Glia Between Resistance and Radiotoxicity in Glioblastoma: Mechanisms and Translational Perspectives—A Narrative Review
Abstract
1. Introduction and Rationale
- (1)
- To synthesize current knowledge on microglia, astrocytes, and the oligodendrocyte lineage in GBM resistance;
- (2)
- To integrate evidence on glia-mediated radiotoxicity;
- (3)
- To outline translational opportunities—including drug targets and trial designs—that jointly pursue tumor control and cognitive preservation.
2. Microglia in Glioblastoma
2.1. Ontogeny, Compartments, and Cellular Burden
2.2. Beyond M1/M2: A Continuum of Functional States
2.3. Crosstalk with Tumor Cells and GSCs: Cytokines, Pathways, and EVs
2.4. Recruitment and Immune Modulation: CCR2/CCL2 and CX3CR1 Axes
2.5. Radio- and Chemoresistance: RT-Driven Remodeling and Therapeutic Rationale
2.6. Therapeutic Implications: Reprogramming over Depletion, Delivery, and Imaging
3. Astrocytes and Astrocytic Reactivity
3.1. Reactive States: Beyond A1/A2
3.2. Paracrine Signaling and Feed-Forward Loops (IL-6/STAT3, TGF-β)
3.3. Gap Junctions and Connexin-43 (Cx43): Invasion and Temozolomide Resistance
3.4. BBB/BTB and the Perivascular Niche
3.5. EVs and Astrocyte Reprogramming
3.6. Therapeutic Implications
4. Oligodendrocyte Lineage and Tumor Progression
4.1. OPC-like Tumor Cell States in GBM
4.2. OPC Programs, Invasion, and Neuronal Mechanisms
4.3. White Matter as a Context-Dependent Niche
4.4. Developmental Transcription Factors of the Oligodendroglial Lineage in GBM
4.5. Intersections with Myelin Biology
4.6. Translational Implications
5. Shared Mechanisms of Resistance and Radiotoxicity
5.1. Inflammation, Cytokine Networks, and EVs
5.2. Metabolic Rewiring and Hypoxia
5.3. DNA Damage Response and Therapy-Induced Senescence
- EVs can be leveraged as biomarkers and as delivery vehicles for radiosensitizers or neuroprotectants across the BBB [65].
- Early clinical progress with brain-penetrant DDR inhibitors (e.g., AZD1390) and preclinical evidence for senescence-targeting interventions motivate trials integrating myeloid/astrocyte reprogramming, DDR modulation, and SASP control, with neurocognitive endpoints and glia-inflammation imaging to monitor on-target effects [74,76].
6. Glia and Radiotoxicity in Healthy Brain Tissue
6.1. Mechanistic Overview and Clinical Relevance
6.2. Microglia, Complement Signaling, and Synaptic Dysfunction
6.3. Astrocytes, BBB/BTB Disruption, and Network Effects
6.4. Hippocampal Neurogenesis and Memory
6.5. Oligodendrocytes, OPCs, and White-Matter Injury
6.6. Time Course: From Acute Inflammation to Delayed Cognitive Decline
6.7. Evidence-Based Mitigation Strategies
- Hippocampal-avoidance whole-brain radiotherapy (HA-WBRT) + memantine. In the phase III NRG-CC001 trial, HA-WBRT with memantine better preserved cognitive function and patient-reported outcomes without compromising intracranial control or survival, confirming earlier phase II findings (RTOG 0933) [87,88].
7. Translational Opportunities
7.1. Targetable Glial Axes for Combination with Radiotherapy
7.2. Precision Delivery Across BBB/BTB and Glial Compartment
7.3. Biomarkers for Selection, Response Monitoring, and Safety
8. Future Directions and Clinical Implications
8.1. Co-Designing Tumor Control and Neuroprotection
8.2. What to Combine with RT (and When)
8.3. Getting Drugs Where They Matter: Delivery Across BBB/BTB
8.4. Safety Guardrails for Glia-Targeted Intensification
8.5. Practical Clinical Implications
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sipos, D.; Raposa, B.L.; Freihat, O.; Simon, M.; Mekis, N.; Cornacchione, P.; Kovács, Á. Glioblastoma: Clinical Presentation, Multidisciplinary Management, and Long-Term Outcomes. Cancers 2025, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Kazda, T.; Dziacky, A.; Burkon, P.; Pospisil, P.; Slavik, M.; Rehak, Z.; Jancalek, R.; Slampa, P.; Slaby, O.; Lakomy, R. Radiotherapy of Glioblastoma 15 Years after the Landmark Stupp’s Trial: More Controversies than Standards? Radiol. Oncol. 2018, 52, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Salgado, M.A.; Villamayor, M.; Albarrán, V.; Alía, V.; Sotoca, P.; Chamorro, J.; Rosero, D.; Barrill, A.M.; Martín, M.; Fernandez, E.; et al. Recurrent Glioblastoma: A Review of the Treatment Options. Cancers 2023, 15, 4279. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef]
- Khan, F.; Pang, L.; Dunterman, M.; Lesniak, M.S.; Heimberger, A.B.; Chen, P. Macrophages and microglia in glioblastoma: Heterogeneity, plasticity, and therapy. J. Clin. Investig. 2023, 133, e163446. [Google Scholar] [CrossRef]
- Tang, F.; Wang, Y.; Zeng, Y.; Xiao, A.; Tong, A.; Xu, J. Tumor-associated macrophage-related strategies for glioma immunotherapy. npj Precis. Oncol. 2023, 7, 78. [Google Scholar] [CrossRef]
- Xiong, J.; Zhou, X.; Su, L.; Jiang, L.; Ming, Z.; Pang, C.; Fuller, C.; Xu, K.; Chi, H.; Zheng, X. The two-sided battlefield of tumour-associated macrophages in glioblastoma: Unravelling their therapeutic potential. Discov. Oncol. 2024, 15, 590. [Google Scholar] [CrossRef]
- Virtuoso, A.; D’Amico, G.; Scalia, F.; de Luca, C.; Papa, M.; Maugeri, G.; D’Agata, V.; Bavisotto, C.C.; D’Amico, A.G. The Interplay between Glioblastoma Cells and Tumor Microenvironment: New Perspectives for Early Diagnosis and Targeted Cancer Therapy. Brain Sci. 2024, 14, 331. [Google Scholar] [CrossRef]
- Wu, J.; Li, R.; Wang, J.; Zhu, H.; Ma, Y.; You, C.; Shu, K. Reactive Astrocytes in Glioma: Emerging Opportunities and Challenges. Int. J. Mol. Sci. 2025, 26, 2907. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, B.Z.; Ellenbogen, Y.; Kant, J.B.Y.; Yu, P.; Li, X.; Caloren, L.; Sotov, V.; Tran, C.; Restrepo, M.; et al. Neurodevelopmental hijacking of oligodendrocyte lineage programs drives glioblastoma infiltration. Dev. Cell 2025, 60, 2420–2433.e12. [Google Scholar] [CrossRef]
- Shamsesfandabadi, P.; Patel, A.; Liang, Y.; Shepard, M.J.; Wegner, R.E. Radiation-Induced Cognitive Decline: Challenges and Solutions. Cancer Manag. Res. 2024, 16, 1043–1052. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Liu, D.; Li, T.; Mao, Y.; Zhu, C. Microglia in radiation-induced brain injury: Cellular and molecular mechanisms and therapeutic potential. CNS Neurosci. Ther. 2024, 30, e14794. [Google Scholar] [CrossRef]
- Voshart, D.C.; Oshima, T.; Jiang, Y.; van der Linden, G.P.; Ainslie, A.P.; Reali Nazario, L.; van Buuren-Broek, F.; Scholma, A.C.; van Weering, H.R.J.; Brouwer, N.; et al. Radiotherapy induces persistent innate immune reprogramming of microglia into a primed state. Cell Rep. 2024, 43, 113764. [Google Scholar] [CrossRef]
- Gibson, E.M.; Monje, M. Microglia in Cancer Therapy-Related Cognitive Impairment. Trends Neurosci. 2021, 44, 441–451. [Google Scholar] [CrossRef]
- Read, R.D.; Tapp, Z.M.; Rajappa, P.; Hambardzumyan, D. Glioblastoma microenvironment-from biology to therapy. Genes Dev. 2024, 38, 360–379. [Google Scholar] [CrossRef]
- Mongeon, B.; Craig, M. Virtual Clinical Trial Reveals Significant Clinical Potential of Targeting Tumor-Associated Macrophages and Microglia to Treat Glioblastoma. CPT Pharmacomet. Syst. Pharmacol. 2025, 14, 1156–1167. [Google Scholar] [CrossRef]
- Krishna, S.; Choudhury, A.; Keough, M.B.; Seo, K.; Ni, L.; Kakaizada, S.; Lee, A.; Aabedi, A.; Popova, G.; Lipkin, B.; et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature 2023, 617, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.K.; Miletic, H.; Hossain, J.A. Tumor-Associated Macrophages in Gliomas-Basic Insights and Treatment Opportunities. Cancers 2022, 14, 1319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Zhang, G.; Wei, S. The diversity and dynamics of tumor-associated macrophages in recurrent glioblastoma. Front. Immunol. 2023, 14, 1238233. [Google Scholar] [CrossRef] [PubMed]
- Ochocka, N.; Segit, P.; Walentynowicz, K.A.; Wojnicki, K.; Cyranowski, S.; Swatler, J.; Mieczkowski, J.; Kaminska, B. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat. Commun. 2021, 12, 1151. [Google Scholar] [CrossRef]
- Buonfiglioli, A.; Hambardzumyan, D. Macrophages and microglia: The cerberus of glioblastoma. Acta Neuropathol. Commun. 2021, 9, 54. [Google Scholar] [CrossRef]
- Russo, M.N.; Whaley, L.A.; Norton, E.S.; Zarco, N.; Guerrero-Cázares, H. Extracellular vesicles in the glioblastoma microenvironment: A diagnostic and therapeutic perspective. Mol. Asp. Med. 2023, 91, 101167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jin, G.; Zhang, J.; Liu, F. Recruitment mechanisms and therapeutic implications of tumor-associated macrophages in the glioma microenvironment. Front. Immunol. 2023, 14, 1067641. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.; Miska, J.; Wainwright, D.A.; Dey, M.; Rivetta, C.V.; Yu, D.; Kanojia, D.; Pituch, K.C.; Qiao, J.; Pytel, P.; et al. CCL2 Produced by the Glioma Microenvironment Is Essential for the Recruitment of Regulatory T Cells and Myeloid-Derived Suppressor Cells. Cancer Res. 2016, 76, 5671–5682. [Google Scholar] [CrossRef]
- Nusraty, S.; Boddeti, U.; Zaghloul, K.A.; Brown, D.A. Microglia in Glioblastomas: Molecular Insight and Immunotherapeutic Potential. Cancers 2024, 16, 1972. [Google Scholar] [CrossRef] [PubMed]
- Akkari, L.; Bowman, R.L.; Tessier, J.; Klemm, F.; Handgraaf, S.M.; de Groot, M.; Quail, D.F.; Tillard, L.; Gadiot, J.; Huse, J.T.; et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci. Transl. Med. 2020, 12, eaaw7843. [Google Scholar] [CrossRef]
- Mendez, J.S.; Cohen, A.L.; Eckenstein, M.; Jensen, R.L.; Burt, L.M.; Salzman, K.L.; Chamberlain, M.; Hsu, H.H.; Hutchinson, M.; Iwamoto, F.; et al. Phase 1b/2 study of orally administered pexidartinib in combination with radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. Neurooncol. Adv. 2024, 6, vdae202. [Google Scholar] [CrossRef]
- Watson, S.S.; Zomer, A.; Fournier, N.; Lourenco, J.; Quadroni, M.; Chryplewicz, A.; Nassiri, S.; Aubel, P.; Avanthay, S.; Croci, D.; et al. Fibrotic response to anti-CSF-1R therapy potentiates glioblastoma recurrence. Cancer Cell 2024, 42, 1507–1527.e11. [Google Scholar] [CrossRef]
- Liaw, K.; Reddy, R.; Sharma, A.; Li, J.; Chang, M.; Sharma, R.; Salazar, S.; Kannan, S.; Kannan, R.M. Targeted systemic dendrimer delivery of CSF-1R inhibitor to tumor-associated macrophages improves outcomes in orthotopic glioblastoma. Bioeng. Transl. Med. 2020, 6, e10205. [Google Scholar] [CrossRef]
- Weidner, L.; Lorenz, J.; Quach, S.; Braun, F.K.; Rothhammer-Hampl, T.; Ammer, L.M.; Vollmann-Zwerenz, A.; Bartos, L.M.; Dekorsy, F.J.; Holzgreve, A.; et al. Translocator protein (18kDA) (TSPO) marks mesenchymal glioblastoma cell populations characterized by elevated numbers of tumor-associated macrophages. Acta Neuropathol. Commun. 2023, 11, 147. [Google Scholar] [CrossRef]
- Albert, N.L.; Nelwan, D.V.; Fleischmann, D.F.; Quach, S.; von Rohr, K.; Kaiser, L.; Teske, N.; Unterrainer, L.M.; Bartos, L.M.; Ruf, V.C.; et al. Prognostic Value of TSPO PET Before Radiotherapy in Newly Diagnosed IDH-Wild-Type Glioblastoma. J. Nucl. Med. 2023, 64, 1519–1525. [Google Scholar] [CrossRef]
- Cumbers, G.A.; Harvey-Latham, E.D.; Kassiou, M.; Werry, E.L.; Danon, J.J. Emerging TSPO-PET Radiotracers for Imaging Neuroinflammation: A Critical Analysis. Semin. Nucl. Med. 2024, 54, 856–874. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Hou, X.; Dong, L.; Hou, W. Roles of STAT3 in the pathogenesis and treatment of glioblastoma. Front. Cell Dev. Biol. 2023, 11, 1098482. [Google Scholar] [CrossRef]
- McCutcheon, S.; Spray, D.C. Glioblastoma-Astrocyte Connexin 43 Gap Junctions Promote Tumor Invasion. Mol. Cancer Res. 2022, 20, 319–331. [Google Scholar] [CrossRef]
- Gui, Y.; Qin, H.; Zhang, X.; Chen, Q.; Ye, F.; Tian, G.; Yang, S.; Ye, Y.; Pan, D.; Zhou, J.; et al. Glioma-astrocyte connexin43 confers temozolomide resistance through activation of the E2F1/ERCC1 axis. Neuro-Oncology 2025, 27, 711–726. [Google Scholar] [CrossRef]
- Murphy, S.F.; Varghese, R.T.; Lamouille, S.; Guo, S.; Pridham, K.J.; Kanabur, P.; Osimani, A.M.; Sharma, S.; Jourdan, J.; Rodgers, C.M.; et al. Connexin 43 Inhibition Sensitizes Chemoresistant Glioblastoma Cells to Temozolomide. Cancer Res. 2016, 76, 139–149. [Google Scholar] [CrossRef]
- Pridham, K.J.; Shah, F.; Hutchings, K.R.; Sheng, K.L.; Guo, S.; Liu, M.; Kanabur, P.; Lamouille, S.; Lewis, G.; Morales, M.; et al. Connexin 43 confers chemoresistance through activating PI3K. Oncogenesis 2022, 11, 2. [Google Scholar] [CrossRef]
- Grek, C.L.; Sheng, Z.; Naus, C.C.; Sin, W.C.; Gourdie, R.G.; Ghatnekar, G.G. Novel approach to temozolomide resistance in malignant glioma: Connexin43-directed therapeutics. Curr. Opin. Pharmacol. 2018, 41, 79–88. [Google Scholar] [CrossRef]
- Schmidt, E.N.C.; Evert, B.O.; Pregler, B.E.F.; Melhem, A.; Hsieh, M.C.; Raspe, M.; Strobel, H.; Roos, J.; Pietsch, T.; Schuss, P.; et al. Tonabersat enhances temozolomide-mediated cytotoxicity in glioblastoma by disrupting intercellular connectivity through connexin 43 inhibition. Mol. Oncol. 2025, 19, 878–898. [Google Scholar] [CrossRef] [PubMed]
- Mokarram, N.; Case, A.; Hossainy, N.N.; Lyon, J.G.; MacDonald, T.J.; Bellamkonda, R. Device-assisted strategies for drug delivery across the blood-brain barrier to treat glioblastoma. Commun. Mater. 2025, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Kitchen, P.; Halsey, A.; Wang, M.X.; Törnroth-Horsefield, S.; Conner, A.C.; Badaut, J.; Iliff, J.J.; Bill, R.M. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 2022, 145, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.M.; McFarland White, K.; Fass, S.B.; Chen, S.; Shi, Z.; Ge, X.; Engelbach, J.A.; Gaines, S.H.; Bice, A.R.; Vasek, M.J.; et al. Evaluation of gliovascular functions of AQP4 readthrough isoforms. Front. Cell Neurosci. 2023, 17, 1272391. [Google Scholar] [CrossRef]
- Lan, Y.L.; Zou, S.; Chen, R. Update on the intriguing roles of AQP4 expression and redistribution in the progression and treatment of glioma. Ann. Med. 2024, 56, 2401111. [Google Scholar] [CrossRef]
- Ngo, M.T.; Sarkaria, J.N.; Harley, B.A.C. Perivascular Stromal Cells Instruct Glioblastoma Invasion, Proliferation, and Therapeutic Response within an Engineered Brain Perivascular Niche Model. Adv. Sci. 2022, 9, e2201888. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, Y.; Hu, H.; Zhang, S.; Chen, Y. Extracellular vesicles as modulators of glioblastoma progression and tumor microenvironment. Pathol. Oncol. Res. 2024, 30, 1611549. [Google Scholar] [CrossRef]
- Kuang, L.; Wu, L.; Li, Y. Extracellular vesicles in tumor immunity: Mechanisms and novel insights. Mol. Cancer 2025, 24, 45. [Google Scholar] [CrossRef]
- Cela, I.; Capone, E.; Trevisi, G.; Sala, G. Extracellular vesicles in glioblastoma: Biomarkers and therapeutic tools. Semin. Cancer Biol. 2024, 101, 25–43. [Google Scholar] [CrossRef]
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849.e21. [Google Scholar] [CrossRef]
- Eberhart, C.G.; Bar, E.E. Spatial enrichment of cellular states in glioblastoma. Acta Neuropathol. 2020, 140, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Drexler, R.; Khatri, R.; Sauvigny, T.; Mohme, M.; Maire, C.L.; Ryba, A.; Zghaibeh, Y.; Dührsen, L.; Salviano-Silva, A.; Lamszus, K.; et al. A prognostic neural epigenetic signature in high-grade glioma. Nat. Med. 2024, 30, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Doroszko, M.; Stockgard, R.; Uppman, I.; Heinold, J.; Voukelatou, F.; Mangukiya, H.B.; Millner, T.O.; Skeppås, M.; Ballester Bravo, M.; Elgendy, R.; et al. The invasion phenotypes of glioblastoma depend on plastic and reprogrammable cell states. Nat. Commun. 2025, 16, 6662. [Google Scholar] [CrossRef]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 2022, 185, 2899–2917.e31. [Google Scholar] [CrossRef]
- Taylor, K.R.; Monje, M. Invasive glioma cells: The malignant pioneers that follow the current. Cell 2022, 185, 2846–2848. [Google Scholar] [CrossRef]
- Brooks, L.J.; Clements, M.P.; Burden, J.J.; Kocher, D.; Richards, L.; Devesa, S.C.; Zakka, L.; Woodberry, M.; Ellis, M.; Jaunmuktane, Z.; et al. The white matter is a pro-differentiative niche for glioblastoma. Nat. Commun. 2021, 12, 2184. [Google Scholar] [CrossRef]
- Myers, B.L.; Brayer, K.J.; Paez-Beltran, L.E.; Villicana, E.; Keith, M.S.; Suzuki, H.; Newville, J.; Anderson, R.H.; Lo, Y.; Mertz, C.M.; et al. Transcription factors ASCL1 and OLIG2 drive glioblastoma initiation and co-regulate tumor cell types and migration. Nat. Commun. 2024, 15, 10363. [Google Scholar] [CrossRef]
- Taylor, K.R.; Monje, M. Neuron-oligodendroglial interactions in health and malignant disease. Nat. Rev. Neurosci. 2023, 24, 733–746. [Google Scholar] [CrossRef]
- Duffau, H. White Matter Tracts and Diffuse Lower-Grade Gliomas: The Pivotal Role of Myelin Plasticity in the Tumor Pathogenesis, Infiltration Patterns, Functional Consequences and Therapeutic Management. Front. Oncol. 2022, 12, 855587. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neurooncol. Adv. 2020, 2, vdaa057. [Google Scholar] [CrossRef] [PubMed]
- Markarian, M.; Krattli RPJr Baddour, J.D.; Alikhani, L.; Giedzinski, E.; Usmani, M.T.; Agrawal, A.; Baulch, J.E.; Tenner, A.J.; Acharya, M.M. Glia-Selective Deletion of Complement C1q Prevents Radiation-Induced Cognitive Deficits and Neuroinflammation. Cancer Res. 2021, 81, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arboledas, A.; Acharya, M.M.; Tenner, A.J. The Role of Complement in Synaptic Pruning and Neurodegeneration. Immunotargets Ther. 2021, 10, 373–386. [Google Scholar] [CrossRef]
- Indira Chandran, V.; Gopala, S.; Venkat, E.H.; Kjolby, M.; Nejsum, P. Extracellular vesicles in glioblastoma: A challenge and an opportunity. npj Precis. Oncol. 2024, 8, 103. [Google Scholar] [CrossRef]
- Singh, S.; Dey, D.; Barik, D.; Mohapatra, I.; Kim, S.; Sharma, M.; Prasad, S.; Wang, P.; Singh, A.; Singh, G. Glioblastoma at the crossroads: Current understanding and future therapeutic horizons. Signal Transduct. Target Ther. 2025, 10, 213. [Google Scholar] [CrossRef]
- Chédeville, A.L.; Madureira, P.A. The Role of Hypoxia in Glioblastoma Radiotherapy Resistance. Cancers 2021, 13, 542. [Google Scholar] [CrossRef]
- Ali, M.Y.; Oliva, C.R.; Noman, A.S.M.; Allen, B.G.; Goswami, P.C.; Zakharia, Y.; Monga, V.; Spitz, D.R.; Buatti, J.M.; Griguer, C.E. Radioresistance in Glioblastoma and the Development of Radiosensitizers. Cancers 2020, 12, 2511. [Google Scholar] [CrossRef]
- de Ruiter Swain, J.; Michalopoulou, E.; Noch, E.K.; Lukey, M.J.; Van Aelst, L. Metabolic partitioning in the brain and its hijacking by glioblastoma. Genes Dev. 2023, 37, 681–702. [Google Scholar] [CrossRef]
- Wang, S.; Huang, T.; Wu, Q.; Yuan, H.; Wu, X.; Yuan, F.; Duan, T.; Taori, S.; Zhao, Y.; Snyder, N.W.; et al. Lactate reprograms glioblastoma immunity through CBX3-regulated histone lactylation. J. Clin. Investig. 2024, 134, e176851. [Google Scholar] [CrossRef]
- Strohm, A.O.; Johnston, C.; Hernady, E.; Marples, B.; O’Banion, M.K.; Majewska, A.K. Cranial irradiation disrupts homeostatic microglial dynamic behavior. J. Neuroinflamm. 2024, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jin, X.; Xue, H.; Zhang, J.; Zeng, L.; Zhou, Y.; Pan, Y.; Zhang, J.; Shao, C. TMEM164 enhances radioresistance of GBM cells by inhibiting the FASN-NADPH-ROS axis. J. Neuro-Oncol. 2025, 175, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Kelly, W.; Diaz Duque, A.E.; Michalek, J.; Konkel, B.; Caflisch, L.; Chen, Y.; Pathuri, S.C.; Madhusudanannair-Kunnuparampil, V.; Floyd, J.; Brenner, A. Phase II Investigation of TVB-2640 (Denifanstat) with Bevacizumab in Patients with First Relapse High-Grade Astrocytoma. Clin. Cancer Res. 2023, 29, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Colclough, N.; Orton, A.L.; Martin, S.; Wild, M.; Reddy, V.P.; Lenz, E.; Markandu, R.; Wilson, J.; Fan, C.; Potter, J.; et al. Achieving human brain exposure with the oral ataxia-telangiectasia mutated kinase inhibitor AZD1390, a substrate of aldehyde oxidase. Drug Metab. Dispos. 2025, 53, 100107. [Google Scholar] [CrossRef]
- Dragojevic, S.; Smith, E.J.; Regan, M.S.; Stopka, S.A.; Baquer, G.; Xue, Z.; Zhang, W.; Connors, M.A.; Kloeber, J.A.; Hu, Z.; et al. DNA-PK Inhibition Shows Differential Radiosensitization in Orthotopic GBM PDX Models Based on DDR Pathway Deficits. Mol. Cancer Ther. 2025, 24, 859–869. [Google Scholar] [CrossRef]
- Fletcher-Sananikone, E.; Kanji, S.; Tomimatsu, N.; Di Cristofaro, L.F.M.; Kollipara, R.K.; Saha, D.; Floyd, J.R.; Sung, P.; Hromas, R.; Burns, T.C.; et al. Elimination of Radiation-Induced Senescence in the Brain Tumor Microenvironment Attenuates Glioblastoma Recurrence. Cancer Res. 2021, 81, 5935–5947. [Google Scholar] [CrossRef]
- Riviere-Cazaux, C.; Carlstrom, L.P.; Neth, B.J.; Olson, I.E.; Rajani, K.; Rahman, M.; Ikram, S.; Mansour, M.A.; Mukherjee, B.; Warrington, A.E.; et al. An untapped window of opportunity for glioma: Targeting therapy-induced senescence prior to recurrence. npj Precis. Oncol. 2023, 7, 126. [Google Scholar] [CrossRef]
- Ji, J.; Ding, K.; Cheng, B.; Zhang, X.; Luo, T.; Huang, B.; Yu, H.; Chen, Y.; Xu, X.; Lin, H.; et al. Radiotherapy-Induced Astrocyte Senescence Promotes an Immunosuppressive Microenvironment in Glioblastoma to Facilitate Tumor Regrowth. Adv. Sci. 2024, 11, e2304609. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, Y.; Peng, B.; Luo, N.; Zhang, Y.; Zhu, W.; Yang, F.; Chen, Z.; Zhang, Q.; Li, Q.; et al. All-trans retinoic acid inhibits glioblastoma progression and attenuates radiation-induced brain injury. JCI Insight 2024, 9, e179530. [Google Scholar] [CrossRef]
- Hinkle, J.J.; Olschowka, J.A.; Love, T.M.; Williams, J.P.; O’Banion, M.K. Cranial irradiation mediated spine loss is sex-specific and complement receptor-3 dependent in male mice. Sci. Rep. 2019, 9, 18899. [Google Scholar] [CrossRef]
- Hinkle, J.J.; Olschowka, J.A.; Williams, J.P.; O’Banion, M.K. Pharmacologic Manipulation of Complement Receptor 3 Prevents Dendritic Spine Loss and Cognitive Impairment After Acute Cranial Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 912–923. [Google Scholar] [CrossRef]
- Wang, C.; Fan, X.; Shi, Y.; Tang, F. Radiation-Induced Brain Injury with Special Reference to Astrocytes as a Therapeutic Target. J. Integr. Neurosci. 2025, 24, 25907. [Google Scholar] [CrossRef] [PubMed]
- Rola, R.; Raber, J.; Rizk, A.; Otsuka, S.; VandenBerg, S.R.; Morhardt, D.R.; Fike, J.R. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 2004, 188, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Mineyeva, O.A.; Bezriadnov, D.V.; Kedrov, A.V.; Lazutkin, A.A.; Anokhin, K.V.; Enikolopov, G.N. Radiation Induces Distinct Changes in Defined Subpopulations of Neural Stem and Progenitor Cells in the Adult Hippocampus. Front. Neurosci. 2019, 12, 1013. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, Z. Cognitive dysfunction induced by cranial radiotherapy: Mechanisms and therapeutic methods. Brain Res. Bull. 2024, 218, 111106. [Google Scholar] [CrossRef]
- Lee, R.X.; Tang, F.R. Radiation-induced neuropathological changes in the oligodendrocyte lineage with relevant clinical manifestations and therapeutic strategies. Int. J. Radiat. Biol. 2022, 98, 1519–1531. [Google Scholar] [CrossRef]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Markarian, M.; Allen, B.D.; Baddour, J.D.; Giedzinski, E.; Jorge, P.G.; Petit, B.; Bailat, C.; Vozenin, M.C.; Limoli, C.; et al. Ultra-High-Dose-Rate FLASH Irradiation Limits Reactive Gliosis in the Brain. Radiat. Res. 2020, 194, 636–645. [Google Scholar] [CrossRef]
- Simmons, D.A.; Lartey, F.M.; Schüler, E.; Rafat, M.; King, G.; Kim, A.; Ko, R.; Semaan, S.; Gonzalez, S.; Jenkins, M.; et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother. Oncol. 2019, 139, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, H.J. FLASH radiotherapy: Bridging revolutionary mechanisms and clinical frontiers in cancer treatment—A narrative review. Ewha Med. J. 2024, 47, e54. [Google Scholar] [CrossRef] [PubMed]
- Groot, J.; Ott, M.; Wei, J.; Kassab, C.; Fang, D.; Najem, H.; O’Brien, B.; Weathers, S.P.; Matsouka, C.K.; Majd, N.K.; et al. A first-in-human Phase I trial of the oral p-STAT3 inhibitor WP1066 in patients with recurrent malignant glioma. CNS Oncol. 2022, 11, CNS87. [Google Scholar] [CrossRef]
- Wick, A.; Desjardins, A.; Suarez, C.; Forsyth, P.; Gueorguieva, I.; Burkholder, T.; Cleverly, A.L.; Estrem, S.T.; Wang, S.; Lahn, M.M.; et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor-beta receptor I, in combination with standard temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma. Investig. New Drugs 2020, 38, 1570–1579. [Google Scholar] [CrossRef]
- Flores-Toro, J.A.; Luo, D.; Gopinath, A.; Sarkisian, M.R.; Campbell, J.J.; Charo, I.F.; Singh, R.; Schall, T.J.; Datta, M.; Jain, R.K.; et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc. Natl. Acad. Sci. USA 2020, 117, 1129–1138. [Google Scholar] [CrossRef]
- Chen, J.; Laverty, D.J.; Talele, S.; Bale, A.; Carlson, B.L.; Porath, K.A.; Bakken, K.K.; Burgenske, D.M.; Decker, P.A.; Vaubel, R.A.; et al. Aberrant ATM signaling and homology-directed DNA repair as a vulnerability of p53-mutant GBM to AZD1390-mediated radiosensitization. Sci. Transl. Med. 2024, 16, eadj5962. [Google Scholar] [CrossRef]
- Carpentier, A.; Stupp, R.; Sonabend, A.M.; Dufour, H.; Chinot, O.; Mathon, B.; Ducray, F.; Guyotat, J.; Baize, N.; Menei, P.; et al. Repeated blood-brain barrier opening with a nine-emitter implantable ultrasound device in combination with carboplatin in recurrent glioblastoma: A phase I/II clinical trial. Nat. Commun. 2024, 15, 1650. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Gould, A.; Amidei, C.; Ward, R.; Schmidt, K.A.; Zhang, D.Y.; Gomez, C.; Bebawy, J.F.; Liu, B.P.; Bouchoux, G.; et al. Repeated blood-brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: A phase 1 trial. Lancet Oncol. 2023, 24, 509–522. [Google Scholar] [CrossRef]
- Zhu, H.; Allwin, C.; Bassous, M.G.; Pouliopoulos, A.N. Focused ultrasound-mediated enhancement of blood-brain barrier permeability for brain tumor treatment: A systematic review of clinical trials. J. Neurooncol. 2024, 170, 235–252. [Google Scholar] [CrossRef]
- Martinez, P.; Nault, G.; Steiner, J.; Wempe, M.F.; Pierce, A.; Brunt, B.; Slade, M.; Song, J.J.; Mongin, A.; Song, K.H.; et al. MRI-guided focused ultrasound blood-brain barrier opening increases drug delivery and efficacy in a diffuse midline glioma mouse model. Neurooncol. Adv. 2023, 5, vdad111. [Google Scholar] [CrossRef]
- Liaw, K.; Sharma, R.; Sharma, A.; Salazar, S.; Appiani La Rosa, S.; Kannan, R.M. Systemic dendrimer delivery of triptolide to tumor-associated macrophages improves anti-tumor efficacy and reduces systemic toxicity in glioblastoma. J. Control. Release 2021, 329, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, M.; Ghazi, F.; Mehrabifard, M.; Alivirdiloo, V.; Hajiabbasi, M.; Rahimi, F.; Mobed, A.; Taheripak, G.; Ramezani Farani, M.; Huh, Y.S.; et al. State-of-the-art application of nanoparticles in radiotherapy: A platform for synergistic effects in cancer treatment. Strahlenther. Onkol. 2025, 201, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Iv, M.; Naya, L.; Sanan, S.; Van Buskirk, S.L.; Nagpal, S.; Thomas, R.P.; Recht, L.D.; Patel, C.B. Tumor treating fields increases blood-brain barrier permeability and relative cerebral blood volume in patients with glioblastoma. Neuroradiol. J. 2024, 37, 107–118. [Google Scholar] [CrossRef]
- Filippi, L.; Frantellizzi, V.; Vincentis, G.; Schillaci, O.; Evangelista, L. Clinical Applications of TSPO PET for Glioma Imaging: Current Evidence and Future Perspective-A Systematic Review. Diagnostics 2023, 13, 1813. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, R.; Huang, X.; Ji, C.; Xia, W.; Qi, Y.; Yang, X.; Lin, L.; Wang, J.; Cheng, H.; et al. MRI-derived radiomics assessing tumor-infiltrating macrophages enable prediction of immune-phenotype, immunotherapy response and survival in glioma. Biomark. Res. 2024, 12, 14. [Google Scholar] [CrossRef]
- Khalili, N.; Kazerooni, A.F.; Familiar, A.; Haldar, D.; Kraya, A.; Foster, J.; Koptyra, M.; Storm, P.B.; Resnick, A.C.; Nabavizadeh, A. Radiomics for characterization of the glioma immune microenvironment. npj Precis. Oncol. 2023, 7, 59. [Google Scholar] [CrossRef]
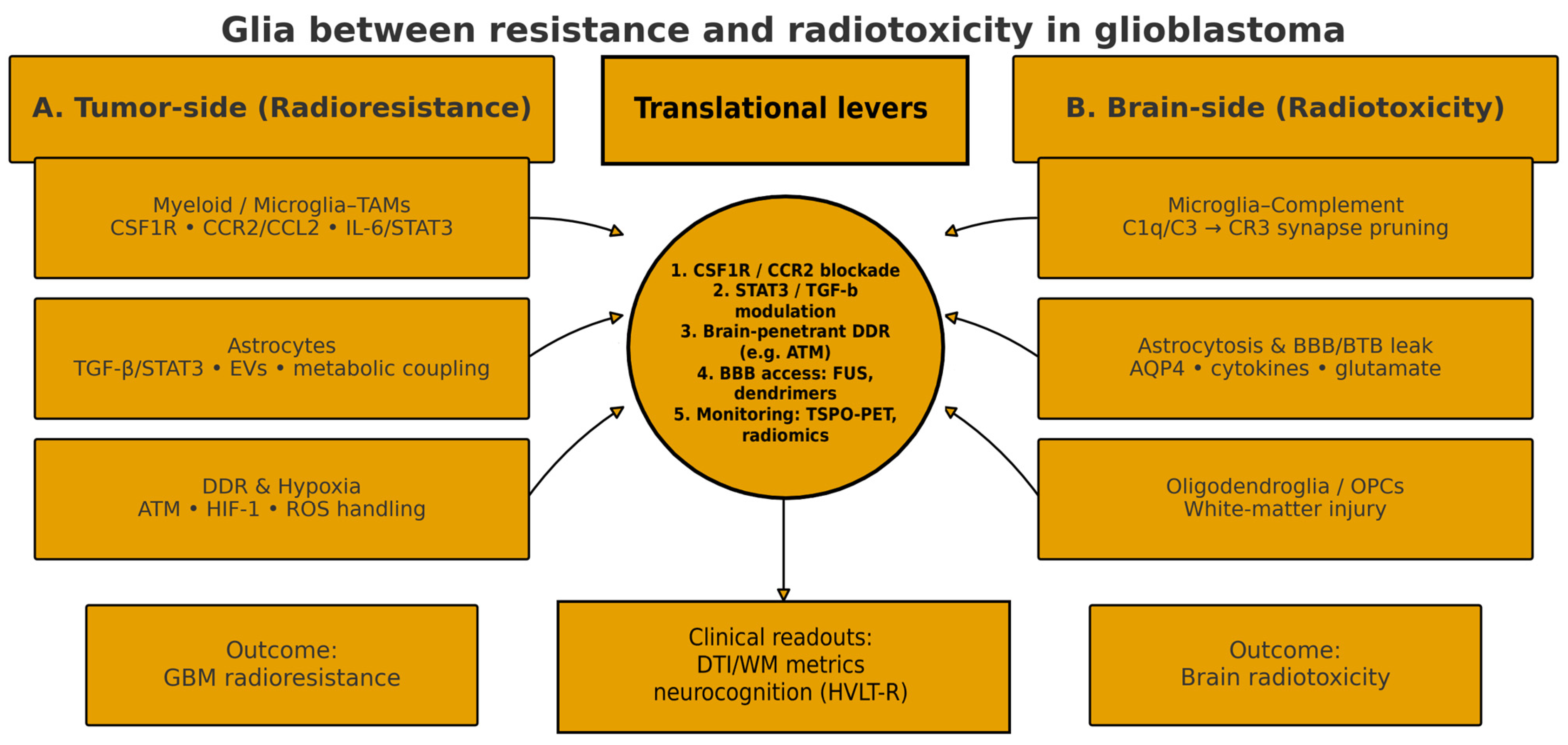
| Pathway/Target | Rationale (Glial Mechanism) | Candidate Agent(s) | Evidence Stage | RT-Integration Notes | Key Refs |
|---|---|---|---|---|---|
| CSF1R (microglia/TAM survival & phenotype) | Limits pro-tumor myeloid support; potential reduction in complement tagging | Pexidartinib (PLX3397); PLX5622 (tool) | Preclinical; early clinical signals | Post-RT CSF1R blockade can overcome resistance; combinations with RT/ICB under study | [5,18,21,23,26,27,28,29]; complement–microglia pruning background [63,64,80,81] |
| STAT3/TGF-β (astrocyte & myeloid signaling) | Dampens pro-tumor inflammation and astrocyte-mediated support | WP1066 (STAT3 inhibitor); Galunisertib (TGF-βRI inhibitor) | Phase I/II signals in GBM | Consider timing with RT/TMZ; monitor toxicity | [33,34,35,82,93,94] |
| CCR2–CCL2 (monocyte recruitment) | Blocks influx of immunosuppressive monocytes/MDSCs | CCR2 inhibitors (e.g., CCX872, PF-04136309) | Preclinical in GBM; clinical exploration | May unmask ICB efficacy; synergy with RT | [6,16,18,23,24,95] |
| ATM/DDR (brain-penetrant) | Sensitizes tumor cells to RT; intersects glia–DDR crosstalk | AZD1390 (ATM inhibitor) | Phase I with RT in GBM | Preliminary efficacy with manageable safety in early studies; alternatives in DDR (e.g., DNA-PK) when indicated | [74,96] (DDR alt [75]) |
| Complement (C1q/C3–CR3) | Mitigates microglia-mediated synapse pruning after RT | C1q/C3 blockers (e.g., ANX005; pegcetacoplan) | Preclinical evidence; clinical in other neuro-indications | Neuroprotection focus to limit radiotoxicity and cognitive decline | [11,14,62,63,64,80,81] |
| BBB access/delivery | Improves CNS exposure of glia-/tumor-targeting agents | Focused Ultrasound BBB opening; dendrimers | Early clinical feasibility in GBM | Windowed/targeted delivery; coordinate with RT sessions | Clinical FUS-BBB [97,98,99]; device-assisted BBB & carriers [42,100]; dendrimers [29,101] |
| Glial Axis | Key Pathway(s) | Lever (Example) | Best Timing vs. RT | Primary Readouts |
|---|---|---|---|---|
| Myeloid/microglia–TAMs | CSF1R; CCR2/CCL2; IL-6/STAT3 [5,6,7,18,19,21,23,24] | Pexidartinib [27]; CCR2 inhibitors [95] | Pre; Post [26,28] | TSPO-PET ↓ [30,31,32,105]; periferic myeloid signature [5,23,24] |
| Astrocytes | STAT3; TGF-β [33,34,35] | WP1066; Galunisertib [93,94] | During; Post | Cytokines (TGF-β–related); MRI edema [43,44,45,94] |
| DDR (tumor/glia) | ATM (brain-penetrant) | AZD1390 [74] | During | Safety/DLT; early RANO response [75] |
| Complement/pruning | C1q/C3→CR3 | C1q/C3 modulators; CR3 pharmacologic manipulation [63,64,80,81] | Post | DTI WM (FA/RD) stabilization; cognitive outcomes (e.g., HVLT-R) [11,87,88] |
| BBB access/delivery | FUS-BBB; dendrimers | MR-guided FUS; dendrimer carriers [42,97,98,99,100,101] | Pre; During | BBB opening success; target drug exposure [97,98,99,100] |
| Oligodendroglia/OPCs | Myelin/WM integrity | Neuroprotection ± remyelination [58,60,61,86] | Post | DTI RD/AD; WM lesion load [11,58,61,86] |
| Glutamate/excitotoxicity | Astrocytic glutamate handling; neuron–glioma synapses [17,53,54] | Memantine-like approaches [87,88] | During; Post | HVLT-R trajectory; cognitive index; edema/WM correlates [11,85,87,88] |
| Metabolic coupling | Lactate shuttle; EV cargo; lipid/LDH axes [22,47,48,49,69,70] | Metformin/LDH-axis (investigational); EV-directed strategies [22,47,48,49,66,69,70,73] | Pre; During; Post | MR-spectroscopy (if available); delta-radiomics/immune-metabolic signatures [69,100,101,106,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donnini, F.; Minniti, G.; Rubino, G.; Battaglia, G.; Pastina, P.; Carfagno, T.; Vannini, M.; Mazzei, M.A.; Tini, P. Glia Between Resistance and Radiotoxicity in Glioblastoma: Mechanisms and Translational Perspectives—A Narrative Review. Neuroglia 2025, 6, 44. https://doi.org/10.3390/neuroglia6040044
Donnini F, Minniti G, Rubino G, Battaglia G, Pastina P, Carfagno T, Vannini M, Mazzei MA, Tini P. Glia Between Resistance and Radiotoxicity in Glioblastoma: Mechanisms and Translational Perspectives—A Narrative Review. Neuroglia. 2025; 6(4):44. https://doi.org/10.3390/neuroglia6040044
Chicago/Turabian StyleDonnini, Flavio, Giuseppe Minniti, Giovanni Rubino, Giuseppe Battaglia, Pierpaolo Pastina, Tommaso Carfagno, Marta Vannini, Maria Antonietta Mazzei, and Paolo Tini. 2025. "Glia Between Resistance and Radiotoxicity in Glioblastoma: Mechanisms and Translational Perspectives—A Narrative Review" Neuroglia 6, no. 4: 44. https://doi.org/10.3390/neuroglia6040044
APA StyleDonnini, F., Minniti, G., Rubino, G., Battaglia, G., Pastina, P., Carfagno, T., Vannini, M., Mazzei, M. A., & Tini, P. (2025). Glia Between Resistance and Radiotoxicity in Glioblastoma: Mechanisms and Translational Perspectives—A Narrative Review. Neuroglia, 6(4), 44. https://doi.org/10.3390/neuroglia6040044







