Mercury Removal from Mining Wastewater by Phytoaccumulation in Autochthonous Aquatic Plant Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Mining Wastewater
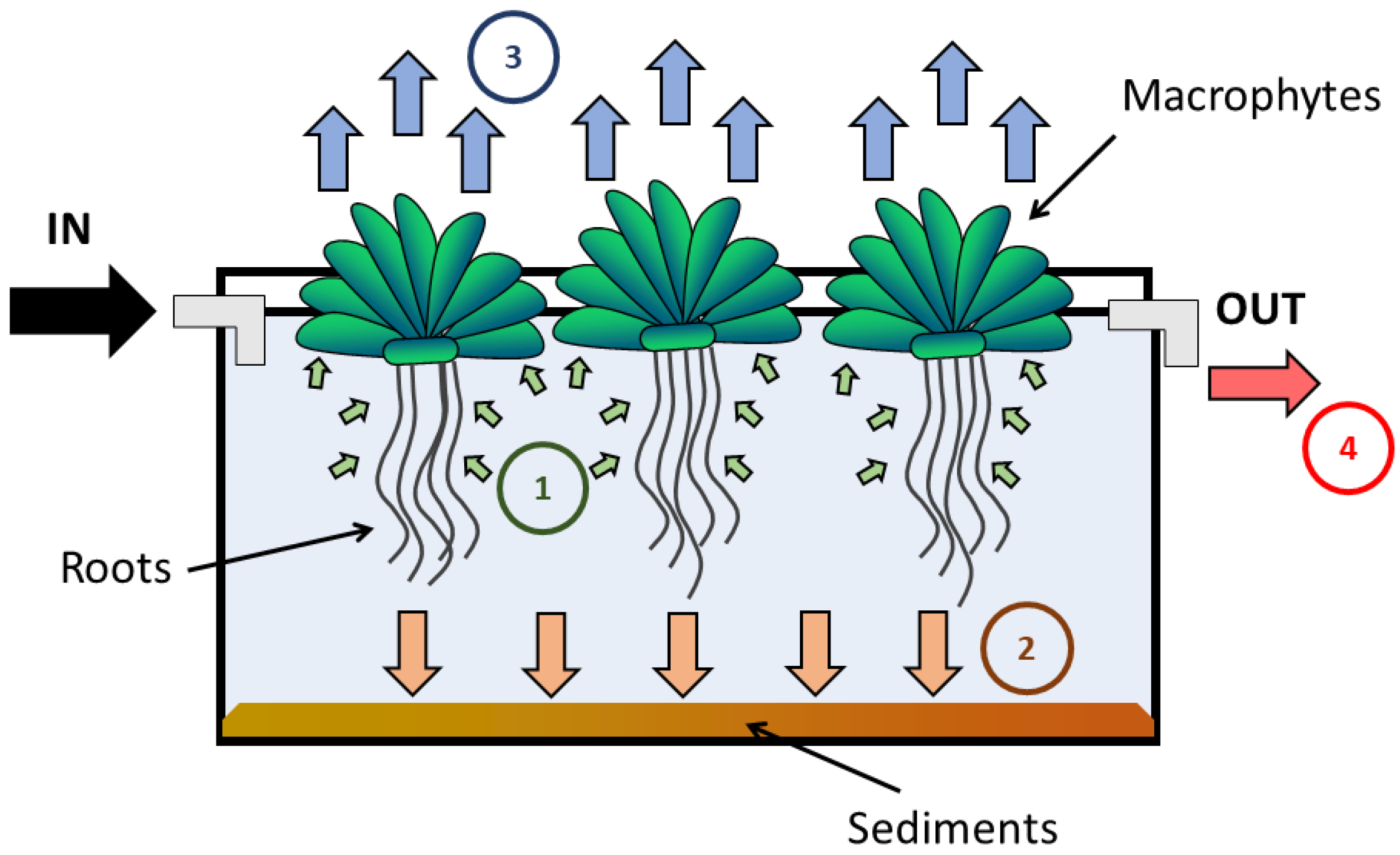
2.2. Pilot-Scale Systems
2.3. Experimental Design and Management Mode
2.4. Balance of Total Mercury in the Systems
2.5. Bioconcentration and Translocation Factors
2.6. Kinetic of THg Removal by Phytoaccumulation
2.7. Analytical Methods
3. Results and Discussion
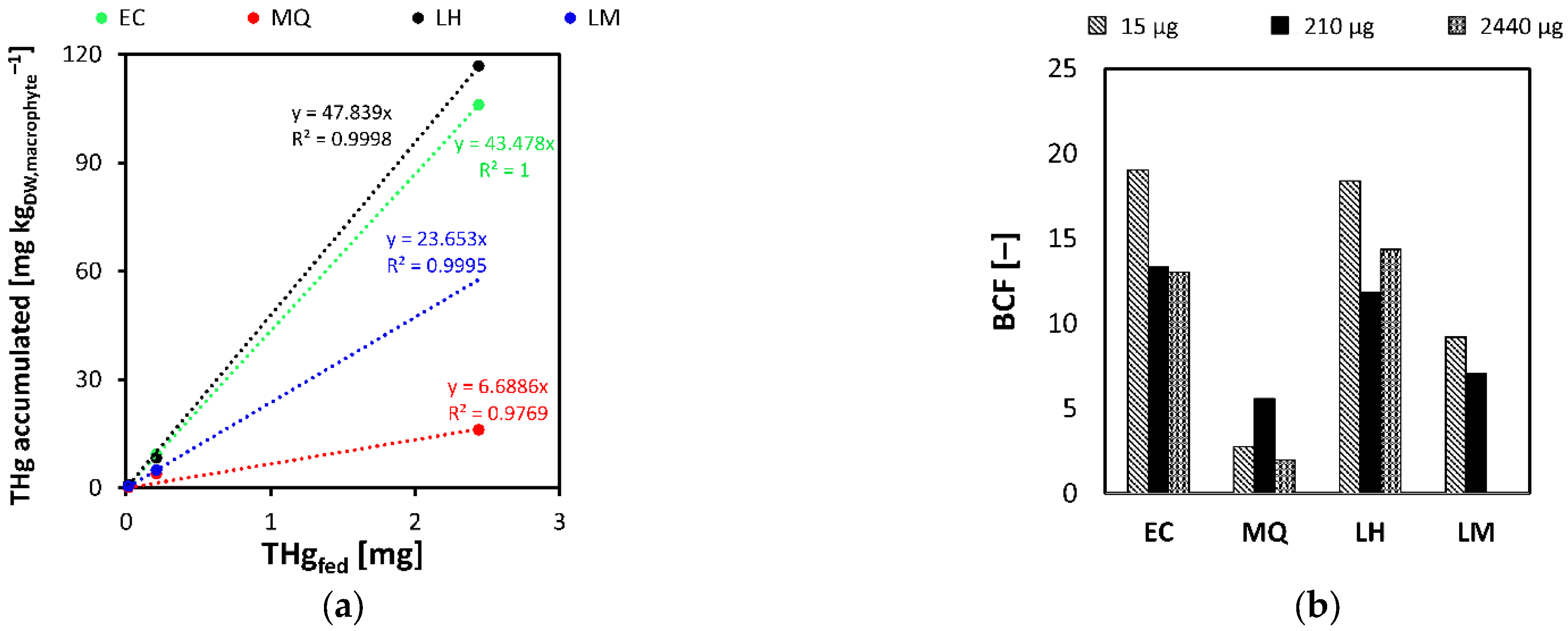
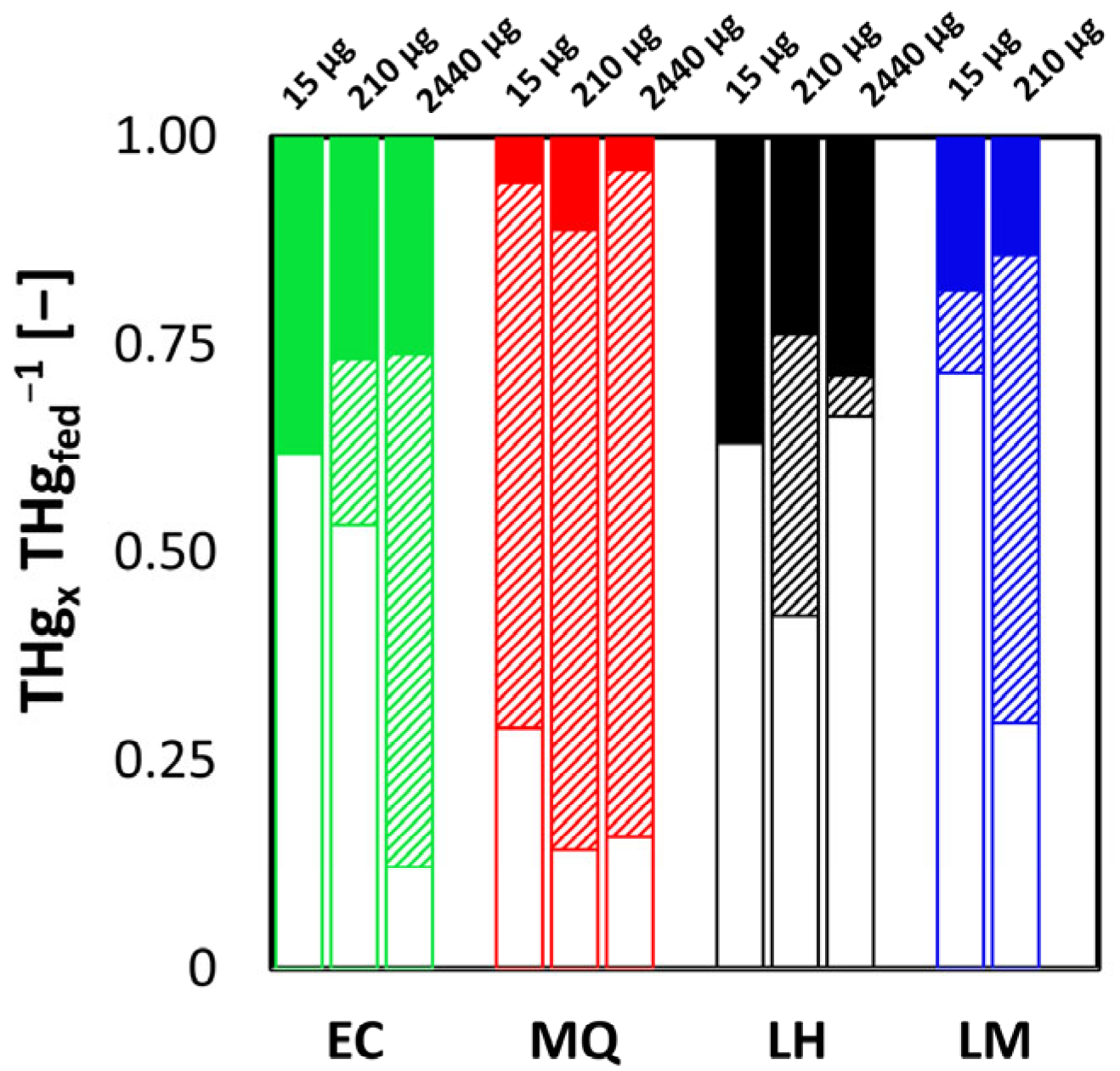
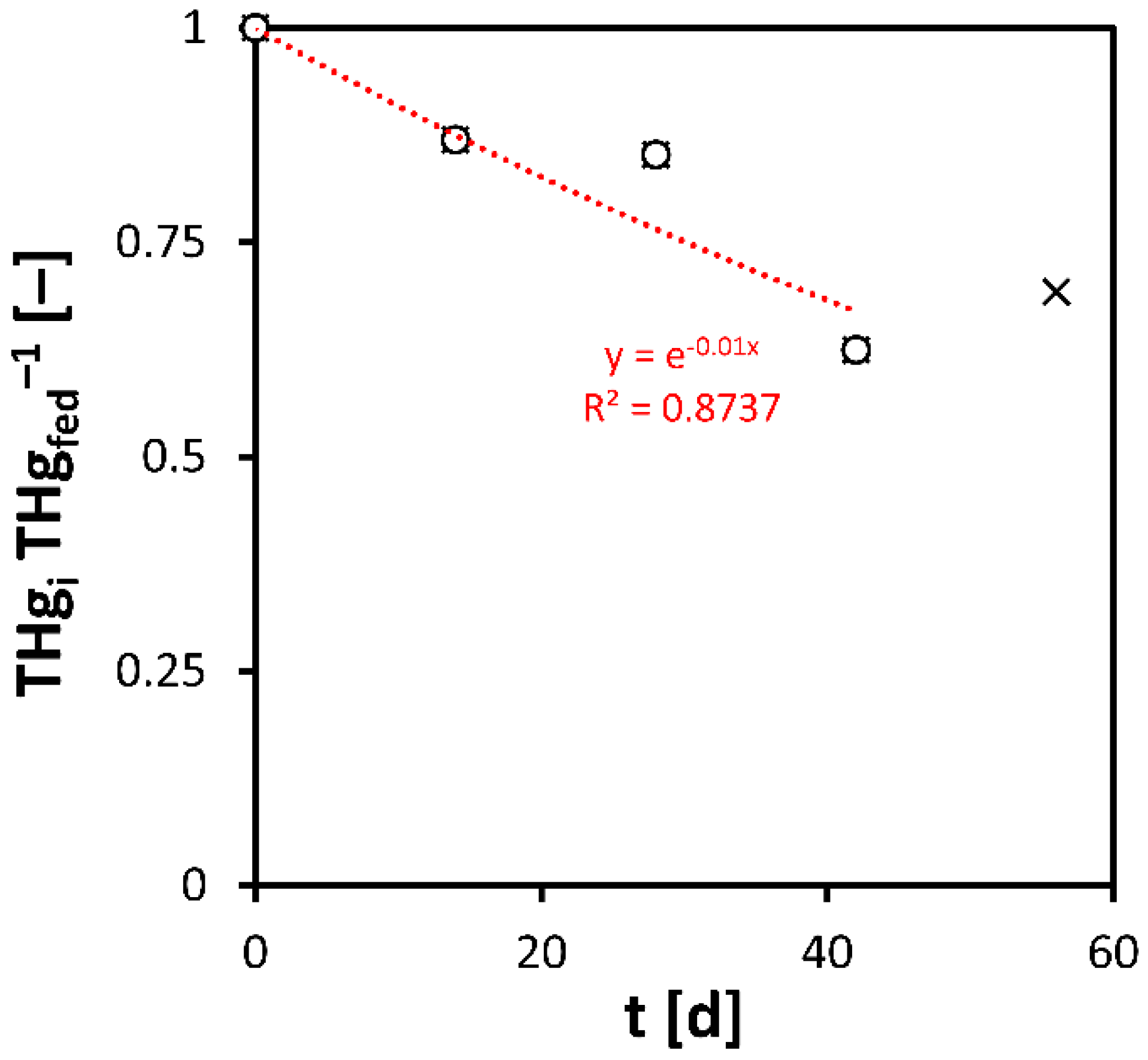
3.1. Phase A
3.2. Phase B
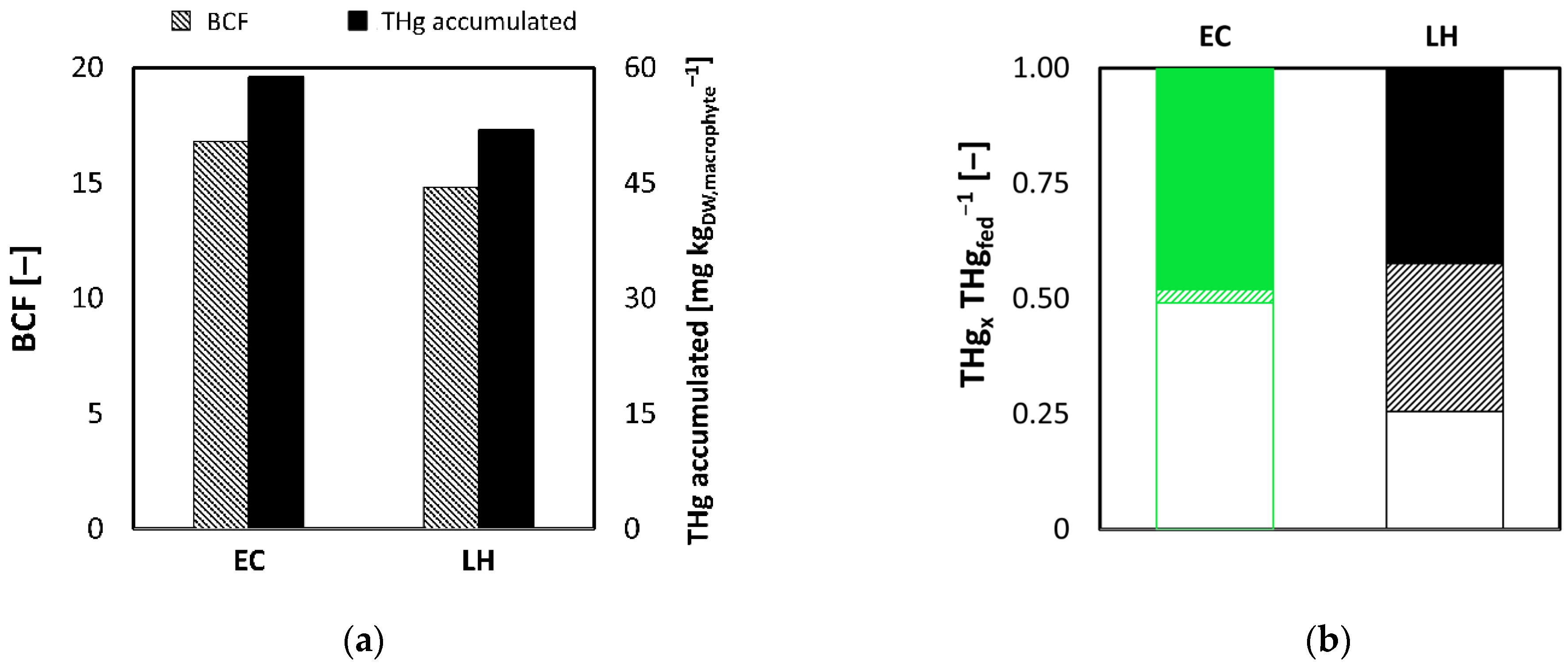
3.3. Phase C
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the Terrestrial Environment: A Review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Park, J.-D.; Zheng, W. Human Exposure and Health Effects of Inorganic and Elemental Mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Sound Tailings Management in Artisanal and Small-Scale Gold Mining—Technical Document; United Nations Environment Programme: Geneva, Switzerland, 2021. [Google Scholar]
- World Bank. State of the Artisanal and Small-Scale Mining Sector; World Bank: Washington, DC, USA, 2019. [Google Scholar]
- Yoshimura, A.; Suemasu, K.; Veiga, M.M. Estimation of Mercury Losses and Gold Production by Artisanal and Small-Scale Gold Mining (ASGM). J. Sustain. Metall. 2021, 7, 1045–1059. [Google Scholar] [CrossRef]
- Seccatore, J.; Veiga, M.; Origliasso, C.; Marin, T.; de Tomi, G. An Estimation of the Artisanal Small-Scale Production of Gold in the World. Sci. Total Environ. 2014, 496, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Cuya, A.; Glikman, J.A.; Groenendijk, J.; Macdonald, D.W.; Swaisgood, R.R.; Barocas, A. Socio-Environmental Perceptions and Barriers to Conservation Engagement among Artisanal Small-Scale Gold Mining Communities in Southeastern Peru. Glob. Ecol. Conserv. 2021, 31, e01816. [Google Scholar] [CrossRef]
- Telmer, K.H.; Veiga, M.M. World Emissions of Mercury from Artisanal and Small Scale Gold Mining and the Knowledge Gaps about Them; World Bank: New York, NY, USA, 2008. [Google Scholar]
- Cordy, P.; Veiga, M.M.; Salih, I.; Al-Saadi, S.; Console, S.; Garcia, O.; Mesa, L.A.; Velásquez-López, P.C.; Roeser, M. Mercury Contamination from Artisanal Gold Mining in Antioquia, Colombia: The World’s Highest per Capita Mercury Pollution. Sci. Total Environ. 2011, 410–411, 154–160. [Google Scholar] [CrossRef]
- Aghaei, E.; Alorro, R.D.; Tadesse, B.; Browner, R. A Review on Current Practices and Emerging Technologies for Sustainable Management, Sequestration and Stabilization of Mercury from Gold Processing Streams. J. Environ. Manag. 2019, 249, 109367. [Google Scholar] [CrossRef]
- Oliveira, L.J.; Hylander, L.D.; e Silva, E.d.C. Mercury Behavior in a Tropical Environment: The Case of Small-Scale Gold Mining in Poconé, Brazil. Environ. Pract. 2004, 6, 121–134. [Google Scholar] [CrossRef]
- República de Colombia. Resolución n. N. 1658/2013—By Means of Which Provisions Are Established for the Marketing and Use of Mercury in the Different Industrial Activities of the Country, Requirements and Incentives Are Established for Its Reduction and Elimination and Other Provisions Are Given; Congreso de la República de Colombia: Bogotá, Colombia, 2013. (In Spanish) [Google Scholar]
- Vélez-Torres, I.; Vanegas, D. Contentious Environmental Governance in Polluted Gold Mining Geographies: The Case of La Toma, Colombia. World Dev. 2022, 157, 105953. [Google Scholar] [CrossRef]
- Su, Y.; Han, F.X.; Chen, J.; Sridhar, B.B.M.; Monts, D.L. Phytoextraction and Accumulation of Mercury in Three Plant Species: Indian Mustard (Brassica juncea), Beard Grass (Polypogon monospeliensis), and Chinese Brake Fern (Pteris vittata). Int. J. Phytoremediation 2008, 10, 547–560. [Google Scholar] [CrossRef]
- Zolnikov, T.R.; Ramirez Ortiz, D. A Systematic Review on the Management and Treatment of Mercury in Artisanal Gold Mining. Sci. Total Environ. 2018, 633, 816–824. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.N.; Xing, Y.; Shang, L. Remediation of Mercury Contaminated Sites—A Review. J. Hazard. Mater. 2012, 221–222, 1–18. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Brumbaugh, W.G.; Krabbenhoft, D.P.; Helsel, D.R.; Wiener, J.G.; Echols, K.R. A National Pilot Study of Mercury Contamination of Aquatic Ecosystems Along Multiple Gradients: Bioaccumulation in Fish (No. 2001-0009); U.S. Fish and Wildlife Service: Arlington, VA, USA, 2001. [Google Scholar]
- Willis, J.M.; Gambrell, R.P.; Hester, M.W. Growth Response and Tissue Accumulation Trends of Herbaceous Wetland Plant Species Exposed to Elevated Aqueous Mercury Levels. Int. J. Phytoremediation 2010, 12, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Núñez, S.E.R.; Negrete, J.L.M.; Rios, J.E.A.; Hadad, H.R.; Maine, M.A. Hg, Cu, Pb, Cd, and Zn Accumulation in Macrophytes Growing in Tropical Wetlands. Water Air Soil Pollut. 2011, 216, 361–373. [Google Scholar] [CrossRef]
- Skinner, K.; Wright, N.; Porter-Goff, E. Mercury Uptake and Accumulation by Four Species of Aquatic Plants. Environ. Pollut. 2007, 145, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.N.; Anderson, C.W.N.; Stewart, R.B.; Robinson, B.H. Phytofiltration of Mercury-Contaminated Water: Volatilisation and Plant-Accumulation Aspects. Env. Exp. Bot. 2008, 62, 78–85. [Google Scholar] [CrossRef]
- Jeevanantham, S.; Saravanan, A.; Hemavathy, R.V.; Kumar, P.S.; Yaashikaa, P.R.; Yuvaraj, D. Removal of Toxic Pollutants from Water Environment by Phytoremediation: A Survey on Application and Future Prospects. Environ. Technol. Innov. 2019, 13, 264–276. [Google Scholar] [CrossRef]
- Quintero, J.A.; Porras, O.O.; Torres, K.C. Plantas Acuáticas—Magdalena Medio Colombiano; Instituto Universitario de la Paz—Dirección de Investigación y Proyección Social: Barrancabermeja, Colombia, 2020; Volume 1. [Google Scholar]
- Carrión, C.; Ponce-de León, C.; Cram, S.; Sommer, I.; Hernández, M.; Vanegas, C. Potential Use of Water Hyacinth (Eichhornia crassipes) in Xochimilco for Metal Phytoremediation. Agrociencia 2012, 46, 609–620. [Google Scholar]
- Carvalho Dos Santos, M.; Lenzi, E. The Use of Aquatic Macrophytes (Eichhornia crassipes) as a Biological Filter in the Treatment of Lead Contaminated Effluents. Environ. Technol. 2000, 21, 615–622. [Google Scholar] [CrossRef]
- Moreno, F.N.; Anderson, C.W.N.; Stewart, R.B.; Robinson, B.H. Mercury Volatilisation and Phytoextraction from Base-Metal Mine Tailings. Environ. Pollut. 2005, 136, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M. Phytoaccumulation of Heavy Metals by Aquatic Plants. Environ. Int. 2004, 29, 1029–1039. [Google Scholar] [CrossRef]
- Abbasi, S.A.; Ponni, G.; Tauseef, S.M. Marsilea Quadrifolia: A New Bioagent for Treating Wastewater. Water Air Soil Pollut. 2018, 229, 133. [Google Scholar] [CrossRef]
- Mishra, V.K.; Upadhyay, A.R.; Pathak, V.; Tripathi, B.D. Phytoremediation of Mercury and Arsenic from Tropical Opencast Coalmine Effluent Through Naturally Occurring Aquatic Macrophytes. Water Air Soil Pollut. 2008, 192, 303–314. [Google Scholar] [CrossRef]
- Arenas, A.D.; Marcó, L.M.; Torres, G. Evaluación de La Planta Lemna Minor Como Biorremediadora de Aguas Contaminadas Con Mercurio. Av. Cienc. Ing. 2011, 2, 1–11. [Google Scholar]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of Common Duckweed (Lemna minor) in Phytoremediation of Chemicals in the Environment: State and Future Perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef]
- Jayampathi, T.; Atugoda, T.; Jayasinghe, C. Uptake and Accumulation of Pharmaceuticals and Personal Care Products in Leafy Vegetables. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–113. [Google Scholar]
- Wang, W.-X. Bioaccumulation and Biomonitoring. In Marine Ecotoxicology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 99–119. [Google Scholar]
- Mishra, T.; Pandey, V.C. Phytoremediation of Red Mud Deposits Through Natural Succession. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–424. [Google Scholar]
- Panja, S.; Sarkar, D.; Zhang, Z.; Datta, R. Removal of Antibiotics and Nutrients by Vetiver Grass (Chrysopogon Zizanioides) from a Plug Flow Reactor Based Constructed Wetland Model. Toxics 2021, 9, 84. [Google Scholar] [CrossRef]
- Dombeck, G.D.; Perry, M.W.; Phinney, J.T. Mass Balance on Water Column Trace Metals in a Free-Surface-Flow-Constructed Wetlands in Sacramento, California. Ecol. Eng. 1998, 10, 313–339. [Google Scholar] [CrossRef]
- Von Sperling, M. Relationship between First-Order Decay Coefficients in Ponds, for Plug Flow, CSTR and Dispersed Flow Regimes. Water Sci. Technol. 2002, 45, 17–24. [Google Scholar] [CrossRef]
- Gatidou, G.; Oursouzidou, M.; Stefanatou, A.; Stasinakis, A.S. Removal Mechanisms of Benzotriazoles in Duckweed Lemna Minor Wastewater Treatment Systems. Sci. Total Environ. 2017, 596–597, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ekperusi, A.O.; Nwachukwu, E.O.; Sikoki, F.D. Assessing and Modelling the Efficacy of Lemna Paucicostata for the Phytoremediation of Petroleum Hydrocarbons in Crude Oil-Contaminated Wetlands. Sci. Rep. 2020, 10, 8489. [Google Scholar] [CrossRef] [PubMed]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of Heavy Metals: Mechanisms, Methods and Enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Leitenmaier, B.; Küpper, H. Compartmentation and Complexation of Metals in Hyperaccumulator Plants. Front. Plant Sci. 2013, 4, 374. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.R.S.; de Oliveira, D.C.M.; Guimarães, J.R.D. Total Mercury Distribution and Volatilization in Microcosms with and Without the Aquatic Macrophyte Eichhornia Crassipes. Aquat. Geochem. 2012, 18, 421–432. [Google Scholar] [CrossRef]
- Sundaramoorthy, P.; Chidambaram, A.; Ganesh, K.S.; Unnikannan, P.; Baskaran, L. Chromium Stress in Paddy: (i) Nutrient Status of Paddy under Chromium Stress; (ii) Phytoremediation of Chromium by Aquatic and Terrestrial Weeds. Comptes Rendus Biol. 2010, 333, 597–607. [Google Scholar] [CrossRef]
- Pelcová, P.; Kopp, R.; Ridošková, A.; Grmela, J.; Štěrbová, D. Evaluation of Mercury Bioavailability and Phytoaccumulation by Means of a DGT Technique and of Submerged Aquatic Plants in an Aquatic Ecosystem Situated in the Vicinity of a Cinnabar Mine. Chemosphere 2022, 288, 132545. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, F.H.; Collivignarelli, M.C.; Masoud, A.M.N.; Carnevale Miino, M.; Torres, K.C.; Quintero, J.A.; Sorlini, S.; Vaccari, M. Mercury Removal from Mining Wastewater by Phytoaccumulation in Autochthonous Aquatic Plant Species. Clean Technol. 2023, 5, 839-851. https://doi.org/10.3390/cleantechnol5030041
Gomez FH, Collivignarelli MC, Masoud AMN, Carnevale Miino M, Torres KC, Quintero JA, Sorlini S, Vaccari M. Mercury Removal from Mining Wastewater by Phytoaccumulation in Autochthonous Aquatic Plant Species. Clean Technologies. 2023; 5(3):839-851. https://doi.org/10.3390/cleantechnol5030041
Chicago/Turabian StyleGomez, Franco Hernan, Maria Cristina Collivignarelli, Ahmed Mohammad Nafea Masoud, Marco Carnevale Miino, Kelly Cristina Torres, Jesus Antonio Quintero, Sabrina Sorlini, and Mentore Vaccari. 2023. "Mercury Removal from Mining Wastewater by Phytoaccumulation in Autochthonous Aquatic Plant Species" Clean Technologies 5, no. 3: 839-851. https://doi.org/10.3390/cleantechnol5030041
APA StyleGomez, F. H., Collivignarelli, M. C., Masoud, A. M. N., Carnevale Miino, M., Torres, K. C., Quintero, J. A., Sorlini, S., & Vaccari, M. (2023). Mercury Removal from Mining Wastewater by Phytoaccumulation in Autochthonous Aquatic Plant Species. Clean Technologies, 5(3), 839-851. https://doi.org/10.3390/cleantechnol5030041










