Elementary Steps in Steady State Kinetic Model Approximation for the Homo-Heterogeneous Photocatalysis of Carbamazepine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Kinetic Analysis
2.3. Description of the Experiments
3. Results and Discussion
3.1. Influence of CBZ Concentration on the Photocatalytic Degradation
3.2. Influence of H2O2 Concentration on the Photocatalytic Degradation of CBZ
3.3. Influence of TiO2 Concentration on the Photocatalytic Degradation of CBZ
3.4. Influence of UVC Irradiation Intensity on the Photocatalytic Degradation of CBZ
3.5. Corrected Kinetic Analysis
4. Conclusions
- Carbamazepine should be pseudo-first-order or pseudo-zero-order at low or at high concentrations, respectively.
- The homogeneous catalysts (H2O2) should be pseudo-first-order at all concentrations, without influencing CBZ pseudo-order.
- The heterogeneous catalysts (TiO2) should be pseudo-first-order at all concentrations, without influencing CBZ pseudo-order.
- The UVC irradiation intensity should be pseudo-first-order at all concentrations, without influencing CBZ pseudo-order.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Pseudo-Order of a Process
References
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Alrashood, S.T. Carbamazepine. Profiles Drug Subst. Excipients Relat. Methodol. 2016, 41, 133–321. [Google Scholar]
- Clara, M.; Strenn, B.; Kreuzinger, N. Carbamazepine as a possible anthropogenic marker in the aquatic environment: Investigations on the behaviour of Carbamazepine in wastewater treatment and during groundwater infiltration. Water Res. 2004, 38, 947–954. [Google Scholar] [CrossRef]
- Oldenkamp, R.; Beusen, A.H.W.; Huijbregts, M.A.J. Aquatic risks from human pharmaceuticals—Modelling temporal trends of carbamazepine and ciprofloxacin at the global scale. Environ. Res. Lett. 2019, 14, 034003. [Google Scholar] [CrossRef]
- Im, J.-K.; Son, H.-S.; Kang, Y.-M.; Zoh, K.-D. Carbamazepine Degradation by Photolysis and Titanium Dioxide Photocatalysis. Water Environ. Res. 2012, 84, 554–561. [Google Scholar] [CrossRef] [PubMed]
- De Oliva, S.U.; Miraglia, S.M. Carbamazepine damage to rat spermatogenesis in different sexual developmental phases. Int. J. Androl. 2009, 32, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, R.; Wang, M.; Zha, J. Carbamazepine at environmentally relevant concentrations caused DNA damage and apoptosis in the liver of Chinese rare minnows (Gobiocypris rarus) by the Ras/Raf/ERK/p53 signaling pathway. Environ. Pollut. 2021, 270, 116245. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef]
- Serrano, D.; Suárez, S.; Lema, J.M.; Omil, F. Removal of persistent pharmaceutical micropollutants from sewage by addition of PAC in a sequential membrane bioreactor. Water Res. 2011, 45, 5323–5333. [Google Scholar] [CrossRef]
- Levakov, I.; Shahar, Y.; Rytwo, G. Carbamazepine Removal by Clay-Based Materials Using Adsorption and Photodegradation. Water 2022, 14, 2047. [Google Scholar] [CrossRef]
- Alharbi, S.K.; Price, W.E. Degradation and Fate of Pharmaceutically Active Contaminants by Advanced Oxidation Processes. Curr. Pollut. Rep. 2017, 3, 268–280. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, W.; Zhao, Z.; Liu, W.; Ye, J.; Tong, M.; Li, Y. Different degradation mechanisms of carbamazepine and diclofenac by single-atom Barium embedded g-C3N4: The role of photosensitation-like mechanism. J. Hazard. Mater. 2021, 416, 125936. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Jurek, A.; Bielan, Z.; Dudziak, S.; Wolak, I.; Sobczak, Z.; Klimczuk, T.; Nowaczyk, G.; Hupka, J. Design and application of magnetic photocatalysts for water treatment. The effect of particle charge on surface functionality. Catalysts 2017, 7, 360. [Google Scholar] [CrossRef]
- Pan, F.; Ji, H.; Du, P.; Huang, T.; Wang, C.; Liu, W. Insights into catalytic activation of peroxymonosulfate for carbamazepine degradation by MnO2 nanoparticles in-situ anchored titanate nanotubes: Mechanism, ecotoxicity and DFT study. J. Hazard. Mater. 2021, 402, 123779. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Tellez, A.; Masson, R.; Robert, D.; Keller, N.; Keller, V. Comparison of Hombikat UV100 and P25 TiO2 performance in gas-phase photocatalytic oxidation reactions. J. Photochem. Photobiol. A Chem. 2012, 250, 58–65. [Google Scholar] [CrossRef]
- Carabin, A.; Drogui, P.; Robert, D. Photo-degradation of carbamazepine using TiO2 suspended photocatalysts. J. Taiwan Inst. Chem. Eng. 2015, 54, 109–117. [Google Scholar] [CrossRef]
- Vogna, D.; Marotta, R.; Andreozzi, R.; Napolitano, A.; d’Ischia, M. Kinetic and chemical assessment of the UV/H2O2 treatment of antiepileptic drug carbamazepine. Chemosphere 2004, 54, 497–505. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, D.W.; Chi, J.; Lee, D.H. Degradation kinetics of recalcitrant organic compounds in a decontamination process with UV/H2O2 and UV/H2O2/TiO2 processes. Korean J. Chem. Eng. 2003, 20, 503–508. [Google Scholar] [CrossRef]
- Yegane Badi, M.; Vosoughi, M.; Sadeghi, H.; Mokhtari, S.A.; Mehralipour, J. Ultrasonic-assisted H2O2/TiO2 process in catechol degradation: Kinetic, synergistic and optimisation via response surface methodology. Int. J. Environ. Anal. Chem. 2022, 102, 757–770. [Google Scholar] [CrossRef]
- Wols, B.A.; Hofman-Caris, C.H.M. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef]
- Lopez, A.; Bozzi, A.; Mascolo, G.; Kiwi, J. Kinetic investigation on UV and UV/H2O2 degradations of pharmaceutical intermediates in aqueous solution. J. Photochem. Photobiol. A Chem. 2003, 156, 121–126. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Reports 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Ge, L. Novel visible-light-driven Pt/BiVO4 photocatalyst for efficient degradation of methyl orange. J. Mol. Catal. A Chem. 2008, 282, 62–66. [Google Scholar] [CrossRef]
- Zhou, X.-T.; Ji, H.-B.; Huang, X.-J. Photocatalytic Degradation of Methyl Orange over Metalloporphyrins Supported on TiO2 Degussa P25. Molecules 2012, 17, 1149–1158. [Google Scholar]
- Li, Y.; Yang, Y.; Lei, J.; Liu, W.; Tong, M.; Liang, J. The degradation pathways of carbamazepine in advanced oxidation process: A mini review coupled with DFT calculation. Sci. Total Environ. 2021, 779, 146498. [Google Scholar] [CrossRef]
- Chieh, C. Steady-State Approximation—Chemistry LibreTexts. Available online: https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/Reaction_Mechanisms/Steady-State_Approximation (accessed on 16 March 2023).
- Rendel, P.M.; Rytwo, G. Degradation kinetics of caffeine in water by UV/H2O2 and UV/TiO2. Desalin. Water Treat. 2020, 173, 231–242. [Google Scholar] [CrossRef]
- Brown, S.S. Applicability of the steady state approximation to the interpretation of atmospheric observations of NO3 and N2O5. J. Geophys. Res. 2003, 108, D17. [Google Scholar] [CrossRef]
- Cole, S.L.; Wilder, J.W. Gas Phase Decomposition by the Lindemann Mechanism. SIAM J. Appl. Math. 1998, 51, 1489–1497. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Physical Chemistry; W.H. Freemand and Co.: New York, NY, USA, 2006; Volume 8, ISBN 0-7167-8759-8. [Google Scholar]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef] [PubMed]
- Flach, E.H.; Schnell, S. Use and abuse of the quasi-steady-state approximation. Syst. Biol. (Stevenage) 2006, 153, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Turányi, T.; Tomlin, A.S.; Pilling, M.J. On the error of the quasi-steady-state approximation. J. Phys. Chem. 1993, 97, 163–172. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Dionysiou, D.D.; Chen, B.; Yang, J.; Li, J. Overlooked Formation of H2O2 during the Hydroxyl Radical-Scavenging Process When Using Alcohols as Scavengers. Environ. Sci. Technol. 2022, 56, 3386–3396. [Google Scholar] [CrossRef]
- Rytwo, G.; Zelkind, A.L. Evaluation of Kinetic Pseudo-Order in the Photocatalytic Degradation of Ofloxacin. Catalysts 2022, 12, 24. [Google Scholar] [CrossRef]
- Efron, B. Bootstrap Methods: Another Look at the Jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Mishra, D.K.; Dolan, K.D.; Yang, L. Bootstrap confidence intervals for the kinetic parameters of degradation of anthocyanins in grape pomace. J. Food Process Eng. 2011, 34, 1220–1233. [Google Scholar] [CrossRef]
- Barnston, A.G. Correspondence among the Correlation, RMSE, and Heidke Forecast Verification Measures; Refinement of the Heidke Score. Weather Forecast. 1992, 7, 699–709. [Google Scholar] [CrossRef]
- Rytwo, G.; Klein, T.; Margalit, S.; Mor, O.; Naftali, A.; Daskal, G. A continuous-flow device for photocatalytic degradation and full mineralization of priority pollutants in water. Desalin. Water Treat. 2016, 57, 16424–16434. [Google Scholar] [CrossRef]
- Larsen, D. 5.7: Using Graphs to Determine Integrated Rate Laws—Chemistry LibreTexts. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/05:_Experimental_Methods/5.07:_Using_Graphs_to_Determine_Integrated_Rate_Laws (accessed on 6 April 2023).
- Ford, C. Is R-squared Useless?|University of Virginia Library Research Data Services + Sciences. Available online: https://data.library.virginia.edu/is-r-squared-useless/ (accessed on 6 April 2023).
- Rendel, P.M.; Rytwo, G. The Effect of Electrolytes on the Photodegradation Kinetics of Caffeine. Catalysts 2020, 10, 644. [Google Scholar] [CrossRef]
- White, D.P. Chapter 14- Chemical Kinetics. Available online: My.ilstu.edu/~ccmclau/che141/materials/outlines/chapter14.ppt (accessed on 6 April 2023).
- UPAC. Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; (the “Gold Book”); Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. Available online: https://goldbook.iupac.org/ (accessed on 20 February 2023).
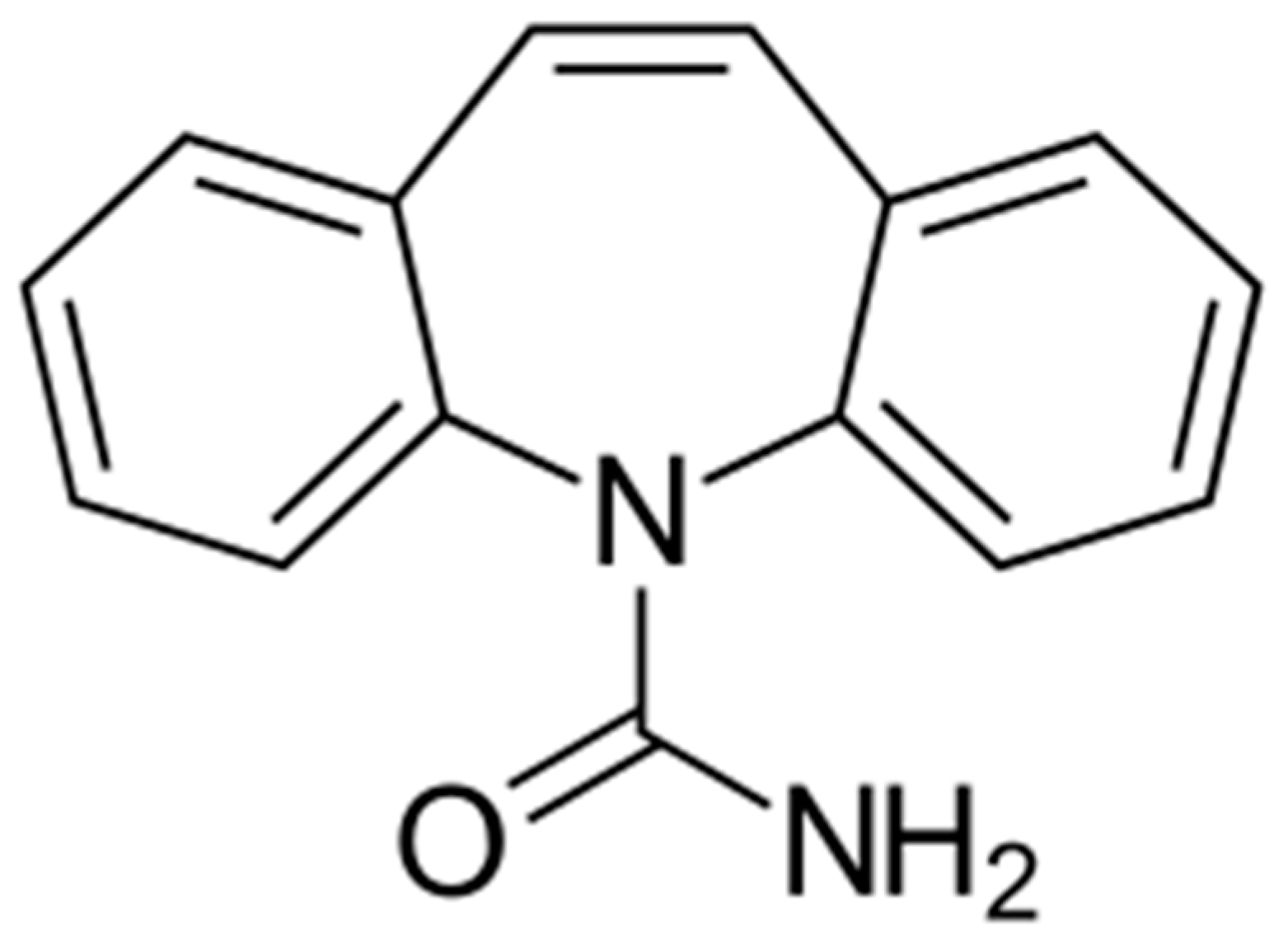

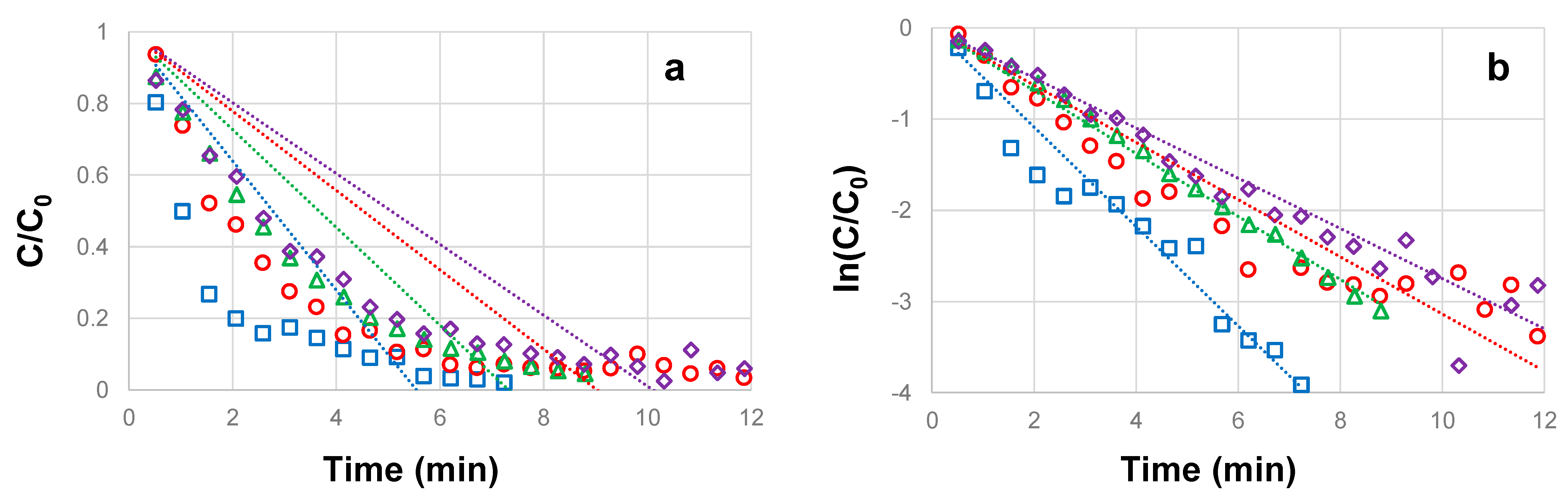

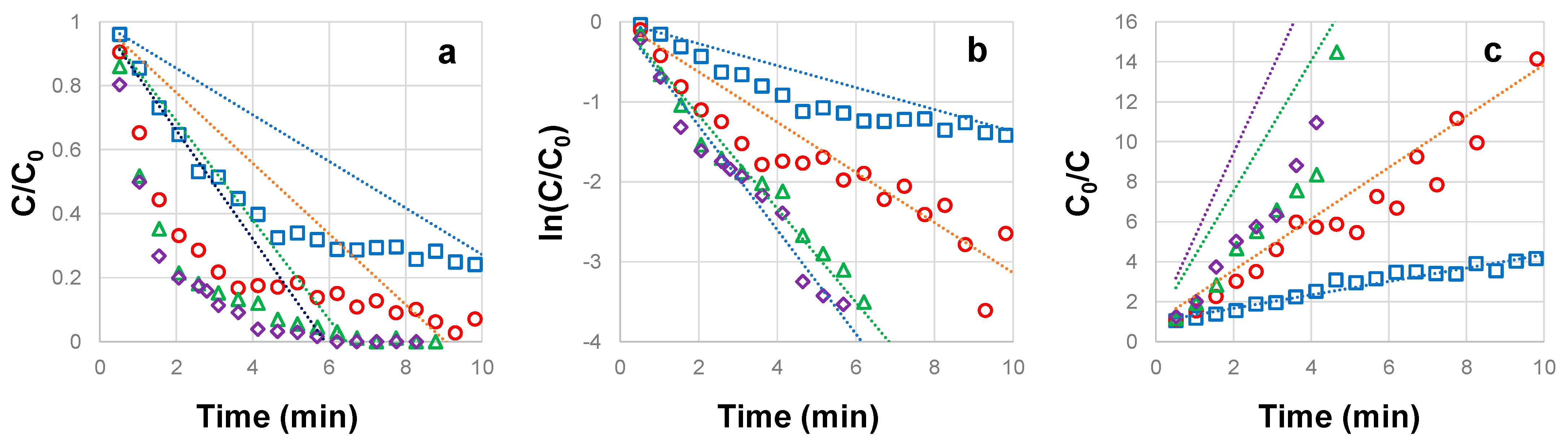
| CBZ Concentration (mg L−1) | CBZ Pseudo-Order na | Half Life t1/2 (min) | RMSE | R2 |
|---|---|---|---|---|
| 1 | 0 | 2.77 ± 17.8% | 0.346 | 0.683 |
| 5 | 0 | 5.09 ± 4.26% | 0.328 | 0.765 |
| 15 | 0 | 6.05 ± 7.41% | 0.142 | 0.925 |
| 20 | 0 | 7.37 ± 4.68% | 0.046 | 0.977 |
| 1 | 1 | 1.08 ± 12.02% | 0.077 | 0.921 |
| 5 | 1 | 2.22 ± 3.64% | 0.037 | 0.995 |
| 15 | 1 | 3.96 ± 1.31% | 0.035 | 0.999 |
| 20 | 1 | 6.27 ± 1.31% | 0.028 | 0.999 |
| 1 | 1.11 ± 4.90% | 0.95 ± 6.05% | 0.072 | 0.955 |
| 5 | 0.83 ± 2.04% | 2.33 ± 1.10% | 0.030 | 0.996 |
| 15 | 0.76 ± 3.25% | 4.18 ± 1.28% | 0.021 | 0.998 |
| 20 | 0.61 ± 8.71% | 6.55 ± 1.15% | 0.018 | 0.998 |
| H2O2 Concentration (mg L−1) | CBZ Pseudo-Order na | Half Life t1/2 (min) | RMSE | R2 |
|---|---|---|---|---|
| 0.5 | 0 | 4.99 ± 6.44% | 0.194 | 0.823 |
| 1 | 0 | 4.29 ± 10.4% | 0.248 | 0.707 |
| 2 | 0 | 2.98 ± 5.32% | 0.249 | 0.618 |
| 5 | 0 | 2.77 ± 17.8% | 0.346 | 0.683 |
| 0.5 | 1 | 2.44 ± 1.33% | 0.028 | 0.991 |
| 1 | 1 | 1.82 ± 3.33% | 0.048 | 0.980 |
| 2 | 1 | 1.31 ± 7.37% | 0.087 | 0.905 |
| 5 | 1 | 1.08 ± 12.02% | 0.077 | 0.921 |
| 0.5 | 0.95 ± 2.57% | 2.48 ± 2.48% | 0.027 | 0.992 |
| 1 | 0.93 ± 4.49% | 1.86 ± 1.37% | 0.047 | 0.979 |
| 2 | 1.05 ± 6.78% | 1.25 ± 6.28% | 0.086 | 0.902 |
| 5 | 1.11 ± 4.90% | 0.95 ± 6.05% | 0.072 | 0.955 |
| TiO2 Concentration (mg L−1) | CBZ Pseudo-Order na | Half Life t1/2 (min) | RMSE | R2 |
|---|---|---|---|---|
| 0.02 | 0 | 2.19 ± 16.3% | 0.302 | 0.518 |
| 0.05 | 0 | 2.47 ± 7.39% | 0.293 | 0.578 |
| 0.2 | 0 | 2.84 ± 13.84% | 0.262 | 0.663 |
| 0.5 | 0 | 2.77 ± 17.8% | 0.346 | 0.683 |
| 0.02 | 1 | 0.83 ± 5.29% | 0.096 | 0.889 |
| 0.05 | 1 | 0.90 ± 4.48% | 0.073 | 0.933 |
| 0.2 | 1 | 1.06 ± 4.55% | 0.086 | 0.902 |
| 0.5 | 1 | 1.08 ± 12.02% | 0.077 | 0.921 |
| 0.02 | 0.81 ± 15.6% | 0.88 ± 4.22% | 0.093 | 0.890 |
| 0.05 | 0.88 ± 11.8% | 0.91 ± 5.70% | 0.070 | 0.936 |
| 0.2 | 1.07 ± 8.56% | 1.02 ± 7.34% | 0.085 | 0.907 |
| 0.5 | 1.11 ± 4.90% | 0.95 ± 6.05% | 0.072 | 0.955 |
| UVC Irradiation Intensity (W m−2) | CBZ Pseudo-Order na | Half Life t1/2 (min) | RMSE | R2 |
|---|---|---|---|---|
| 901 (2 lamps) | 0 | 9.68 ± 2.52% | 0.202 | 0.747 |
| 1803 (4 lamps) | 0 | 5.12 ± 3.36% | 0.254 | 0.689 |
| 2704 (6 lamps) | 0 | 3.42 ± 3.95% | 0.286 | 0.684 |
| 3605 (8 lamps) | 0 | 2.77 ± 17.8% | 0.346 | 0.671 |
| 901 (2 lamps) | 1 | 5.02 ± 5.63% | 0.096 | 0.938 |
| 1803 (4 lamps) | 1 | 1.78 ± 6.06% | 0.068 | 0.947 |
| 2704 (6 lamps) | 1 | 1.18 ± 4.03% | 0.051 | 0.966 |
| 3605 (8 lamps) | 1 | 0.98 ± 17.8% | 0.083 | 0.968 |
| 901 (2 lamps) | 2 | 3.43 ± 1.22% | 0.044 | 0.985 |
| 1803 (4 lamps) | 2 | 1.25 ± 5.26% | 0.075 | 0.979 |
| 2704 (6 lamps) | 2 | 0.89 ± 5.83% | 0.098 | 0.982 |
| 3605 (8 lamps) | 2 | 0.71 ± 1.82% | 0.103 | 0.980 |
| 901 (2 lamps) | 1.92 ± 3.41% | 3.46 ± 4.97% | 0.041 | 0.955 |
| 1803 (4 lamps) | 1.41 ± 2.65% | 1.50 ± 3.53% | 0.057 | 0.950 |
| 2704 (6 lamps) | 1.03 ± 2.86% | 1.16 ± 2.93% | 0.050 | 0.967 |
| 3605 (8 lamps) | 1.11 ± 4.90% | 0.95 ± 6.05% | 0.072 | 0.955 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahar, Y.; Rytwo, G. Elementary Steps in Steady State Kinetic Model Approximation for the Homo-Heterogeneous Photocatalysis of Carbamazepine. Clean Technol. 2023, 5, 866-880. https://doi.org/10.3390/cleantechnol5030043
Shahar Y, Rytwo G. Elementary Steps in Steady State Kinetic Model Approximation for the Homo-Heterogeneous Photocatalysis of Carbamazepine. Clean Technologies. 2023; 5(3):866-880. https://doi.org/10.3390/cleantechnol5030043
Chicago/Turabian StyleShahar, Yuval, and Giora Rytwo. 2023. "Elementary Steps in Steady State Kinetic Model Approximation for the Homo-Heterogeneous Photocatalysis of Carbamazepine" Clean Technologies 5, no. 3: 866-880. https://doi.org/10.3390/cleantechnol5030043
APA StyleShahar, Y., & Rytwo, G. (2023). Elementary Steps in Steady State Kinetic Model Approximation for the Homo-Heterogeneous Photocatalysis of Carbamazepine. Clean Technologies, 5(3), 866-880. https://doi.org/10.3390/cleantechnol5030043







