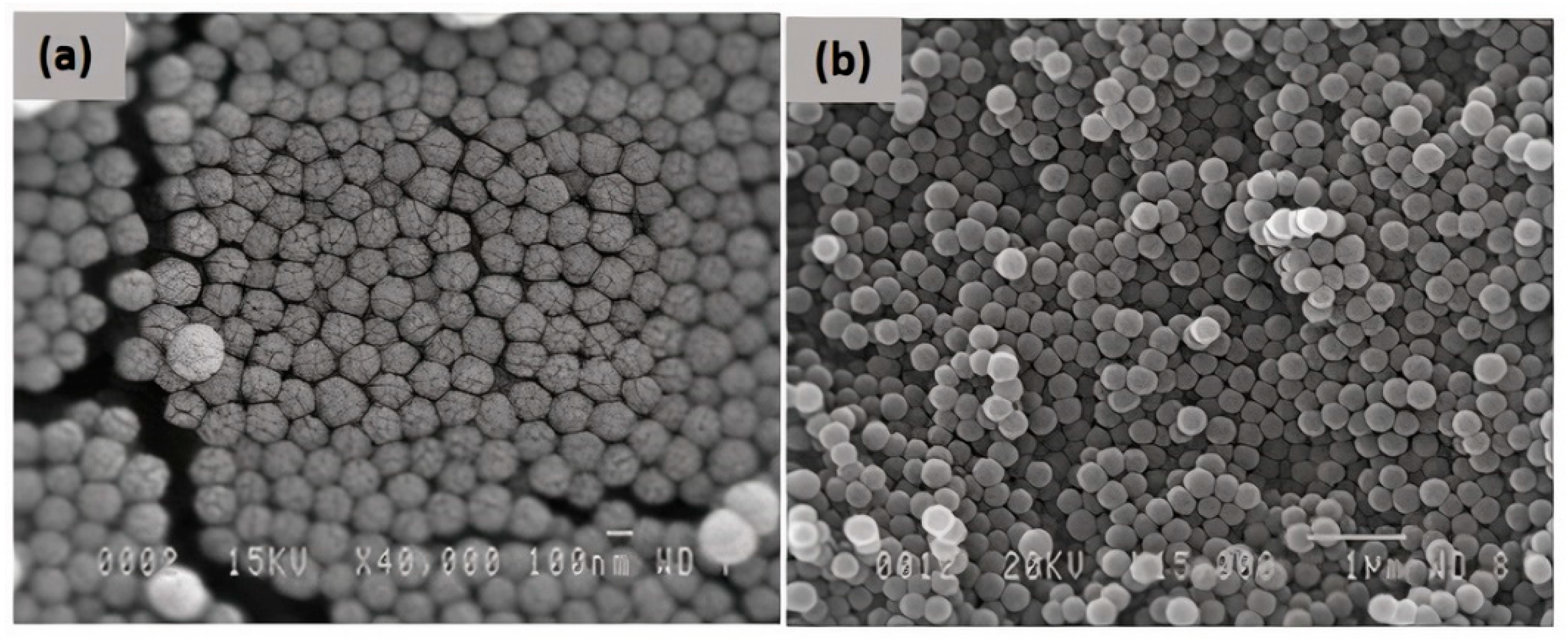
3.1. Microscopic Analysis of Prepared Samples
3.1.1. TNDs
3.1.2. SiO2
3.1.3. TND/SiO2
3.2. Analysis of the Effect of the Loaded Metal Type
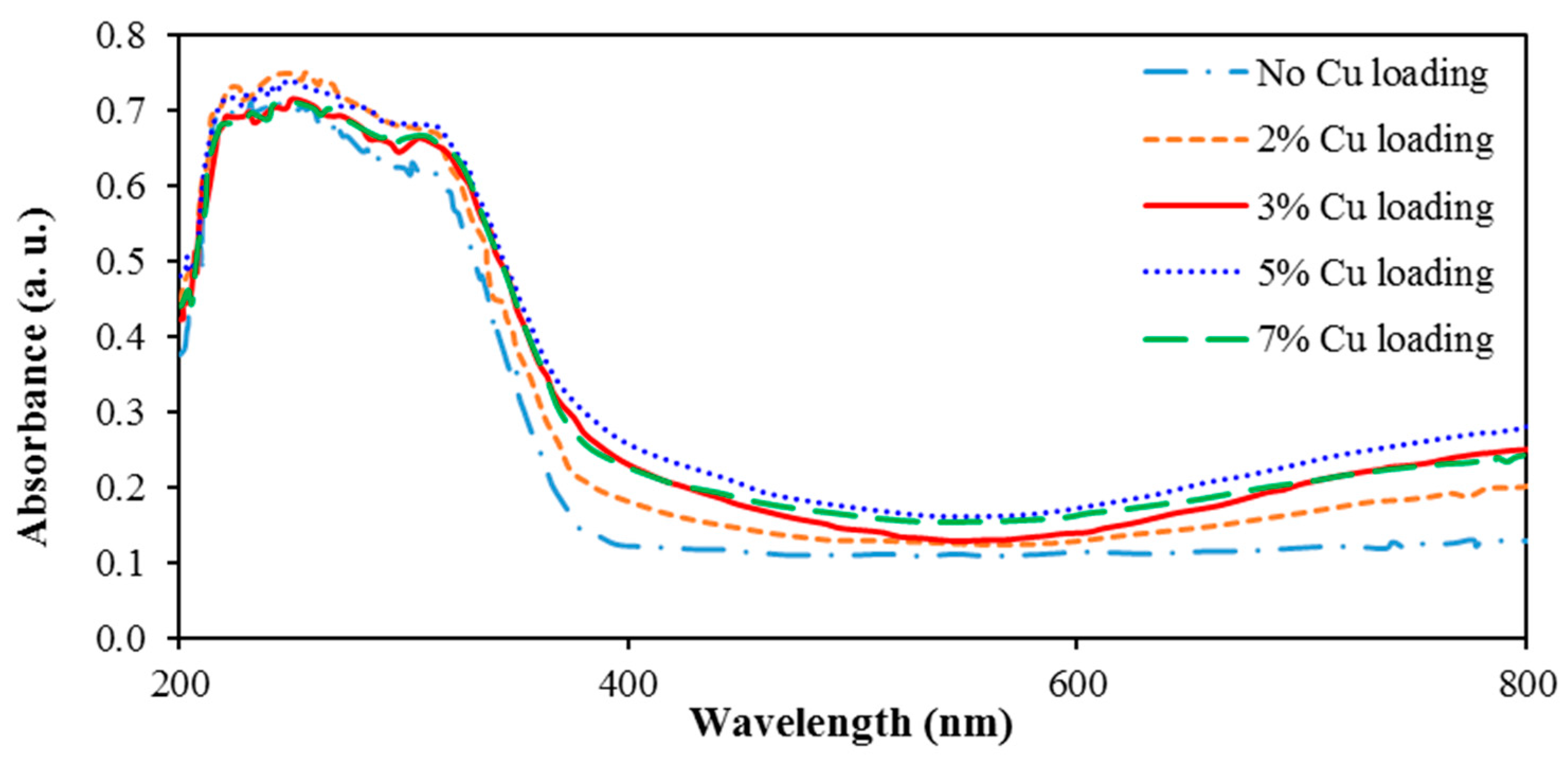
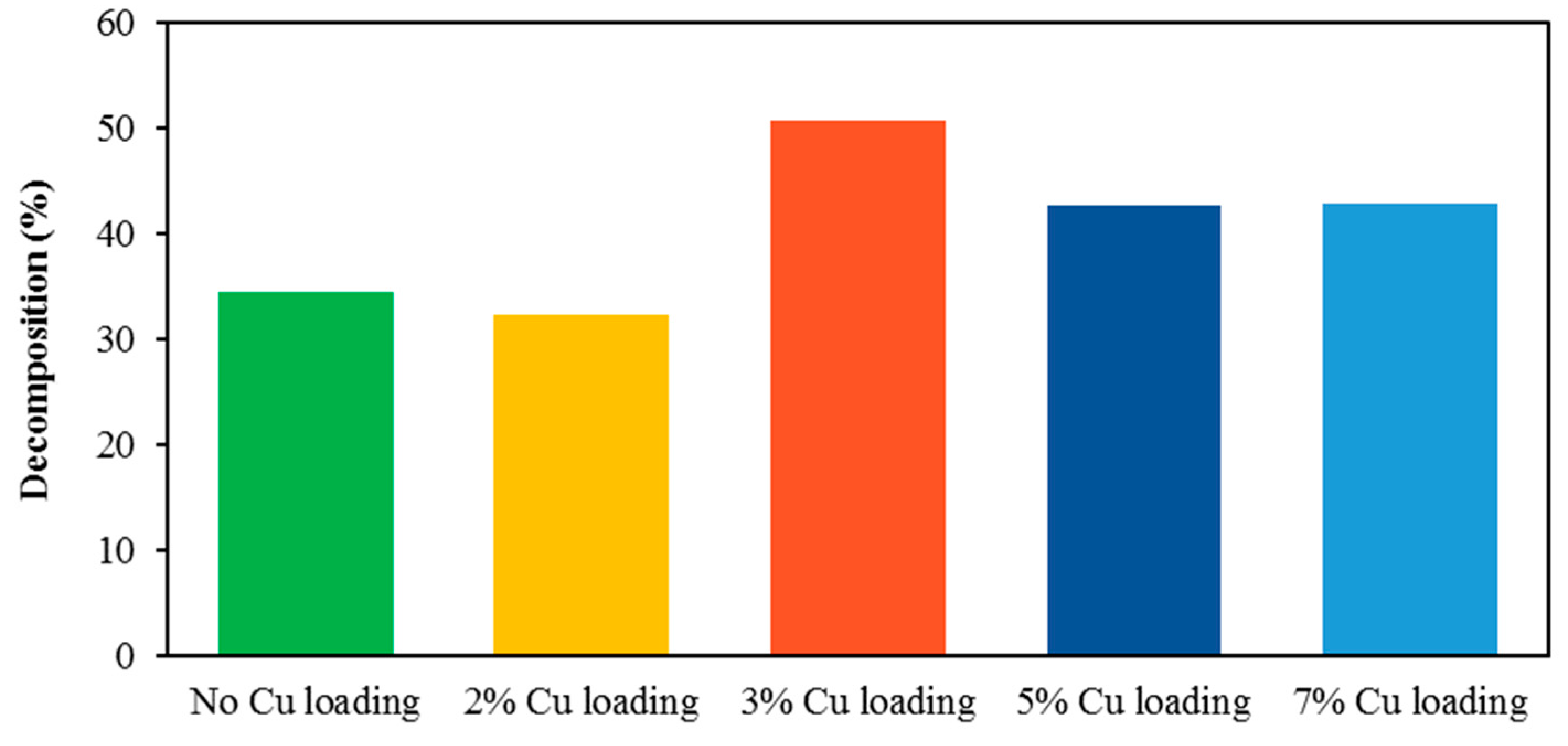
3.3. Analysis of the Effect of the Loaded Metal Content
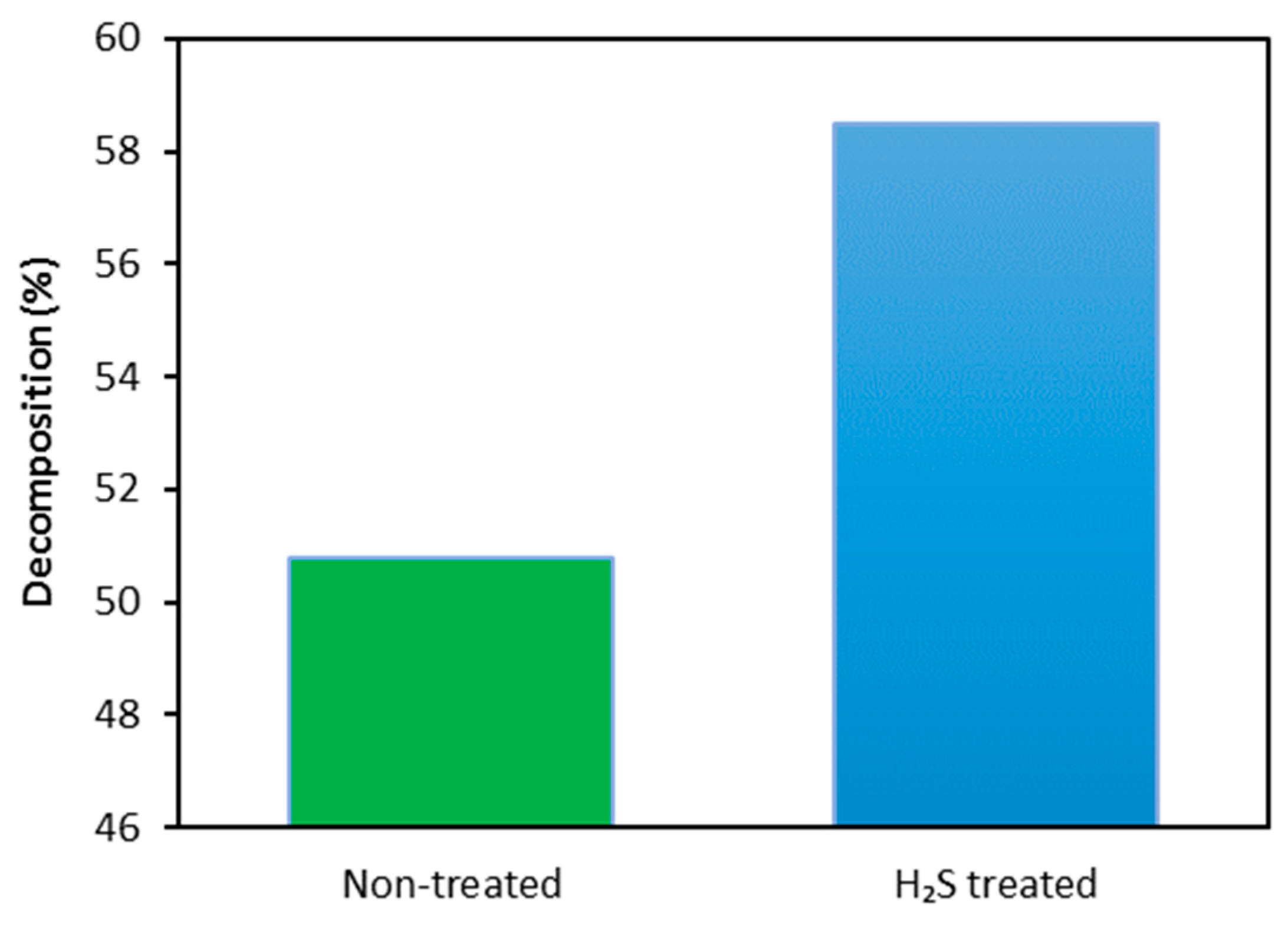
3.4. Analysis of the Effect of H2S Treatment
4. Conclusions
- The Cu-loaded TiO2/SiO2 NSs had the highest surface area of 109 m2/g, while it was 98 and 67 m2/g for the Ni- and Pt-loaded samples, respectively;
- Loading Cu, Ni, and Pt extended the absorption wavelength to 420, 400, and 390 nm, respectively;
- The photocatalytic activity of the Cu-deposited sample was 4% and 63% higher than the Pt- and Ni-deposited samples, respectively.
- The deposition of 3, 5, and 7% Cu fairly similarly extended the absorption wavelength from 390 nm (for blank TiO2) up to 420 nm;
- The highest photocatalytic activity was obtained for 3% Cu deposition content.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, S.D. Environmental mass spectrometry: Emerging contaminants and current issues. Anal. Chem. 2012, 84, 747–778. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Cueto, R.; Balamurugan, S.; Romeo, L.D.; Kuttruff, J.T.; Marx, B.D.; Negulescu, I.I. Removal of Acid Dyes from Textile Wastewaters Using Fish Scales by Absorption Process. Clean Technol. 2019, 1, 311–324. [Google Scholar] [CrossRef]
- Silva, J.; Morante, L.; Demeke, T.; Baah-Twum, J.; Navarro, A.E. Preparation and Characterization of Chemically-Modified Biomaterials and Their Application as Adsorbents of Penicillin G. Clean Technol. 2019, 1, 114–124. [Google Scholar] [CrossRef]
- Voigt, M.; Hentschel, B.; Theiss, N.; Savelsberg, C.; Bartels, I.; Nickisch-Hartfiel, A.; Jaeger, M. Lomefloxacin—Occurrence in the German River Erft, Its Photo-Induced Elimination, and Assessment of Ecotoxicity. Clean Technol. 2020, 2, 74–90. [Google Scholar] [CrossRef]
- Atul, W.V.; Gaikwad, G.; Dhonde, M.; Khaty, N.; Thakare, S. Removal of organic pollutant from water by heterogenous photocatalysis: A review. Res. J. Chem. Environ. 2013, 17, 84–94. [Google Scholar]
- Zendegi-Shiraz, A.; Feilizadeh, M.; Iranbakhsh, A.; Attar, F.; Karimi Estahbanati, M.R.; Nikparast, Y.; Zendegi-Shiraz, M. Removal and degradation of triazole fungicides using Ag/PEG-CuO: An efficient adsorbent-catalyst coupling process: An ACC process for triazole fungicides treatment. Int. J. Environ. Anal. Chem. 2021, 1–16. [Google Scholar] [CrossRef]
- Yadav, B.; Chavan, S.; Tyagi, R.D.; Drogui, P. Occurrence, fate, and persistence of per-and poly-fluoroalkyl substances (PFASs) during municipal sludge treatment. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 227–245. [Google Scholar]
- Feilizadeh, M.; Attar, F.; Mahinpey, N. Hydrogen peroxide-assisted photocatalysis under solar light irradiation: Interpretation of interaction effects between an active photocatalyst and H2O2. Can. J. Chem. Eng. 2019, 97, 2009–2014. [Google Scholar] [CrossRef]
- Karimi Estahbanati, M.R.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Current developments and future trends in photocatalytic glycerol valorization: Process analysis. React. Chem. Eng. 2021, 6, 197–219. [Google Scholar] [CrossRef]
- Feilizadeh, M.; Alemzadeh, I.; Delparish, A.; Karimi Estahbanati, M.R.; Soleimani, M.; Jangjou, Y.; Vosoughi, A. Optimization of operating parameters for efficient photocatalytic inactivation of Escherichia coli based on a statistical design of experiments. Water Sci. Technol. 2015, 71, 823–831. [Google Scholar] [CrossRef]
- Feilizadeh, M.; Delparish, A.; Toufigh Bararpour, S.; Abedini Najafabadi, H.; Mohammad Esmaeil Zakeri, S.; Vossoughi, M. Photocatalytic removal of 2-nitrophenol using silver and sulfur co-doped TiO2 under natural solar light. Water Sci. Technol. 2015, 72, 339–346. [Google Scholar] [CrossRef]
- Karimi Estahbanati, M.R.; Babin, A.; Feilizadeh, M.; Nayernia, Z.; Mahinpey, N.; Iliuta, M.C. Photocatalytic conversion of alcohols to hydrogen and carbon-containing products: A cleaner alcohol valorization approach. J. Clean. Prod. 2021, 318, 128546. [Google Scholar] [CrossRef]
- Karimi Estahbanati, M.R.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Current developments and future trends in photocatalytic glycerol valorization: Photocatalyst development. Ind. Eng. Chem. Res. 2020, 59, 22330–22352. [Google Scholar] [CrossRef]
- Feilizadeh, M.; Vossoughi, M.; Zakeri, S.M.E.; Rahimi, M. Enhancement of Efficient Ag–S/TiO2 Nanophotocatalyst for Photocatalytic Degradation under Visible Light. Ind. Eng. Chem. 2014, 53, 9578–9586. [Google Scholar]
- Karimi Estahbanati, M.R. Photocatalytic Valorization of Biobased Alcoholic Wastes: A Sustainable Approach for the Generation of Green Products. Ph.D. Thesis, Université Laval, Qubec City, QC, Canada, 2019. [Google Scholar]
- Karimi Estahbanati, M.R.; Feilizadeh, M.; Iliuta, M.C. Photocatalytic valorization of glycerol to hydrogen: Optimization of operating parameters by artificial neural network. Appl. Catal. B Environ. 2017, 209, 483–492. [Google Scholar] [CrossRef]
- Barahimi, V.; Moghimi, H.; Taheri, R.A. Cu doped TiO2-Bi2O3 nanocomposite for degradation of azo dye in aqueous solution: Process modeling and optimization using central composite design. J. Environ. Chem. Eng. 2019, 7, 103078. [Google Scholar] [CrossRef]
- Shafei, A.; Sheibani, S. Visible light photocatalytic activity of Cu doped TiO2-CNT nanocomposite powder prepared by sol–gel method. Mater. Res. Bull. 2019, 110, 198–206. [Google Scholar] [CrossRef]
- Wu, M.-C.; Wu, P.-Y.; Lin, T.-H.; Lin, T.-F. Photocatalytic performance of Cu-doped TiO2 nanofibers treated by the hydrothermal synthesis and air-thermal treatment. Appl. Surf. Sci. 2018, 430, 390–398. [Google Scholar] [CrossRef]
- Anju, K.R.; Thankapan, R.; Rajabathar, J.R.; Al-Lohedan, H.A. Hydrothermal synthesis of nanosized (Fe, Co, Ni)-TiO2 for enhanced visible light photosensitive applications. Optik 2018, 165, 408–415. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Guo, S.; Yu, S.; Han, S. Porous nickel doped titanium dioxide nanoparticles with improved visible light photocatalytic activity. Nanoscale Adv. 2020, 2, 1352–1357. [Google Scholar] [CrossRef]
- Kongsong, P.; Jantaporn, W.; Masae, M. Enhanced photocatalytic activity of Ni doped TiO2 nanowire–nanoparticle hetero–structured films prepared by hydrothermal and sol–gel methods. Surf. Interface Anal. 2020, 52, 486–492. [Google Scholar] [CrossRef]
- Tasbihi, M.; Fresno, F.; Simon, U.; Villar-Garcia, I.J.; Perez-Dieste, V.; Escudero, C.; Víctor, A. On the selectivity of CO2 photoreduction towards CH4 using Pt/TiO2 catalysts supported on mesoporous silica. Appl. Catal. B Environ. 2018, 239, 68–76. [Google Scholar] [CrossRef]
- Wang, M.; Zhen, W.; Tian, B.; Ma, J.; Lu, G. The inhibition of hydrogen and oxygen recombination reaction by halogen atoms on over-all water splitting over Pt-TiO2 photocatalyst. Appl. Catal. B Environ. 2018, 236, 240–252. [Google Scholar] [CrossRef]
- Khan, H.; Rigamonti, M.G.; Boffito, D.C. Enhanced photocatalytic activity of Pt-TiO2/WO3 hybrid material with energy storage ability. Appl. Catal. B Environ. 2019, 252, 77–85. [Google Scholar] [CrossRef]
- Hu, Y.; Song, X.; Jiang, S.; Wei, C. Enhanced photocatalytic activity of Pt-doped TiO2 for NOx oxidation both under UV and visible light irradiation: A synergistic effect of lattice Pt4+ and surface PtO. Chem. Eng. J. 2015, 274, 102–112. [Google Scholar] [CrossRef]
- Karimi Estahbanati, M.K.; Feilizadeh, M.; Babin, A.; Mei, B.; Mul, G.; Iliuta, M.C. Selective photocatalytic oxidation of cyclohexanol to cyclohexanone: A spectroscopic and kinetic study. Chem. Eng. J. 2020, 382, 122732. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Optimization of Alachlor Photocatalytic Degradation with Nano-TiO2 in Water under Solar Illumination: Reaction Pathway and Mineralization. Clean Technol. 2019, 1, 141–153. [Google Scholar] [CrossRef]
- Dinh, C.T.; Seo, Y.; Nguyen, T.D.; Kleitz, F.; Do, T.O. Controlled synthesis of titanate nanodisks as versatile building blocks for the design of hybrid nanostructures. Angew. Chem. Int. Ed. 2012, 51, 6608–6612. [Google Scholar] [CrossRef]
- Dinh, C.-T.; Pham, M.-H.; Seo, Y.; Kleitz, F.; Do, T.-O. Design of multicomponent photocatalysts for hydrogen production under visible light using water-soluble titanate nanodisks. Nanoscale 2014, 6, 4819–4829. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Vu, T.-D. Décomposition Photocatalytique du Méthanol sur des Nanosphères de TiO₂ Chargées de Métal. Master’s Thesis, Université Laval, Quebec City, QC, Canada, 2019. [Google Scholar]
- Nguyen, D.-T.; Chouat, A.; Do, T.-O. Highly efficient proton-assisted photocatalytic CO2 reduction over 3-mercaptopropionic acid-capped quantums dots. Sustain. Energy Fuels 2021, 5, 4015–4022. [Google Scholar] [CrossRef]
- Iler, R. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Dabbaghian, M.; Babalou, A.; Hadi, P.; Jannatdoust, E. A parametric study of the synthesis of silica nanoparticles via sol-gel precipitation method. Int. J. Nanosci. Nanotechnol. 2010, 6, 104–113. [Google Scholar]
- Elango, G.; Roopan, S.M.; Dhamodaran, K.I.; Elumalai, K.; Al-Dhabi, N.A.; Arasu, M.V. Spectroscopic investigation of biosynthesized nickel nanoparticles and its larvicidal, pesticidal activities. J. Photochem. Photobiol. 2016, 162, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Rajathi, F.A.A.; Nambaru, V. Phytofabrication of nano-crystalline platinum particles by leaves of Cerbera manghas and its antibacterial efficacy. Int. J. Pharm. Biol. Sci. 2014, 5, 619–628. [Google Scholar]
- Nguyen, C.-C.; Nguyen, D.T.; Do, T.-O. A novel route to synthesize C/Pt/TiO2 phase tunable anatase–Rutile TiO2 for efficient sunlight-driven photocatalytic applications. Appl. Catal. B Environ. 2018, 226, 46–52. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Vu, N.N.; Chabot, S.; Kaliaguine, S.; Do, T.O. Role of CxNy-Triazine in Photocatalysis for Efficient Hydrogen Generation and Organic Pollutant Degradation Under Solar Light Irradiation. Solar RRL 2017, 1, 1700012. [Google Scholar] [CrossRef]
- Mejía, M.I.; Marín, J.M.; Restrepo, G.; Rios, L.A.; Pulgarín, C.; Kiwi, J. Preparation, testing and performance of a TiO2/polyester photocatalyst for the degradation of gaseous methanol. Appl. Catal. B Environ. 2010, 94, 166–172. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Liu, S.; Duan, X.; Hu, Z. Synthesis and characterization of Cu2O/TiO2 photocatalysts for H2 evolution from aqueous solution with different scavengers. Appl. Surf. Sci. 2015, 324, 736–744. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, J.; Li, G.; Chen, J.; Zhang, Z.; Deng, Z.; Jiang, Y.; Guo, W.; Cao, Y. Effective water splitting using CuOx/TiO2 composite films: Role of Cu species and content in hydrogen generation. Appl. Surf. Sci. 2016, 369, 201–206. [Google Scholar] [CrossRef]
- Davydov, A.; Chuang, K.T.; Sanger, A.R. Mechanism of H2S oxidation by ferric oxide and hydroxide surfaces. J. Phys. Chem. B 1998, 102, 4745–4752. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi Estahbanati, M.R.; Vu, T.-D.; Do, T.-O.; Nayernia, Z.; Iliuta, M.C. Metal-Supported TiO2/SiO2 Core-Shell Nanosphere Photocatalyst for Efficient Sunlight-Driven Methanol Degradation. Clean Technol. 2023, 5, 828-838. https://doi.org/10.3390/cleantechnol5030040
Karimi Estahbanati MR, Vu T-D, Do T-O, Nayernia Z, Iliuta MC. Metal-Supported TiO2/SiO2 Core-Shell Nanosphere Photocatalyst for Efficient Sunlight-Driven Methanol Degradation. Clean Technologies. 2023; 5(3):828-838. https://doi.org/10.3390/cleantechnol5030040
Chicago/Turabian StyleKarimi Estahbanati, M. R., Thuy-Dung Vu, Trong-On Do, Zahra Nayernia, and Maria C. Iliuta. 2023. "Metal-Supported TiO2/SiO2 Core-Shell Nanosphere Photocatalyst for Efficient Sunlight-Driven Methanol Degradation" Clean Technologies 5, no. 3: 828-838. https://doi.org/10.3390/cleantechnol5030040
APA StyleKarimi Estahbanati, M. R., Vu, T.-D., Do, T.-O., Nayernia, Z., & Iliuta, M. C. (2023). Metal-Supported TiO2/SiO2 Core-Shell Nanosphere Photocatalyst for Efficient Sunlight-Driven Methanol Degradation. Clean Technologies, 5(3), 828-838. https://doi.org/10.3390/cleantechnol5030040