The Role of Oligodendrocytes in Alzheimer’s Disease Pathogenesis and Therapy
Abstract
1. Introduction
2. Oligodendrocytes
2.1. The Origin of Oligodendrocytes
2.1.1. Prenatal Oligodendrogenesis
2.1.2. Postnatal Oligodendrogenesis
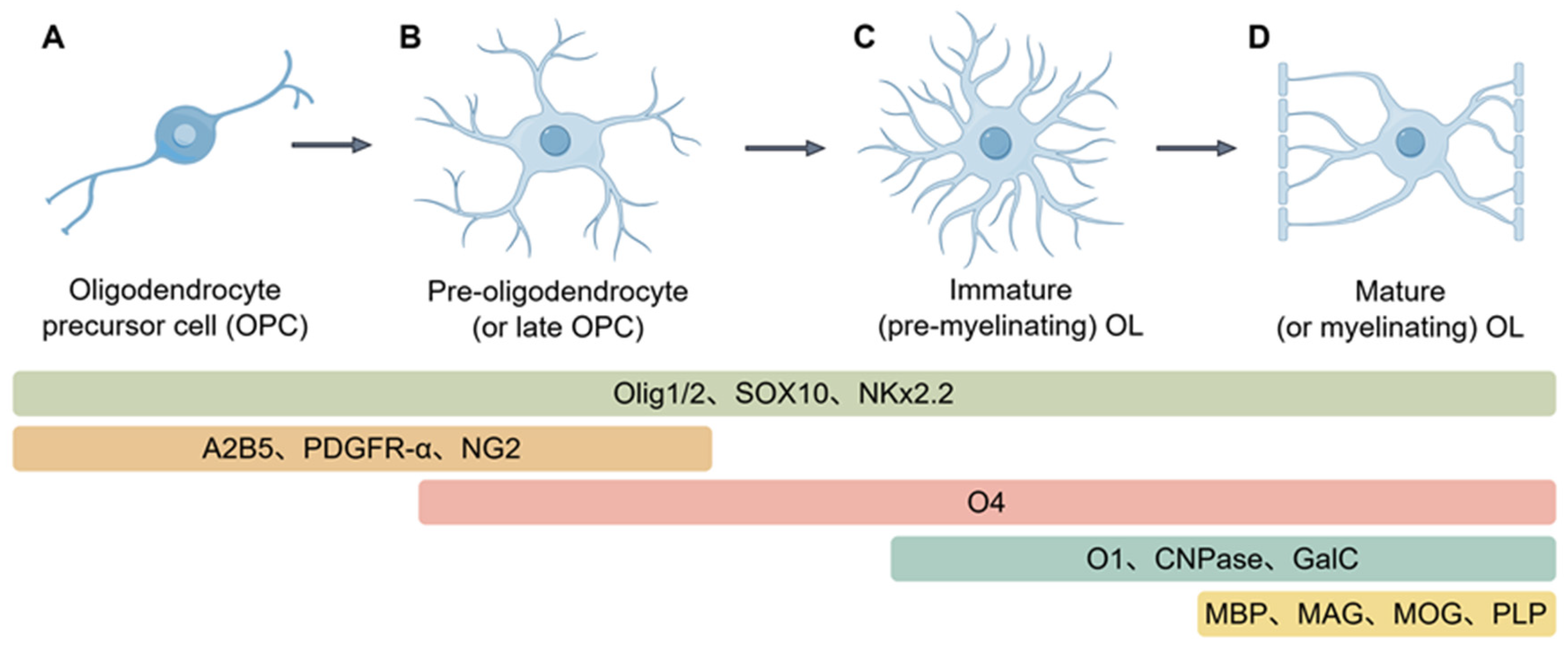
2.2. Oligodendrocyte Differentiation
2.3. Oligodendrocyte Regeneration
2.4. Oligodendrocyte Senescence
2.5. Oligodendrocyte Functions
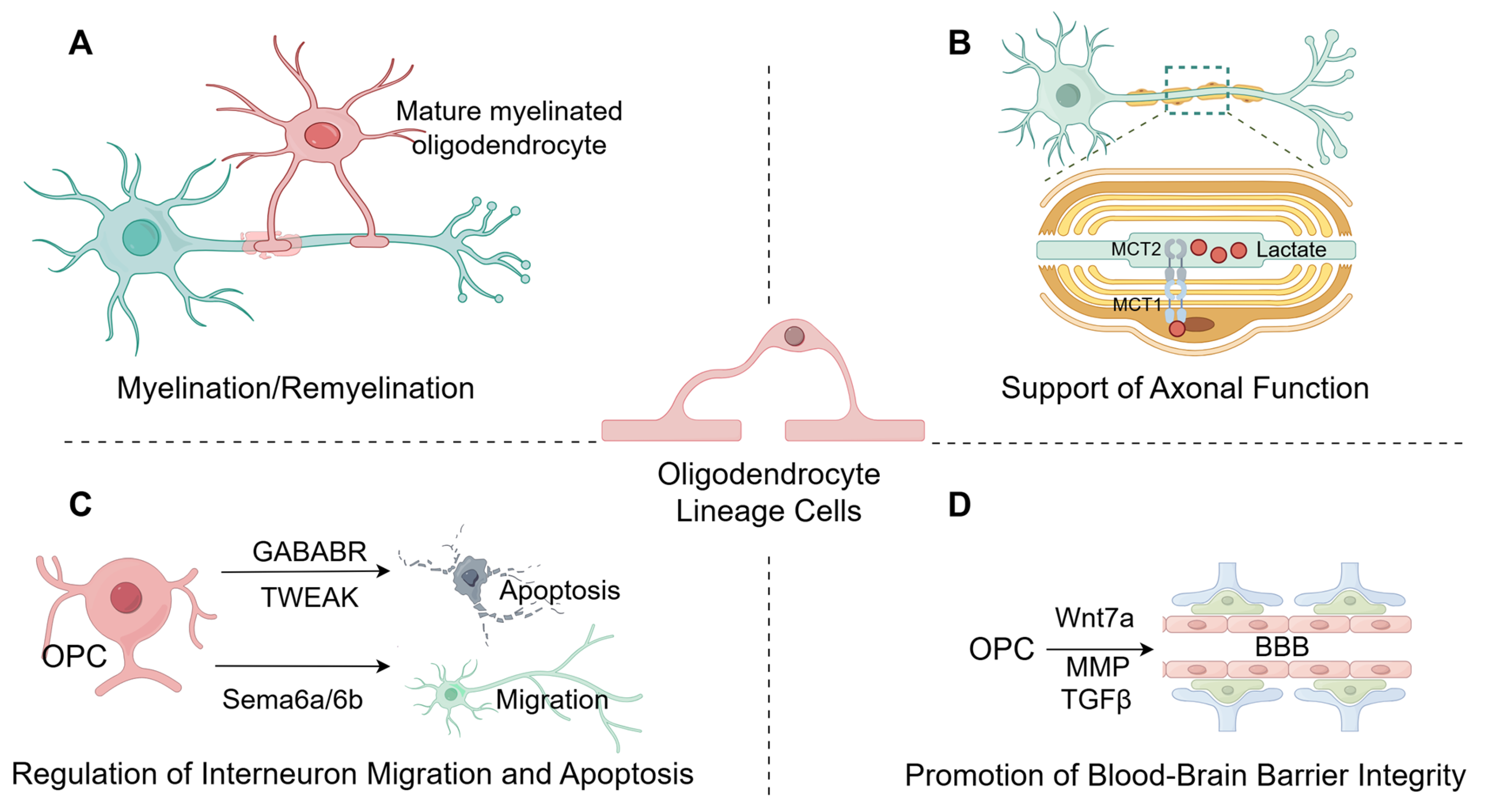
2.5.1. Myelination
2.5.2. Axonal Support
2.5.3. Regulation of Interneuron Migration and Apoptosis
2.5.4. Promotion of Blood–Brain Barrier Integrity
2.5.5. Intercellular Communication via Gap Junctions
3. Oligodendrocytes and AD
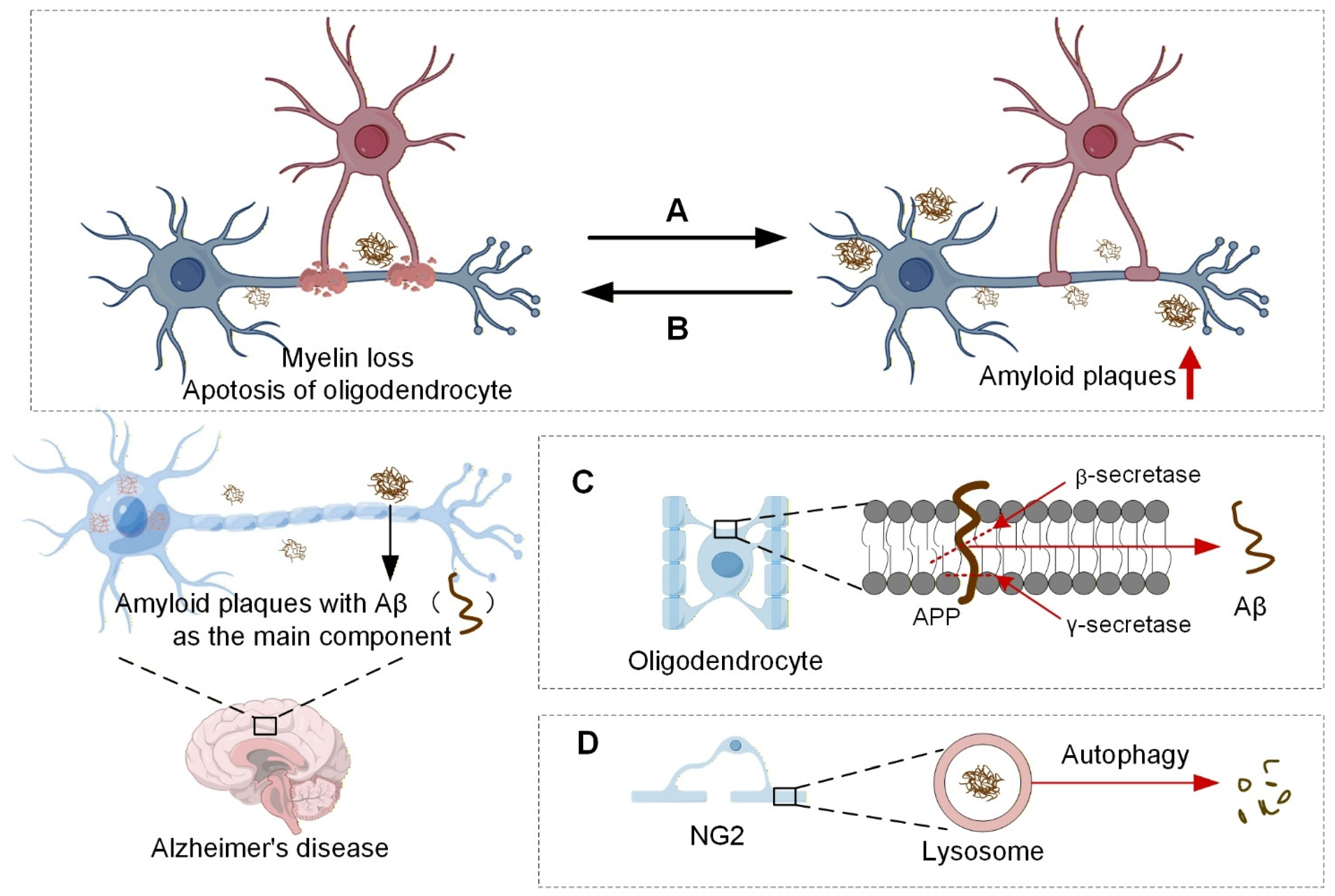
3.1. Oligodendrocytes and Aβ Plaques
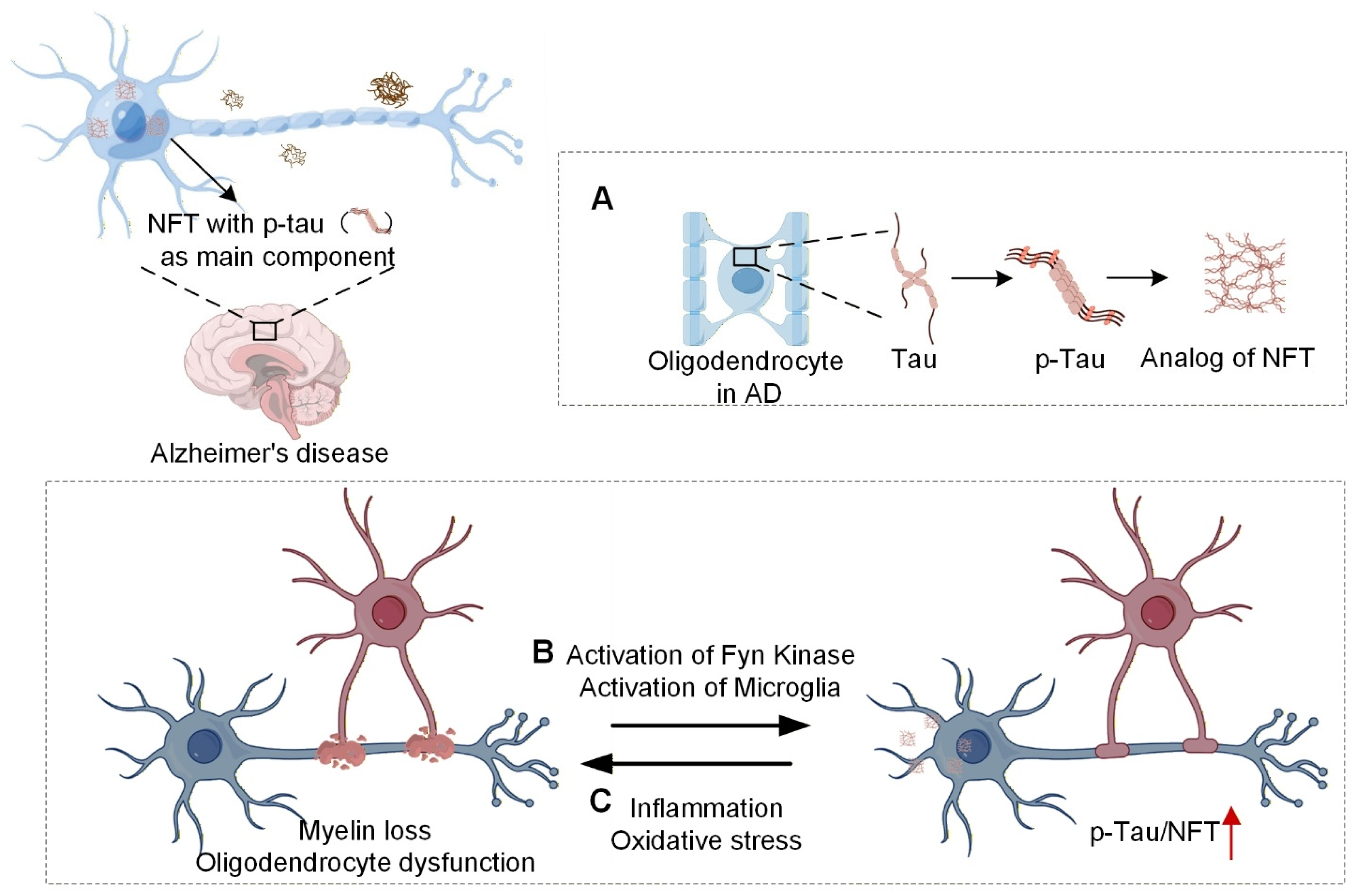
3.2. Oligodendrocytes and Tau Neurofibrillary Tangles
4. Potential Applications of Oligodendrocytes in AD Therapeutics
4.1. Antiaging Strategies
4.2. Strategies for Promoting Differentiation and Proliferation
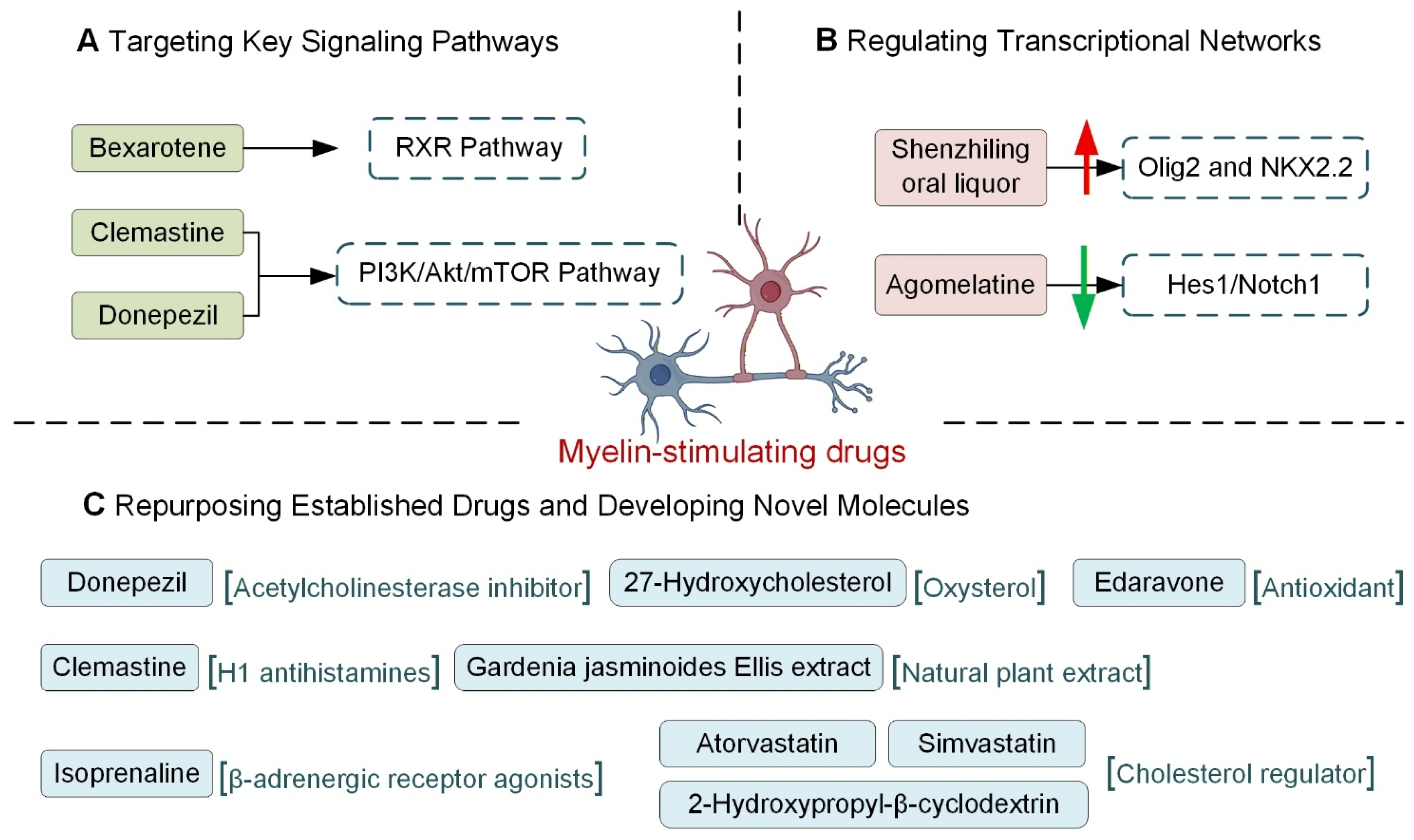
4.2.1. Targeting Key Signaling Pathways
4.2.2. Regulating Transcriptional Networks
4.2.3. Epigenetic Regulation via miRNAs
4.2.4. Repurposing Established Drugs and Developing Novel Molecules
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| Aβ | Amyloid-β |
| AD | Alzheimer’s Disease |
| APOE | Apolipoprotein E |
| APP | Amyloid Precursor Protein |
| BDNF | Brain-Derived Neurotrophic Factor |
| CNPase | 2′,3′-cyclic nucleotide 3′-phosphodiesterase |
| CNS | Central Nervous System |
| DOL | Disease-Associated Oligodendrocyte |
| FGF | Fibroblast Growth Factor |
| GalC | Galactocerebroside |
| GM | Gray Matter |
| MAG | Myelin-Associated Glycoprotein |
| MBP | Myelin Basic Protein |
| miRNA | MicroRNA |
| MOG | Myelin Oligodendrocyte Glycoprotein |
| NFT | Neurofibrillary Tangle |
| OL | Oligodendrocyte |
| OPC | Oligodendrocyte Progenitor Cell |
| OSVZ | Outer Subventricular Zone |
| PDGF | Platelet-Derived Growth Factor |
| PHF | Paired Helical Filament |
| PLP | Proteolipid Protein |
| RXR | Retinoid X Receptor |
| Shh | Sonic Hedgehog |
| SVZ | Subventricular Zone |
| VZ | Ventricular Zone |
| WM | White Matter |
References
- Von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef]
- Yeung, M.S.; Zdunek, S.; Bergmann, O.; Bernard, S.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Brundin, L.; et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 2014, 159, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Czopka, T. Myelination-independent functions of oligodendrocyte precursor cells in health and disease. Nat. Neurosci. 2023, 26, 1663–1669. [Google Scholar] [CrossRef]
- Heemels, M.T. Neurodegenerative diseases. Nature 2016, 539, 179. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, Y.; Zou, P.; Zong, X. Myelin dysfunction in aging and brain disorders: Mechanisms and therapeutic opportunities. Mol. Neurodegener. 2025, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Vallstedt, A.; Klos, J.M.; Ericson, J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron 2005, 45, 55–67. [Google Scholar] [CrossRef]
- Azim, K.; Fischer, B.; Hurtado-Chong, A.; Draganova, K.; Cantu, C.; Zemke, M.; Sommer, L.; Butt, A.; Raineteau, O. Persistent Wnt/beta-catenin signaling determines dorsalization of the postnatal subventricular zone and neural stem cell specification into oligodendrocytes and glutamatergic neurons. Stem Cells 2014, 32, 1301–1312. [Google Scholar] [CrossRef]
- Richardson, W.D.; Kessaris, N.; Pringle, N. Oligodendrocyte wars. Nat. Rev. Neurosci. 2006, 7, 11–18. [Google Scholar] [CrossRef]
- Huang, W.; Bhaduri, A.; Velmeshev, D.; Wang, S.; Wang, L.; Rottkamp, C.A.; Alvarez-Buylla, A.; Rowitch, D.H.; Kriegstein, A.R. Origins and Proliferative States of Human Oligodendrocyte Precursor Cells. Cell 2020, 182, 594–608.e11. [Google Scholar] [CrossRef]
- Jakovcevski, I.; Filipovic, R.; Mo, Z.; Rakic, S.; Zecevic, N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front. Neuroanat. 2009, 3, 5. [Google Scholar] [CrossRef]
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006, 9, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Gao, M.Y.; Gao, R.; Zhao, K.H.; Zhang, Y.; Li, X. Oligodendrocyte lineage cells: Advances in development, disease, and heterogeneity. J. Neurochem. 2023, 164, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, D.; Pohl, F.; Langseth, C.M.; Kukanja, P.; Lee, H.; Albiach, A.M.; Kabbe, M.; Meijer, M.; Linnarsson, S.; Hilscher, M.M. Developmental landscape of human forebrain at a single-cell level identifies early waves of oligodendrogenesis. Dev. Cell 2022, 57, 1421–1436.e5. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xu, R.; Padmashri, R.; Dunaevsky, A.; Liu, Y.; Dreyfus, C.F.; Jiang, P. Pluripotent Stem Cell-Derived Cerebral Organoids Reveal Human Oligodendrogenesis with Dorsal and Ventral Origins. Stem Cell Rep. 2019, 12, 890–905. [Google Scholar] [CrossRef]
- Naruse, M.; Ishizaki, Y.; Ikenaka, K.; Tanaka, A.; Hitosh, S. Origin of oligodendrocytes in mammalian forebrains: A revised perspective. J. Physiol. Sci. 2017, 67, 63–70. [Google Scholar] [CrossRef]
- Warf, B.C.; Fok-Seang, J.; Miller, R.H. Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. J. Neurosci. 1991, 11, 2477–2488. [Google Scholar] [CrossRef]
- Sussman, C.R.; Dyer, K.L.; Marchionni, M.; Miller, R.H. Local control of oligodendrocyte development in isolated dorsal mouse spinal cord. J. Neurosci. Res. 2000, 59, 413–420. [Google Scholar] [CrossRef]
- Cai, J.; Qi, Y.; Hu, X.; Tan, M.; Liu, Z.; Zhang, J.; Li, Q. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron 2005, 45, 41–53. [Google Scholar] [CrossRef]
- Rowitch, D.H.; Kriegstein, A.R. Developmental genetics of vertebrate glial-cell specification. Nature 2010, 468, 214–222. [Google Scholar] [CrossRef]
- Fang, M.; Yu, Q.; Ou, B.; Huang, H.; Yi, M.; Xie, B.; Yang, A.; Qiu, M.; Xu, X. Genetic Evidence that Dorsal Spinal Oligodendrocyte Progenitor Cells are Capable of Myelinating Ventral Axons Effectively in Mice. Neurosci. Bull. 2020, 36, 1474–1483. [Google Scholar] [CrossRef]
- Tripathi, R.B.; Clarke, L.E.; Burzomato, V.; Kessaris, N.; Anderson, P.N.; Attwell, D.; Richardson, W.D. Dorsally and ventrally derived oligodendrocytes have similar electrical properties but myelinate preferred tracts. J. Neurosci. 2011, 31, 6809–6819. [Google Scholar] [CrossRef] [PubMed]
- Valerio-Gomes, B.; Guimaraes, D.M.; Szczupak, D.; Lent, R. The Absolute Number of Oligodendrocytes in the Adult Mouse Brain. Front. Neuroanat. 2018, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Gotz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef]
- Hughes, E.G.; Kang, S.H.; Fukaya, M.; Bergles, D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013, 16, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Baldassarro, V.A.; Flagelli, A.; Sannia, M.; Calza, L. Nuclear receptors and differentiation of oligodendrocyte precursor cells. Vitam. Horm. 2021, 116, 389–407. [Google Scholar]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef]
- Hilscher, M.M.; Langseth, C.M.; Kukanja, P.; Yokota, C.; Nilsson, M.; Castelo-Branco, G. Spatial and temporal heterogeneity in the lineage progression of fine oligodendrocyte subtypes. BMC Biol. 2022, 20, 122. [Google Scholar] [CrossRef]
- Levine, J.M.; Reynolds, R.; Fawcett, J.W. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001, 24, 39–47. [Google Scholar] [CrossRef]
- Dwivedi, S.; Ranjan, S.; Das, S.; Kumari, J.; Singh, S. Role of growth factors and their interplay during oligodendroglial differentiation and maturation. Cytokine Growth Factor Rev. 2025, 84, 47–58. [Google Scholar] [CrossRef]
- Wang, S.; Sdrulla, A.; Johnson, J.E.; Yokota, Y.; Barres, B.A. A role for the helix-loop-helix protein Id2 in the control of oligodendrocyte development. Neuron 2001, 29, 603–614. [Google Scholar] [CrossRef]
- Tripathi, R.B.; Jackiewicz, M.; McKenzie, I.A.; Kougioumtzidou, E.; Grist, M.; Richardson, W.D. Remarkable Stability of Myelinating Oligodendrocytes in Mice. Cell Rep. 2017, 21, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Shimizu, T.; Sherafat, A.; Richardson, W.D. Life-long oligodendrocyte development and plasticity. Semin. Cell Dev. Biol. 2021, 116, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lewis, R.; Miller, R.H. Interactions between oligodendrocyte precursors control the onset of CNS myelination. Dev. Biol. 2011, 350, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Fukaya, M.; Yang, J.K.; Rothstein, J.D.; Bergles, D.E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010, 68, 668–681. [Google Scholar] [CrossRef]
- Noble, M.; Arhin, A.; Gass, D.; Mayer-Pröschel, M. The cortical ancestry of oligodendrocytes: Common principles and novel features. Dev. Neurosci. 2003, 25, 217–233. [Google Scholar] [CrossRef]
- Raff, M.C.; Abney, E.R.; Miller, R.H. Two glial cell lineages diverge prenatally in rat optic nerve. Dev. Biol. 1984, 106, 53–60. [Google Scholar] [CrossRef]
- Trotter, J.; Karram, K.; Nishiyama, A. NG2 cells: Properties, progeny and origin. Brain Res. Rev. 2010, 63, 72–82. [Google Scholar] [CrossRef]
- Boda, E.; Vigano, F.; Rosa, P.; Fumagalli, M.; Labat-Gest, V.; Tempia, F.; Abbracchio, M.P.; Dimou, L.; Buffo, A. The GPR17 receptor in NG2 expressing cells: Focus on in vivo cell maturation and participation in acute trauma and chronic damage. Glia 2011, 59, 1958–1973. [Google Scholar] [CrossRef]
- Yu, W.P.; Collarini, E.J.; Pringle, N.P.; Richardson, W.D. Embryonic expression of myelin genes: Evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron 1994, 12, 1353–1362. [Google Scholar] [CrossRef]
- Braun, P.E.; Sandillon, F.; Edwards, A.; Matthieu, J.M.; Privat, A. Immunocytochemical localization by electron microscopy of 2′3′-cyclic nucleotide 3′-phosphodiesterase in developing oligodendrocytes of normal and mutant brain. J. Neurosci. 1988, 8, 3057–3066. [Google Scholar] [CrossRef]
- Armstrong, R.C.; Dorn, H.H.; Kufta, C.V.; Friedman, E.; Dubois-Dalcq, M.E. Pre-oligodendrocytes from adult human CNS. J. Neurosci. 1992, 12, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Lassmann, H.; Waehneldt, T.V.; Matthieu, J.M.; Linington, C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2′,3′-cyclic nucleotide 3′-phosphodiesterase in the CNS of adult rats. J. Neurochem. 1989, 52, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Timsit, S.; Martinez, S.; Allinquant, B.; Peyron, F.; Puelles, L.; Zalc, B. Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. J. Neurosci. 1995, 15, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D. Myelin-associated glycoprotein. Location and potential functions. Ann. N. Y. Acad. Sci. 1990, 605, 29–43. [Google Scholar] [CrossRef]
- Scolding, N.J.; Frith, S.; Linington, C.; Morgan, B.P.; Campbell, A.K.; Compston, D.A. Myelin-oligodendrocyte glycoprotein (MOG) is a surface marker of oligodendrocyte maturation. J. Neuroimmunol. 1989, 22, 169–176. [Google Scholar] [CrossRef]
- Dubois-Dalcq, M.; Behar, T.; Hudson, L.; Lazzarini, R.A. Emergence of three myelin proteins in oligodendrocytes cultured without neurons. J. Cell Biol. 1986, 102, 384–392. [Google Scholar] [CrossRef]
- Koenning, M.; Jackson, S.; Hay, C.M.; Faux, C.; Kilpatrick, T.J.; Willingham, M.; Emery, B. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J. Neurosci. 2012, 32, 12528–12542. [Google Scholar] [CrossRef]
- Wang, S.Z.; Dulin, J.; Wu, H.; Hurlock, E.; Lee, S.E.; Jansson, K.; Lu, Q.R. An oligodendrocyte-specific zinc-finger transcription regulator cooperates with Olig2 to promote oligodendrocyte differentiation. Development 2006, 133, 3389–3398. [Google Scholar] [CrossRef]
- Sharifi, K.; Ebrahimi, M.; Kagawa, Y.; Islam, A.; Tuerxun, T.; Yasumoto, Y.; Hara, T.; Yamamoto, Y.; Miyazaki, H.; Tokuda, N.; et al. Differential expression and regulatory roles of FABP5 and FABP7 in oligodendrocyte lineage cells. Cell Tissue Res. 2013, 354, 683–695. [Google Scholar] [CrossRef]
- Mitew, S.; Hay, C.M.; Peckham, H.; Xiao, J.; Koenning, M.; Emery, B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 2014, 276, 29–47. [Google Scholar] [CrossRef]
- Zhou, Q.; Anderson, D.J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 2002, 109, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Maire, C.L.; Wegener, A.; Kerninon, C. Gain-of-function of Olig transcription factors enhances oligodendrogenesis and myelination. Stem Cells 2010, 28, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Paes de Faria, J.; Kessaris, N.; Andrew, P.; Richardson, W.D.; Li, H. New Olig1 null mice confirm a non-essential role for Olig1 in oligodendrocyte development. BMC Neurosci. 2014, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Hornig, J.; Frob, F.; Vogl, M.R.; Hermans-Borgmeyer, I.; Tamm, E.R.; Wegner, M. The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 2013, 9, e1003907. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, X.; Cai, J.; Liu, B.; Peng, X.; Wegner, M.; Qiu, M. Induction of oligodendrocyte differentiation by Olig2 and Sox10: Evidence for reciprocal interactions and dosage-dependent mechanisms. Dev. Biol. 2007, 302, 683–693. [Google Scholar] [CrossRef]
- Küspert, M.; Hammer, A.; Bösl, M.R.; Wegner, M. Olig2 regulates Sox10 expression in oligodendrocyte precursors through an evolutionary conserved distal enhancer. Nucleic Acids Res. 2011, 39, 1280–1293. [Google Scholar] [CrossRef]
- Weider, M.; Wegener, A.; Schmitt, C.; Kuspert, M.; Hillgartner, S.; Bosl, M.R.; Hermans-Borgmeyer, I.; Nait-Oumesmar, B.; Wegner, M. Elevated in vivo levels of a single transcription factor directly convert satellite glia into oligodendrocyte-like cells. PLoS Genet. 2015, 11, e1005008. [Google Scholar] [CrossRef]
- Weider, M.; Starost, L.J.; Groll, K.; Kuspert, M.; Sock, E.; Wedel, M.; Frob, F.; Schmitt, C.; Baroti, T.; Hartwig, A.C.; et al. Nfat/calcineurin signaling promotes oligodendrocyte differentiation and myelination by transcription factor network tuning. Nat. Commun. 2018, 9, 899. [Google Scholar] [CrossRef]
- Franklin, R.J.; Goldman, S.A. Glia Disease and Repair-Remyelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020594. [Google Scholar] [CrossRef]
- Shen, S.; Sandoval, J.; Swiss, V.A.; Li, J.; Dupree, J.; Franklin, R.J.; Casaccia-Bonnefil, P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 2008, 11, 1024–1034. [Google Scholar] [CrossRef]
- Watanabe, M.; Hadzic, T.; Nishiyama, A. Transient upregulation of Nkx2.2 expression in oligodendrocyte lineage cells during remyelination. Glia 2004, 46, 311–322. [Google Scholar] [CrossRef]
- Fancy, S.P.; Zhao, C.; Franklin, R.J. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol. Cell Neurosci. 2004, 27, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Boda, E.; Di Maria, S.; Rosa, P.; Taylor, V.; Abbracchio, M.P.; Buffo, A. Early phenotypic asymmetry of sister oligodendrocyte progenitor cells after mitosis and its modulation by aging and extrinsic factors. Glia 2015, 63, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Piaton, G.; Aigrot, M.S.; Williams, A.; Moyon, S.; Tepavcevic, V.; Moutkine, I.; Gras, J.; Matho, K.S.; Schmitt, A.; Soellner, H.; et al. Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain 2011, 134, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.C.; Le, T.Q.; Frost, E.E.; Borke, R.C.; Vana, A.C. Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. J. Neurosci. 2002, 22, 8574–8585. [Google Scholar] [CrossRef]
- Mason, J.L.; Xuan, S.; Dragatsis, I.; Efstratiadis, A.; Goldman, J.E. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J. Neurosci. 2003, 23, 7710–7718. [Google Scholar] [CrossRef]
- Pang, Y.; Zheng, B.; Fan, L.W.; Rhodes, P.G.; Cai, Z. IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia 2007, 55, 1099–1107. [Google Scholar] [CrossRef]
- Wang, S.; Sdrulla, A.D.; diSibio, G.; Bush, G.; Nofziger, D.; Hicks, C.; Weinmaster, G.; Barres, B.A. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 1998, 21, 63–75. [Google Scholar] [CrossRef]
- Glezer, I.; Lapointe, A.; Rivest, S. Innate immunity triggers oligodendrocyte progenitor reactivity and confines damages to brain injuries. Faseb J. 2006, 20, 750–752. [Google Scholar] [CrossRef]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 2010, 6, 578–590. [Google Scholar] [CrossRef]
- Luo, Y.; Coskun, V.; Liang, A.; Yu, J.; Cheng, L.; Ge, W.; Shi, Z.; Zhang, K.; Li, C.; Cui, Y.; et al. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell 2015, 161, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Moyon, S.; Dubessy, A.L.; Aigrot, M.S.; Trotter, M.; Huang, J.K.; Dauphinot, L.; Potier, M.C.; Kerninon, C.; Parsadaniantz, S.M.; Franklin, R.J.; et al. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J. Neurosci. 2015, 35, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, D.W.; Kim, E.; Jeong, I.; Kim, S.; Kim, B.J.; Park, H.C. Notch Signaling Controls Oligodendrocyte Regeneration in the Injured Telencephalon of Adult Zebrafish. Exp. Neurobiol. 2020, 29, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, Y.; Ren, Y.; Yin, S.; Yu, L.; Huang, R.; Song, S.; Hu, X.; Zhu, R.; Cheng, L.; et al. Neurogenesis potential of oligodendrocyte precursor cells from oligospheres and injured spinal cord. Front. Cell. Neurosci. 2022, 16, 1049562. [Google Scholar] [CrossRef]
- Bechler, M.E.; Byrne, L.; Ffrench-Constant, C. CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr. Biol. 2015, 25, 2411–2416. [Google Scholar] [CrossRef]
- Khandker, L.; Jeffries, M.A.; Chang, Y.J.; Mather, M.L.; Evangelou, A.V.; Bourne, J.N.; Tafreshi, A.K.; Ornelas, I.M.; Bozdagi-Gunal, O.; Macklin, W.B.; et al. Cholesterol biosynthesis defines oligodendrocyte precursor heterogeneity between brain and spinal cord. Cell Rep. 2022, 38, 110423. [Google Scholar] [CrossRef]
- Kujuro, Y.; Suzuki, N.; Kondo, T. Esophageal cancer-related gene 4 is a secreted inducer of cell senescence expressed by aged CNS precursor cells. Proc. Natl. Acad. Sci. USA 2010, 107, 8259–8264. [Google Scholar] [CrossRef]
- Neumann, B.; Baror, R.; Zhao, C.; Segel, M.; Dietmann, S.; Rawji, K.S.; Foerster, S.; McClain, C.R.; Chalut, K.; van Wijngaarden, P.; et al. Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells. Cell Stem Cell 2019, 25, 473–485.e8. [Google Scholar] [CrossRef]
- Soreq, L.; UK Brain Expression Consortium; North American Brain Expression Consortium; Rose, J.; Soreq, E.; Hardy, J.; Trabzuni, D.; Cookson, M.R.; Smith, C.; Ryten, M.; et al. Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging. Cell Rep. 2017, 18, 557–570. [Google Scholar] [CrossRef]
- Spitzer, S.O.; Sitnikov, S.; Kamen, Y.; Evans, K.A.; Kronenberg-Versteeg, D.; Dietmann, S.; de Faria, O., Jr.; Agathou, S.; Karadottir, R.T. Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron 2019, 101, 459–471.e5. [Google Scholar] [CrossRef]
- Rivera, A.D.; Pieropan, F.; Chacon-De-La-Rocha, I.; Lecca, D.; Abbracchio, M.P.; Azim, K.; Butt, A.M. Functional genomic analyses highlight a shift in Gpr17-regulated cellular processes in oligodendrocyte progenitor cells and underlying myelin dysregulation in the aged mouse cerebrum. Aging Cell 2021, 20, e13335. [Google Scholar] [CrossRef]
- Falcao, A.M.; van Bruggen, D.; Marques, S.; Meijer, M.; Jakel, S.; Agirre, E.; Samudyata; Floriddia, E.M.; Vanichkina, D.P.; Ffrench-Constant, C.; et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2018, 24, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Marisca, R.; Hoche, T.; Agirre, E.; Hoodless, L.J.; Barkey, W.; Auer, F.; Castelo-Branco, G.; Czopka, T. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci. 2020, 23, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef]
- Kang, C.; Xu, Q.; Martin, T.D.; Li, M.Z.; Demaria, M.; Aron, L.; Lu, T.; Yankner, B.A.; Campisi, J.; Elledge, S.J. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 2015, 349, aaa5612. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An. emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Tse, K.H.; Herrup, K. DNA damage in the oligodendrocyte lineage and its role in brain aging. Mech. Ageing Dev. 2017, 161 Pt A, 37–50. [Google Scholar] [CrossRef]
- Baker, D.J.; Petersen, R.C. Cellular senescence in brain aging and neurodegenerative diseases: Evidence and perspectives. J. Clin. Investig. 2018, 128, 1208–1216. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Sen, J.M.; Gorospe, M.; et al. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Neumann, B.; Segel, M.; Ghosh, T.; Zhao, C.; Tourlomousis, P.; Young, A.; Förster, S.; Sharma, A.; Chen, C.Z.; Cubillos, J.F.; et al. Myc determines the functional age state of oligodendrocyte progenitor cells. Nat. Aging 2021, 1, 826–837. [Google Scholar] [CrossRef]
- Rivellini, C.; Porrello, E.; Dina, G.; Mrakic-Sposta, S.; Vezzoli, A.; Bacigaluppi, M.; Gullotta, G.S.; Chaabane, L.; Leocani, L.; Marenna, S.; et al. JAB1 deletion in oligodendrocytes causes senescence-induced inflammation and neurodegeneration in mice. J. Clin. Investig. 2022, 132, e145071. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, M.E.; Hu, J.; Sutter, P.A.; Kim, H.W.; Huang, J.K.; Crocker, S.J. The Cellular Senescence Factor Extracellular HMGB1 Directly Inhibits Oligodendrocyte Progenitor Cell Differentiation and Impairs CNS Remyelination. Front. Cell. Neurosci. 2022, 16, 833186. [Google Scholar] [CrossRef] [PubMed]
- Saab, A.S.; Nave, K.A. Myelin dynamics: Protecting and shaping neuronal functions. Curr. Opin. Neurobiol. 2017, 47, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Pease-Raissi, S.E.; Chan, J.R. Building a (w)rapport between neurons and oligodendroglia: Reciprocal interactions underlying adaptive myelination. Neuron 2021, 109, 1258–1273. [Google Scholar] [CrossRef]
- Fang, L.P.; Bai, X. Oligodendrocyte precursor cells: The multitaskers in the brain. Pflugers Arch. 2023, 475, 1035–1044. [Google Scholar] [CrossRef]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Vitali, F.; Shang, Y.; Raikes, A.C.; Wang, T.; Jin, Y.; Brinton, R.D.; Gu, H.; Yin, F. Loss of fatty acid degradation by astrocytic mitochondria triggers neuroinflammation and neurodegeneration. Nat. Metab. 2023, 5, 445–465. [Google Scholar] [CrossRef]
- Looser, Z.J.; Faik, Z.; Ravotto, L.; Zanker, H.S.; Jung, R.B.; Werner, H.B.; Ruhwedel, T.; Möbius, W.; Bergles, D.E.; Barros, L.F.; et al. Oligodendrocyte-axon metabolic coupling is mediated by extracellular K(+) and maintains axonal health. Nat. Neurosci. 2024, 27, 433–448. [Google Scholar] [CrossRef]
- Liu, M.; Jin, S.; Fu, X.; Xie, C.; Chen, Y.; Chang, L.; Fan, Y.; He, D.; Hong, X.; Shen, X.; et al. Activation of Kir4.1 Channels by 2-D08 Promotes Myelin Repair in Multiple Sclerosis. Adv. Sci. 2025, 12, e02032. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Rinholm, J.E.; Hamilton, N.B.; Kessaris, N.; Richardson, W.D.; Bergersen, L.H.; Attwell, D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 2011, 31, 538–548. [Google Scholar] [CrossRef]
- Saab, A.S.; Tzvetavona, I.D.; Trevisiol, A.; Baltan, S.; Dibaj, P.; Kusch, K.; Möbius, W.; Goetze, B.; Jahn, H.M.; Huang, W.; et al. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 2016, 91, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, E.; Trevisiol, A.; Saab, A.S.; Looser, Z.J.; Dibaj, P.; Ebrahimi, R.; Kusch, K.; Ruhwedel, T.; Möbius, W.; Jahn, O.; et al. Oligodendroglial fatty acid metabolism as a central nervous system energy reserve. Nat. Neurosci. 2024, 27, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Lepiemme, F.; Stoufflet, J.; Javier-Torrent, M.; Mazzucchelli, G.; Silva, C.G.; Nguyen, L. Oligodendrocyte precursors guide interneuron migration by unidirectional contact repulsion. Science 2022, 376, eabn6204. [Google Scholar] [CrossRef] [PubMed]
- Orduz, D.; Benamer, N.; Ortolani, D.; Coppola, E.; Vigier, L.; Pierani, A.; Angulo, M.C. Developmental cell death regulates lineage-related interneuron-oligodendroglia functional clusters and oligodendrocyte homeostasis. Nat. Commun. 2019, 10, 4249. [Google Scholar] [CrossRef]
- Fang, L.P.; Zhao, N.; Caudal, L.C.; Chang, H.F.; Zhao, R.; Lin, C.H.; Hainz, N.; Meier, C.; Bettler, B.; Huang, W.; et al. Impaired bidirectional communication between interneurons and oligodendrocyte precursor cells affects social cognitive behavior. Nat. Commun. 2022, 13, 1394. [Google Scholar] [CrossRef]
- Tsai, H.H.; Niu, J.; Munji, R.; Davalos, D.; Chang, J.; Zhang, H.; Tien, A.C.; Kuo, C.J.; Chan, J.R.; Daneman, R.; et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 2016, 351, 379–384. [Google Scholar] [CrossRef]
- Minocha, S.; Valloton, D.; Brunet, I.; Eichmann, A.; Hornung, J.P.; Lebrand, C. NG2 glia are required for vessel network formation during embryonic development. Elife 2015, 4, e09102. [Google Scholar] [CrossRef]
- Wang, L.P.; Pan, J.; Li, Y.; Geng, J.; Liu, C.; Zhang, L.Y.; Zhou, P.; Tang, Y.H.; Wang, Y.; Zhang, Z.; et al. Oligodendrocyte precursor cell transplantation promotes angiogenesis and remyelination via Wnt/beta-catenin pathway in a mouse model of middle cerebral artery occlusion. J. Cereb. Blood Flow. Metab. 2022, 42, 757–770. [Google Scholar] [CrossRef]
- Niu, J.; Tsai, H.H.; Hoi, K.K.; Huang, N.; Yu, G.; Kim, K.; Baranzini, S.E.; Xiao, L.; Chan, J.R.; Fancy, S.P.J. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat. Neurosci. 2019, 22, 709–718. [Google Scholar] [CrossRef]
- Seo, J.H.; Maki, T.; Maeda, M.; Miyamoto, N.; Liang, A.C.; Hayakawa, K.; Pham, L.D.; Suwa, F.; Taguchi, A.; Matsuyama, T.; et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS ONE 2014, 9, e103174. [Google Scholar] [CrossRef]
- Seo, J.H.; Miyamoto, N.; Hayakawa, K.; Pham, L.D.; Maki, T.; Ayata, C.; Kim, K.W.; Lo, E.H.; Arai, K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J. Clin. Investig. 2013, 123, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Naus, C.C.; Sáez, J.C.; Leybaert, L. Glial Connexins and Pannexins in the Healthy and Diseased Brain. Physiol. Rev. 2021, 101, 93–145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yamasaki, R.; Yamaguchi, H.; Nagata, S.; Une, H.; Cui, Y.; Masaki, K.; Nakamuta, Y.; Iinuma, K.; Watanabe, M.; et al. Oligodendroglial connexin 47 regulates neuroinflammation upon autoimmune demyelination in a novel mouse model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Abrams, C.K.; Flores-Obando, R.E.; Dungan, G.D.; Cherepanova, E.; Freidin, M.M. Investigating oligodendrocyte connexins: Heteromeric interactions between Cx32 and mutant or wild-type forms of Cx47 do not contribute to or modulate gap junction function. Glia 2021, 69, 1882–1896. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Liu, J.; Chen, J.; Shen, K.; Chen, N.; Xiang, S.; Wang, D.; Xiao, N.; Li, T. Cx47 Phosphorylation Exacerbates White Matter Damage and Kainic Acid Induced Epilepsy. CNS Neurosci. Ther. 2025, 31, e70672. [Google Scholar] [CrossRef]
- Wasseff, S.K.; Scherer, S.S. Activated immune response in an inherited leukodystrophy disease caused by the loss of oligodendrocyte gap junctions. Neurobiol. Dis. 2015, 82, 86–98. [Google Scholar] [CrossRef]
- Pechlivanidou, M.; Kousiappa, I.; Angeli, S.; Sargiannidou, I.; Koupparis, A.M.; Papacostas, S.S.; Kleopa, K.A. Glial Gap Junction Pathology in the Spinal Cord of the 5xFAD Mouse Model of Early-Onset Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 15597. [Google Scholar] [CrossRef]
- Angeli, S.; Kousiappa, I.; Stavrou, M.; Sargiannidou, I.; Georgiou, E.; Papacostas, S.S.; Kleopa, K.A. Altered Expression of Glial Gap Junction Proteins Cx43, Cx30, and Cx47 in the 5XFAD Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 582934. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, X. Alzheimer’s disease: Insights into pathology, molecular mechanisms, and therapy. Protein Cell 2025, 16, 83–120. [Google Scholar] [CrossRef]
- Chen, Y.; Song, S.; Parhizkar, S.; Lord, J.; Zhu, Y.; Strickland, M.R.; Wang, C.; Park, J.; Tabor, G.T.; Jiang, H.; et al. APOE3ch alters microglial response and suppresses Aβ-induced tau seeding and spread. Cell 2024, 187, 428–445.e20. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, M.M.; Nissen, A.; Eckermann, K.; Khlistunova, I.; Biernat, J.; Drexler, D.; Petrova, O.; Schönig, K.; Bujard, H.; Mandelkow, E.; et al. The potential for beta-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy. J. Neurosci. 2008, 28, 737–748. [Google Scholar] [CrossRef]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Sadick, J.S.; O’Dea, M.R.; Hasel, P.; Dykstra, T.; Faustin, A.; Liddelow, S.A. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron 2022, 110, 1788–1805.e10. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Lu, A.; Craessaerts, K.; Pavie, B.; Frigerio, C.S.; Corthout, N.; Qian, X.; Lalakova, J.; Kuhnemund, M.; Voytyuk, I.; et al. Spatial Transcriptomics and In Situ Sequencing to Study Alzheimer’s Disease. Cell 2020, 182, 976–991.e19. [Google Scholar] [CrossRef]
- Walter, S.; Jumpertz, T.; Huttenrauch, M.; Ogorek, I.; Gerber, H.; Storck, S.E.; Zampar, S.; Dimitrov, M.; Lehmann, S.; Lepka, K.; et al. The metalloprotease ADAMTS4 generates N-truncated Abeta4-x species and marks oligodendrocytes as a source of amyloidogenic peptides in Alzheimer’s disease. Acta Neuropathol. 2019, 137, 239–257. [Google Scholar] [CrossRef]
- Sasmita, A.O.; Depp, C.; Nazarenko, T.; Sun, T.; Siems, S.B.; Ong, E.C.; Nkeh, Y.B.; Böhler, C.; Yu, X.; Bues, B.; et al. Oligodendrocytes produce amyloid-β and contribute to plaque formation alongside neurons in Alzheimer’s disease model mice. Nat. Neurosci. 2024, 27, 1668–1674. [Google Scholar] [CrossRef]
- Ishii, A.; Pathoulas, J.A.; Omar, O.M.; Ge, Y.; Yao, A.Y.; Pantalena, T.; Singh, N.; Zhou, J.; He, W.; Murphy, P.; et al. Contribution of amyloid deposition from oligodendrocytes in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 83. [Google Scholar] [CrossRef]
- Rajani, R.M.; Ellingford, R.; Hellmuth, M.; Harris, S.S.; Taso, O.S.; Graykowski, D.; Lam, F.K.W.; Arber, C.; Fertan, E.; Danial, J.S.H.; et al. Selective suppression of oligodendrocyte-derived amyloid beta rescues neuronal dysfunction in Alzheimer’s disease. PLoS Biol. 2024, 22, e3002727. [Google Scholar] [CrossRef]
- Li, W.; Tang, Y.; Fan, Z.; Meng, Y.; Yang, G.; Luo, J.; Ke, Z.J. Autophagy is involved in oligodendroglial precursor-mediated clearance of amyloid peptide. Mol. Neurodegener. 2013, 8, 27. [Google Scholar] [CrossRef]
- Depp, C.; Sun, T.; Sasmita, A.O.; Spieth, L.; Berghoff, S.A.; Nazarenko, T.; Overhoff, K.; Steixner-Kumar, A.A.; Subramanian, S.; Arinrad, S.; et al. Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease. Nature 2023, 618, 349–357. [Google Scholar] [CrossRef]
- Park, H.; Cho, B.; Kim, H.; Saito, T.; Saido, T.C.; Won, K.J.; Kim, J. Single-cell RNA-sequencing identifies disease-associated oligodendrocytes in male APP NL-G-F and 5XFAD mice. Nat. Commun. 2023, 14, 802. [Google Scholar] [CrossRef]
- Mitew, S.; Kirkcaldie, M.T.; Halliday, G.M.; Shepherd, C.E.; Vickers, J.C.; Dickson, T.C. Focal demyelination in Alzheimer’s disease and transgenic mouse models. Acta Neuropathol. 2010, 119, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Lee, J.T.; Chen, H.; Chen, S.; Hsu, C.Y.; Xu, J. Amyloid-beta peptide enhances tumor necrosis factor-alpha-induced iNOS through neutral sphingomyelinase/ceramide pathway in oligodendrocytes. J. Neurochem. 2005, 94, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.K.; Sudol, K.L.; Janelsins, M.C.; Mastrangelo, M.A.; Frazer, M.E.; Bowers, W.J. Triple-transgenic Alzheimer’s disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia 2009, 57, 54–65. [Google Scholar] [PubMed]
- Desai, M.K.; Mastrangelo, M.A.; Ryan, D.A.; Sudol, K.L.; Narrow, W.C.; Bowers, W.J. Early oligodendrocyte/myelin pathology in Alzheimer’s disease mice constitutes a novel therapeutic target. Am. J. Pathol. 2010, 177, 1422–1435. [Google Scholar] [CrossRef]
- Horiuchi, M.; Maezawa, I.; Itoh, A.; Wakayama, K.; Jin, L.W.; Itoh, T.; Decarli, C. Amyloid beta1-42 oligomer inhibits myelin sheet formation in vitro. Neurobiol. Aging 2012, 33, 499–509. [Google Scholar]
- Kedia, S.; Simons, M. Oligodendrocytes in Alzheimer’s disease pathophysiology. Nat. Neurosci. 2025, 28, 446–456. [Google Scholar] [CrossRef]
- Richter-Landsberg, C. Protein aggregate formation in oligodendrocytes: Tau and the cytoskeleton at the intersection of neuroprotection and neurodegeneration. Biol. Chem. 2016, 397, 185–194. [Google Scholar] [CrossRef]
- Götz, J.; Tolnay, M.; Barmettler, R.; Chen, F.; Probst, A.; Nitsch, R.M. Oligodendroglial tau filament formation in transgenic mice expressing G272V tau. Eur. J. Neurosci. 2001, 13, 2131–2140. [Google Scholar] [CrossRef]
- Higuchi, M.; Zhang, B.; Forman, M.S.; Yoshiyama, Y.; Trojanowski, J.Q.; Lee, V.M. Axonal degeneration induced by targeted expression of mutant human tau in oligodendrocytes of transgenic mice that model glial tauopathies. J. Neurosci. 2005, 25, 9434–9443. [Google Scholar] [CrossRef] [PubMed]
- Rubinski, A.; Dewenter, A.; Zheng, L.; Franzmeier, N.; Stephenson, H.; Deming, Y.; Duering, M.; Gesierich, B.; Denecke, J.; Pham, A.V.; et al. Florbetapir PET-assessed demyelination is associated with faster tau accumulation in an APOE ε4-dependent manner. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1035–1049. [Google Scholar] [PubMed]
- Rubinski, A.; Franzmeier, N.; Dewenter, A.; Luan, Y.; Smith, R.; Strandberg, O.; Ossenkoppele, R.; Dichgans, M.; Hansson, O.; Ewers, M. Higher levels of myelin are associated with higher resistance against tau pathology in Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 139. [Google Scholar] [CrossRef]
- Schneider, A.; Araújo, G.W.; Trajkovic, K.; Herrmann, M.M.; Merkler, D.; Mandelkow, E.M.; Weissert, R.; Simons, M. Hyperphosphorylation and aggregation of tau in experimental autoimmune encephalomyelitis. J. Biol. Chem. 2004, 279, 55833–55839. [Google Scholar] [CrossRef] [PubMed]
- Briner, A.; Götz, J.; Polanco, J.C. Fyn Kinase Controls Tau Aggregation In Vivo. Cell Rep. 2020, 32, 108045. [Google Scholar] [CrossRef]
- Safaiyan, S.; Kannaiyan, N.; Snaidero, N.; Brioschi, S.; Biber, K.; Yona, S.; Edinger, A.L.; Jung, S.; Rossner, M.J.; Simons, M. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016, 19, 995–998. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Fitzner, D.; Bosch-Queralt, M.; Weil, M.T.; Su, M.; Sen, P.; Ruhwedel, T.; Mitkovski, M.; Trendelenburg, G.; Lütjohann, D.; et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 2018, 359, 684–688. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Papuc, E.; Rejdak, K. The role of myelin damage in Alzheimer’s disease pathology. Arch. Med. Sci. 2020, 16, 345–351. [Google Scholar]
- Shi, Y.; Andhey, P.S.; Ising, C.; Wang, K.; Snipes, L.L.; Boyer, K.; Lawson, S.; Yamada, K.; Qin, W.; Manis, M.; et al. Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to apoE-linked mechanisms. Neuron 2021, 109, 2413–2426.e7. [Google Scholar] [CrossRef]
- Vanzulli, I.; Papanikolaou, M.; De-La-Rocha, I.C.; Pieropan, F.; Rivera, A.D.; Gomez-Nicola, D.; Verkhratsky, A.; Rodríguez, J.J.; Butt, A.M. Disruption of oligodendrocyte progenitor cells is an early sign of pathology in the triple transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging 2020, 94, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Koutsodendris, N.; Blumenfeld, J.; Agrawal, A.; Traglia, M.; Grone, B.; Zilberter, M.; Yip, O.; Rao, A.; Nelson, M.R.; Hao, Y.; et al. Neuronal APOE4 removal protects against tau-mediated gliosis, neurodegeneration and myelin deficits. Nat. Aging 2023, 3, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lee, C.; Kodama, L.; Fan, L.; Zhu, D.; Zhu, J.; Wong, M.Y.; Ye, P.; Norman, K.; Foxe, N.R.; Ijaz, L.; et al. Tlr7 drives sex differences in age- and Alzheimer’s disease-related demyelination. Science 2024, 386, eadk7844. [Google Scholar] [CrossRef]
- Wu, C.; Yang, L.; Feng, S.; Zhu, L.; Yang, L.; Liu, T.C.; Duan, R. Therapeutic non-invasive brain treatments in Alzheimer’s disease: Recent advances and challenges. Inflamm. Regen. 2022, 42, 31. [Google Scholar] [CrossRef] [PubMed]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H.; et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef]
- Vandenberghe, R.; Rinne, J.O.; Boada, M.; Katayama, S.; Scheltens, P.; Vellas, B.; Tuchman, M.; Gass, A.; Fiebach, J.B.; Hill, D.; et al. Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimer’s Res. Ther. 2016, 8, 18. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, S.; Nam, Y.; Park, Y.H.; Shin, S.M.; Moon, M. Second-generation anti-amyloid monoclonal antibodies for Alzheimer’s disease: Current landscape and future perspectives. Transl. Neurodegener. 2025, 14, 6. [Google Scholar] [CrossRef]
- Loera-Valencia, R.; Cedazo-Minguez, A.; Kenigsberg, P.A.; Page, G.; Duarte, A.I.; Giusti, P.; Zusso, M.; Robert, P.; Frisoni, G.B.; Cattaneo, A.; et al. Current and emerging avenues for Alzheimer’s disease drug targets. J. Intern. Med. 2019, 286, 398–437. [Google Scholar]
- Arroyo-Pacheco, N.; Sarmiento-Blanco, S.; Vergara-Cadavid, G.; Castro-Leones, M.; Contreras-Puentes, N. Monoclonal therapy with lecanemab in the treatment of mild Alzheimer’s disease: A systematic review and meta-analysis. Ageing Res. Rev. 2025, 104, 102620. [Google Scholar]
- Zou, P.; Wu, C.; Liu, T.C.; Duan, R.; Yang, L. Oligodendrocyte progenitor cells in Alzheimer’s disease: From physiology to pathology. Transl. Neurodegener. 2023, 12, 52. [Google Scholar] [CrossRef]
- Dimovasili, C.; Fair, A.E.; Garza, I.R.; Batterman, K.V.; Mortazavi, F.; Moore, T.L.; Rosene, D.L. Aging compromises oligodendrocyte precursor cell maturation and efficient remyelination in the monkey brain. Geroscience 2023, 45, 249–264. [Google Scholar] [CrossRef]
- Lee, J.Y.; Harney, D.J.; Teo, J.D.; Kwok, J.B.; Sutherland, G.T.; Larance, M.; Don, A.S. The major TMEM106B dementia risk allele affects TMEM106B protein levels, fibril formation, and myelin lipid homeostasis in the ageing human hippocampus. Mol. Neurodegener. 2023, 18, 63. [Google Scholar] [CrossRef]
- Saez-Atienzar, S.; Masliah, E. Cellular senescence and Alzheimer disease: The egg and the chicken scenario. Nat. Rev. Neurosci. 2020, 21, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Segel, M.; Neumann, B.; Hill, M.F.E.; Weber, I.P.; Viscomi, C.; Zhao, C.; Young, A.; Agley, C.C.; Thompson, A.J.; Gonzalez, G.A.; et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 2019, 573, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Iram, T.; Kern, F.; Kaur, A.; Myneni, S.; Morningstar, A.R.; Shin, H.; Garcia, M.A.; Yerra, L.; Palovics, R.; Yang, A.C.; et al. Young CSF restores oligodendrogenesis and memory in aged mice via Fgf17. Nature 2022, 605, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Neitzel, J.; Franzmeier, N.; Rubinski, A.; Dichgans, M.; Brendel, M.; Malik, R.; Ewers, M. KL-VS heterozygosity is associated with lower amyloid-dependent tau accumulation and memory impairment in Alzheimer’s disease. Nat. Commun. 2021, 12, 3825. [Google Scholar] [CrossRef]
- Belloy, M.E.; Eger, S.J.; Le Guen, Y.; Napolioni, V.; Deters, K.D.; Yang, H.S.; Scelsi, M.A.; Porter, T.; James, S.N.; Wong, A.; et al. KL∗VS heterozygosity reduces brain amyloid in asymptomatic at-risk APOE∗4 carriers. Neurobiol. Aging 2021, 101, 123–129. [Google Scholar] [CrossRef]
- Chen, C.D.; Sloane, J.A.; Li, H.; Aytan, N.; Giannaris, E.L.; Zeldich, E.; Hinman, J.D.; Dedeoglu, A.; Rosene, D.L.; Bansal, R.; et al. The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J. Neurosci. 2013, 33, 1927–1939. [Google Scholar] [CrossRef]
- Zeldich, E.; Chen, C.D.; Avila, R.; Medicetty, S. The Anti-Aging Protein Klotho Enhances Remyelination Following Cuprizone-Induced Demyelination. J. Mol. Neurosci. 2015, 57, 185–196. [Google Scholar] [CrossRef]
- Huang, J.K.; Jarjour, A.A.; Oumesmar, B.N.; Kerninon, C.; Williams, A.; Krezel, W.; Kagechika, H.; Bauer, J.; Zhao, C.; Evercooren, A.B.-V.; et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011, 14, 45–53. [Google Scholar] [CrossRef]
- Mariani, M.M.; Malm, T.; Lamb, R.; Jay, T.R.; Neilson, L.; Casali, B.; Medarametla, L.; Landreth, G.E. Neuronally-directed effects of RXR activation in a mouse model of Alzheimer’s disease. Sci. Rep. 2017, 7, 42270. [Google Scholar] [CrossRef] [PubMed]
- Keough, M.B.; Yong, V.W. Remyelination therapy for multiple sclerosis. Neurotherapeutics 2013, 10, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gil, D.F.; Arboleda, G.; Sandoval-Hernández, A.G. Retinoid X receptor activation promotes re-myelination in a very old triple transgenic mouse model of Alzheimer’s disease. Neurosci. Lett. 2021, 750, 135764. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Guo, Y.E.; Fang, J.H.; Shi, C.J.; Suo, N.; Zhang, R.; Xie, X. Donepezil, a drug for Alzheimer’s disease, promotes oligodendrocyte generation and remyelination. Acta Pharmacol. Sin. 2019, 40, 1386–1393. [Google Scholar] [CrossRef]
- Chen, J.F.; Liu, K.; Hu, B.; Li, R.R.; Xin, W.; Chen, H.; Wang, F.; Chen, L.; Li, R.X.; Ren, S.Y.; et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 2021, 109, 2292–2307.e5. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Pan, T.T.; Xu, D.E.; Huang, X.; Tang, Y.; Huang, W.; Chen, R.; Lu, L.; Chi, H.; Ma, Q.H. Clemastine Ameliorates Myelin Deficits via Preventing Senescence of Oligodendrocytes Precursor Cells in Alzheimer’s Disease Model Mouse. Front. Cell Dev. Biol. 2021, 9, 733945. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Song, Q.; Hou, Y.; Liu, J.; Sun, Y.; Wang, P. Characterization of the chromatin accessibility in an Alzheimer’s disease (AD) mouse model. Alzheimer’s Res. Ther. 2020, 12, 29. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Z.; Mana, L.; Qin, G.; Huang, S.; Gong, Z.; Tian, M.; He, Y.; Wang, P. Shenzhiling oral liquid protects the myelin sheath against Alzheimer’s disease through the PI3K/Akt-mTOR pathway. J. Ethnopharmacol. 2021, 278, 114264. [Google Scholar] [CrossRef]
- Ji, Q.; Lv, Y.; Hu, B.; Su, Y.; Shaikh, I.I.; Zhu, X. Study on the therapeutic potential of induced neural stem cells for Alzheimer’s disease in mice. Biol. Res. 2024, 57, 89. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, K.I.; Chen, C.H.; Lee, T.S. Genetic deletion of soluble epoxide hydrolase delays the progression of Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 267. [Google Scholar] [CrossRef]
- Perna, A.; Marathe, S.; Dreos, R.; Falquet, L.; Egger, H.A.; Auber, L.A. Revealing NOTCH-dependencies in synaptic targets associated with Alzheimer’s disease. Mol. Cell. Neurosci. 2021, 115, 103657. [Google Scholar]
- Yang, X.B.; Zu, H.B.; Zhao, Y.F.; Yao, K. Agomelatine Prevents Amyloid Plaque Deposition, Tau Phosphorylation, and Neuroinflammation in APP/PS1 Mice. Front. Aging Neurosci. 2021, 13, 766410. [Google Scholar]
- Ngo, C.; Kothary, R. MicroRNAs in oligodendrocyte development and remyelination. J. Neurochem. 2022, 162, 310–321. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.K.; Shao, W.Z.; Liu, Y.Q.; Tang, C.; Deng, S.S.; Tang, C.F.; Zheng, L.; Guo, W. miR-34a/TAN1/CREB Axis Engages in Alleviating Oligodendrocyte Trophic Factor-Induced Myelin Repair Function and Astrocyte-Dependent Neuroinflammation in the Early Stages of Alzheimer’s Disease: The Anti-Neurodegenerative Effect of Treadmill Exercise. Neurochem. Res. 2024, 49, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tan, L.; Wang, X. Circular HDAC9/microRNA-138/Sirtuin-1 Pathway Mediates Synaptic and Amyloid Precursor Protein Processing Deficits in Alzheimer’s Disease. Neurosci. Bull. 2019, 35, 877–888. [Google Scholar] [PubMed]
- Boscher, E.; Husson, T.; Quenez, O.; Laquerrière, A.; Marguet, F.; Cassinari, K.; Wallon, D.; Martinaud, O.; Charbonnier, C.; Nicolas, G.; et al. Copy Number Variants in miR-138 as a Potential Risk Factor for Early-Onset Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 68, 1243–1255. [Google Scholar]
- Imamura, O.; Arai, M.; Dateki, M.; Oishi, K.; Takishima, K. Donepezil-induced oligodendrocyte differentiation is mediated through estrogen receptors. J. Neurochem. 2020, 155, 494–507. [Google Scholar] [CrossRef]
- Feng, T.; Yamashita, T.; Sasaki, R.; Tadokoro, K.; Matsumoto, N.; Hishikawa, N.; Abe, K. Protective effects of edaravone on white matter pathology in a novel mouse model of Alzheimer’s disease with chronic cerebral hypoperfusion. J. Cereb. Blood Flow. Metab. 2021, 41, 1437–1448. [Google Scholar]
- Chen, S.; Wang, T.; Yao, J.; Brinton, R.D. Allopregnanolone Promotes Neuronal and Oligodendrocyte Differentiation In Vitro and In Vivo: Therapeutic Implication for Alzheimer’s Disease. Neurotherapeutics 2020, 17, 1813–1824. [Google Scholar]
- Alanko, V.; Gaminde-Blasco, A.; Quintela-López, T.; Loera-Valencia, R.; Solomon, A.; Björkhem, I.; Cedazo-Minguez, A.; Maioli, S.; Tabacaru, G.; Latorre-Leal, M.; et al. 27-hydroxycholesterol promotes oligodendrocyte maturation: Implications for hypercholesterolemia-associated brain white matter changes. Glia 2023, 71, 1414–1428. [Google Scholar]
- Zang, C.; Liu, H.; Ju, C.; Yuan, F.; Ning, J.; Shang, M.; Bao, X.; Yu, Y.; Yao, X.; Zhang, D. Gardenia jasminoides J. Ellis extract alleviated white matter damage through promoting the differentiation of oligodendrocyte precursor cells via suppressing neuroinflammation. Food Funct. 2022, 13, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.W.; Akay, L.A.; Davila-Velderrain, J.; von Maydell, D.; Mathys, H.; Davidson, S.M.; Effenberger, A.; Chen, C.Y.; Maner-Smith, K.; Hajjar, I.; et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 2022, 611, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, W.M.; Andhey, P.S.; Swain, A.; Levy, T.; Miller, K.R.; Poliani, P.L.; Cominelli, M.; Grover, S.; Gilfillan, S.; et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 2020, 26, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Antone, J.; Alsop, E.; Reiman, R.; Funk, C.; Bendl, J.; Dudley, J.T.; Liang, W.S.; Karr, T.L.; Roussos, P.; et al. Single cell transcriptomes and multiscale networks from persons with and without Alzheimer’s disease. Nat. Commun. 2024, 15, 5815. [Google Scholar] [CrossRef]
- Garton, T.; Gadani, S.P.; Gill, A.J.; Calabresi, P.A. Neurodegeneration and demyelination in multiple sclerosis. Neuron 2024, 112, 3231–3251. [Google Scholar] [CrossRef]
- Ndayisaba, A.; Halliday, G.M.; Khurana, V. Multiple System Atrophy: Pathology, Pathogenesis, and Path Forward. Annu. Rev. Pathol. 2025, 20, 245–273. [Google Scholar] [CrossRef]
- Bryois, J.; Skene, N.G.; Hansen, T.F.; Kogelman, L.J.A.; Watson, H.J.; Liu, Z.; Brueggeman, L.; Breen, G.; Bulik, C.M.; Arenas, E.; et al. Genetic identification of cell types underlying brain complex traits yields insights into the etiology of Parkinson’s disease. Nat. Genet. 2020, 52, 482–493. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Yu, X.; Zhang, H. The Role of Oligodendrocytes in Alzheimer’s Disease Pathogenesis and Therapy. Neuroglia 2025, 6, 46. https://doi.org/10.3390/neuroglia6040046
Guo S, Yu X, Zhang H. The Role of Oligodendrocytes in Alzheimer’s Disease Pathogenesis and Therapy. Neuroglia. 2025; 6(4):46. https://doi.org/10.3390/neuroglia6040046
Chicago/Turabian StyleGuo, Shihui, Xinyi Yu, and Hongsheng Zhang. 2025. "The Role of Oligodendrocytes in Alzheimer’s Disease Pathogenesis and Therapy" Neuroglia 6, no. 4: 46. https://doi.org/10.3390/neuroglia6040046
APA StyleGuo, S., Yu, X., & Zhang, H. (2025). The Role of Oligodendrocytes in Alzheimer’s Disease Pathogenesis and Therapy. Neuroglia, 6(4), 46. https://doi.org/10.3390/neuroglia6040046





