Electron-Beam Processing of Aluminum-Containing Ceramics in the Forevacuum Pressure Range
Abstract
1. Introduction
2. Materials and Investigation Techniques
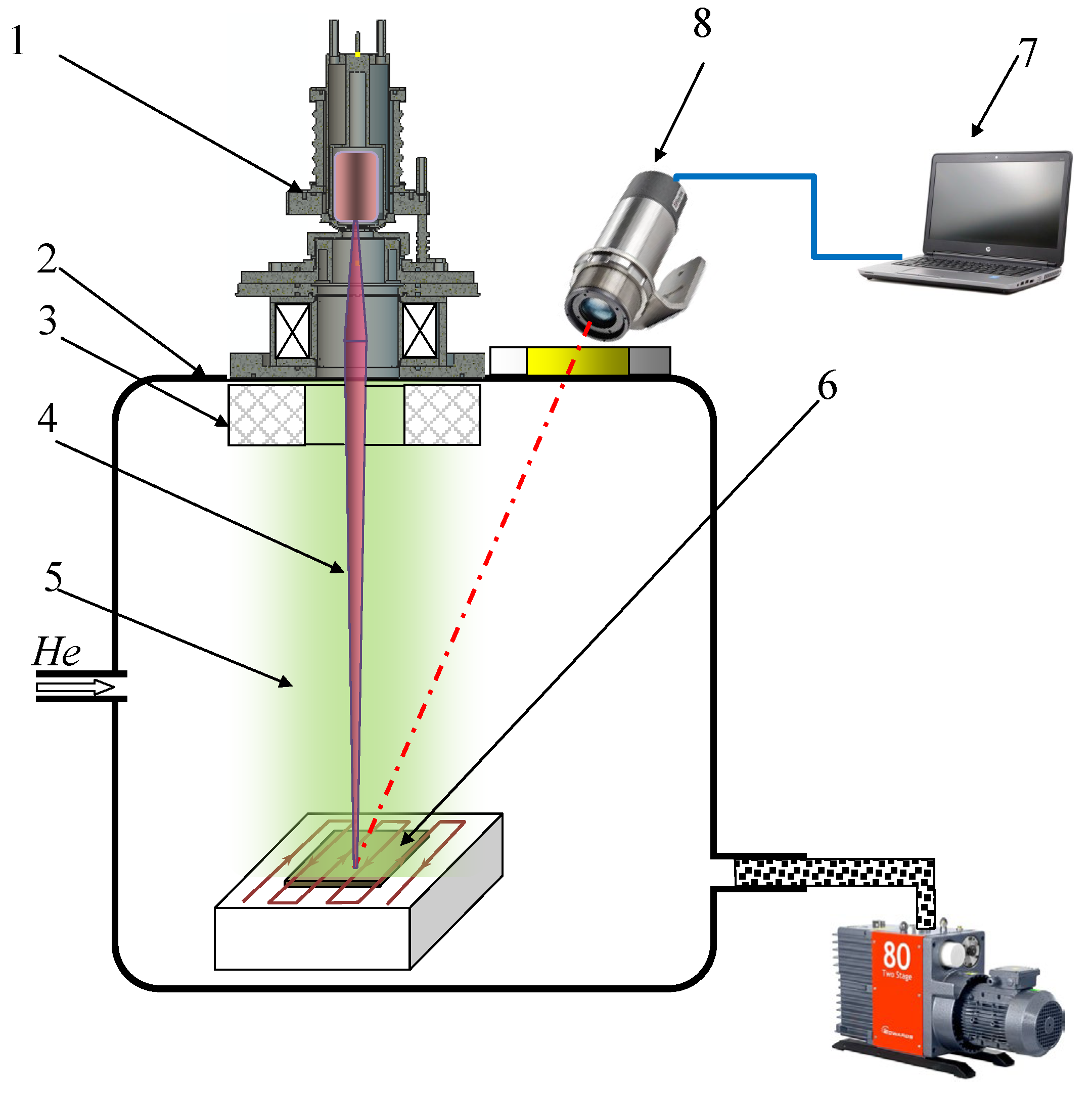
3. Experimental Setup and Methods
4. Results and Discussion
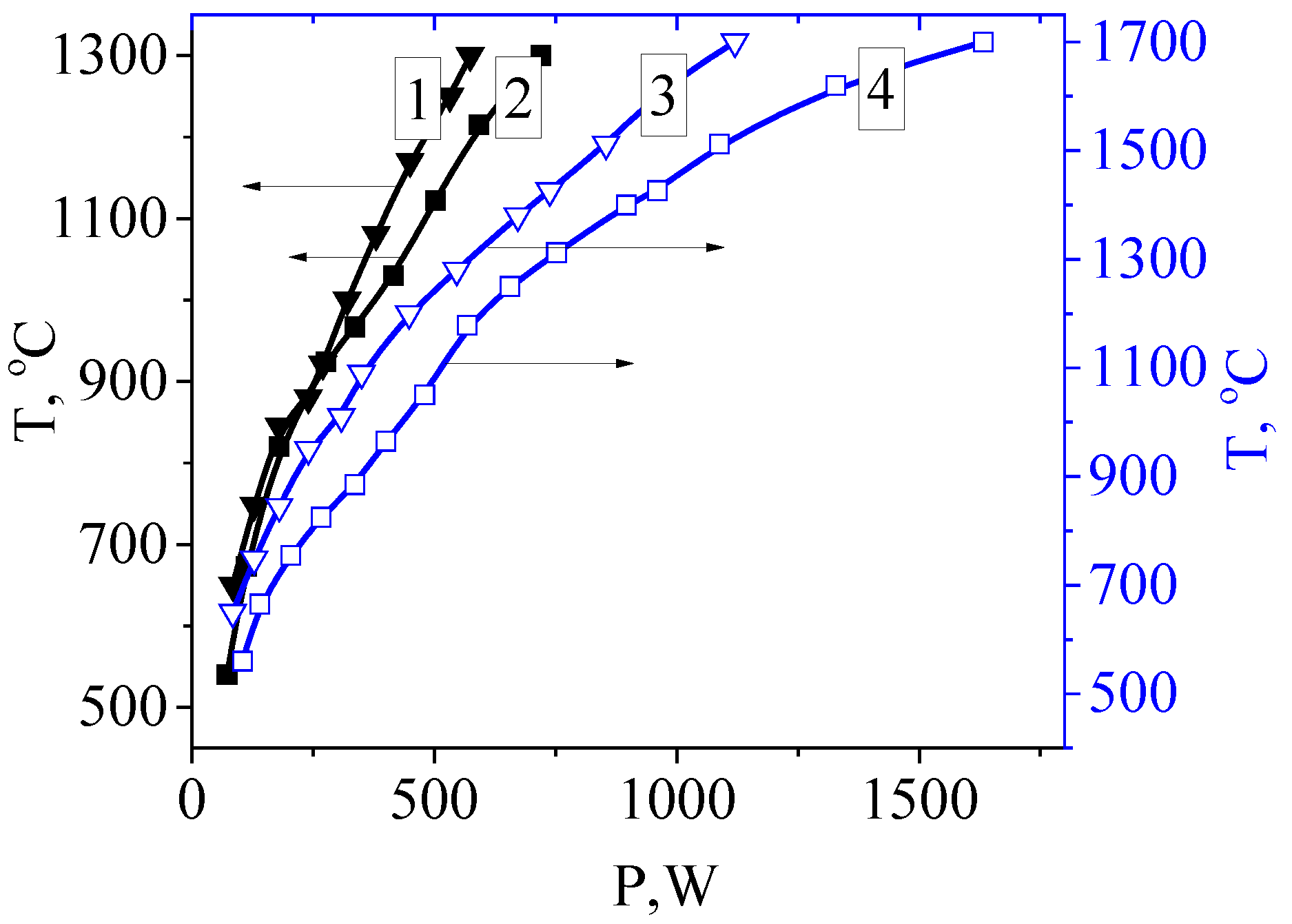
4.1. Electron-Beam Irradiation Regime
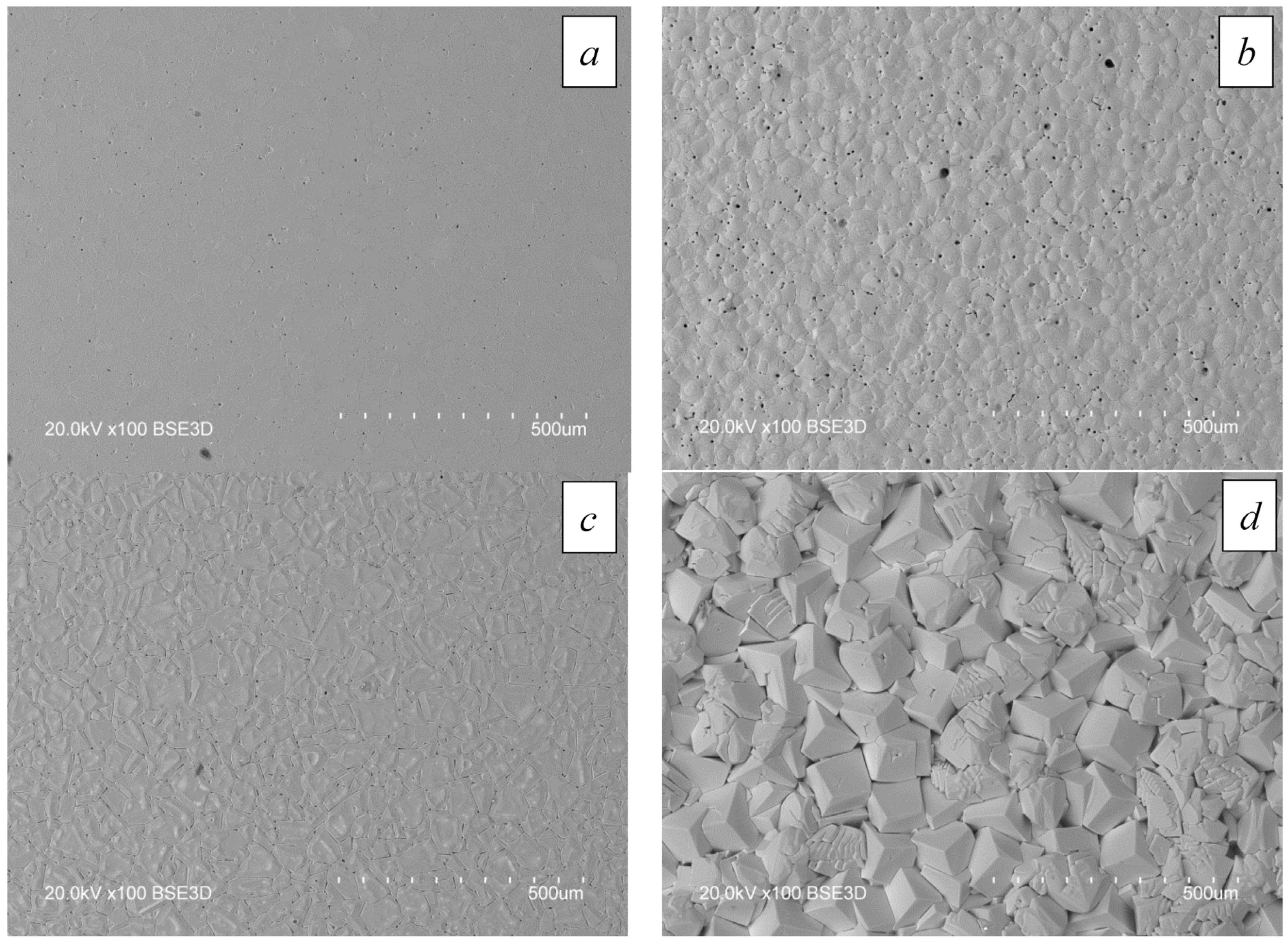
4.2. Effect of Electron-Beam Treatment on the Surface of the Al2O3 Ceramic
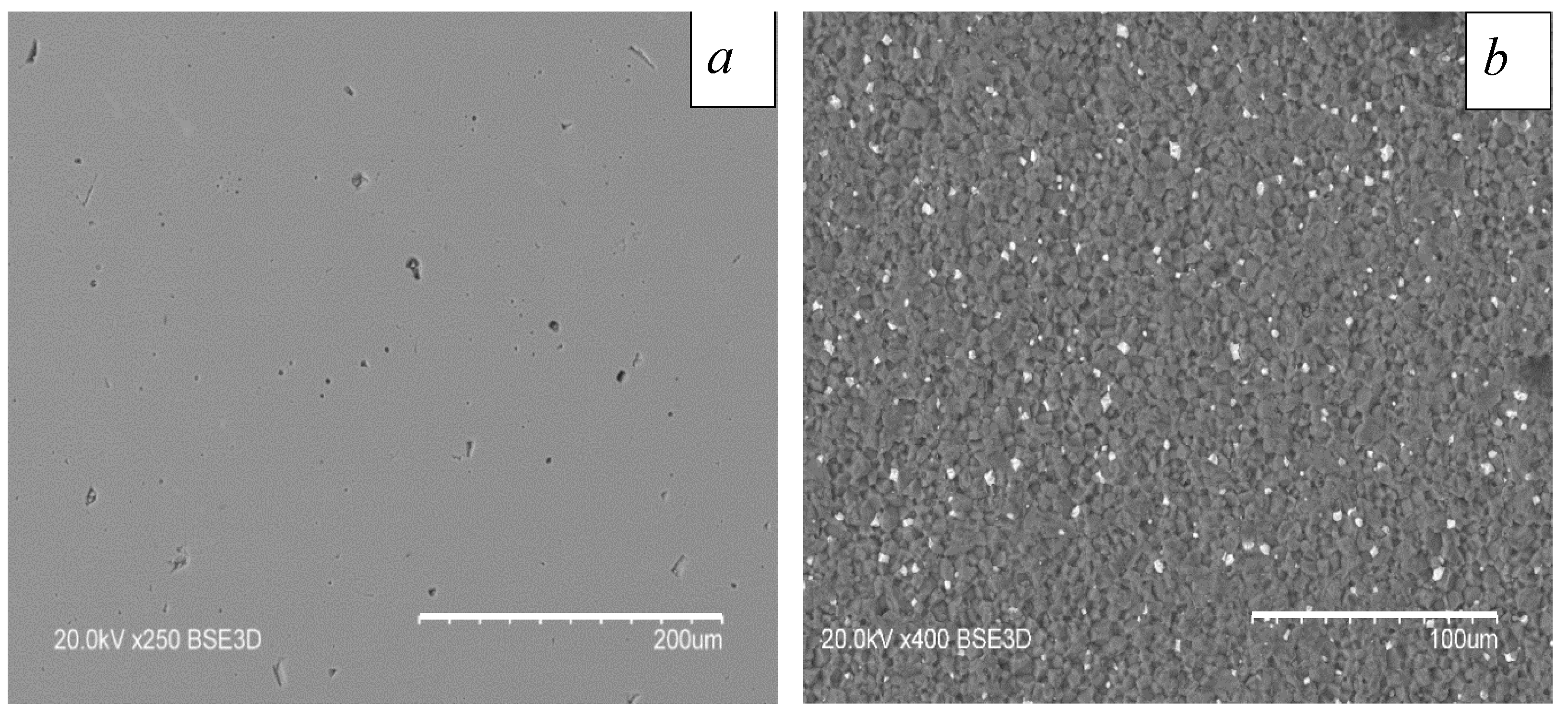
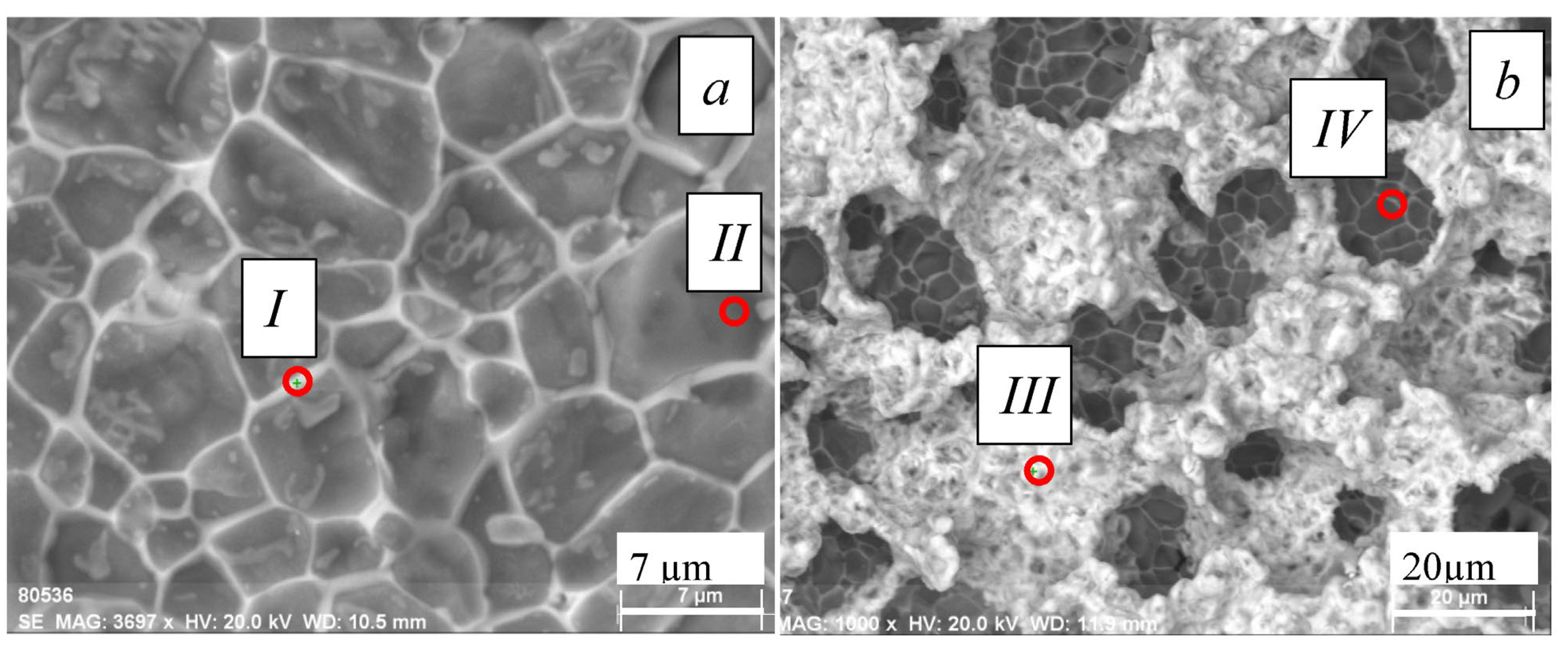
4.3. Effect of Electron-Beam Treatment on the Surface of the AlN Ceramic
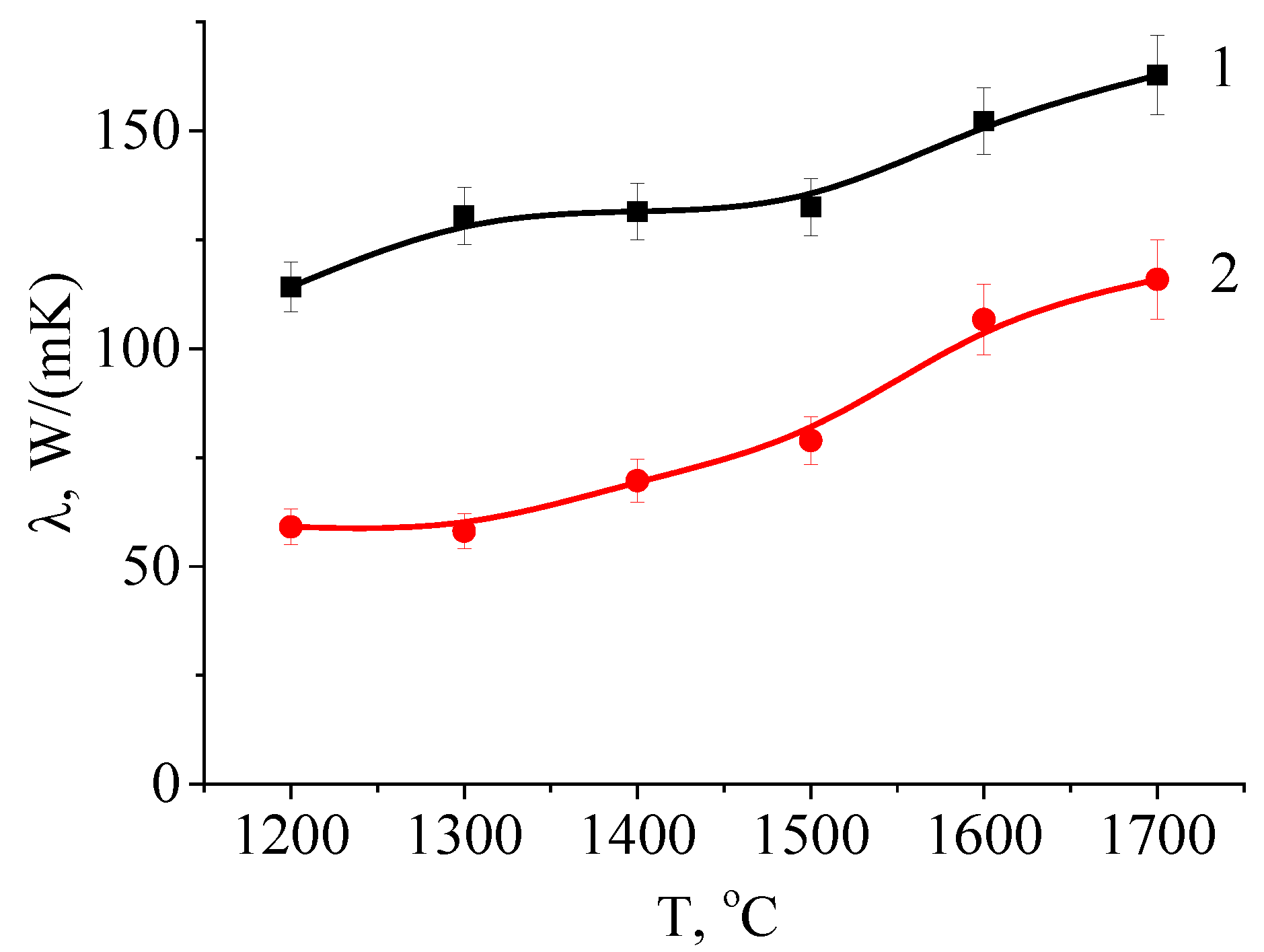
4.4. Thermal Conductivity Measurement of the AlN Ceramic after Electron-Beam Processing
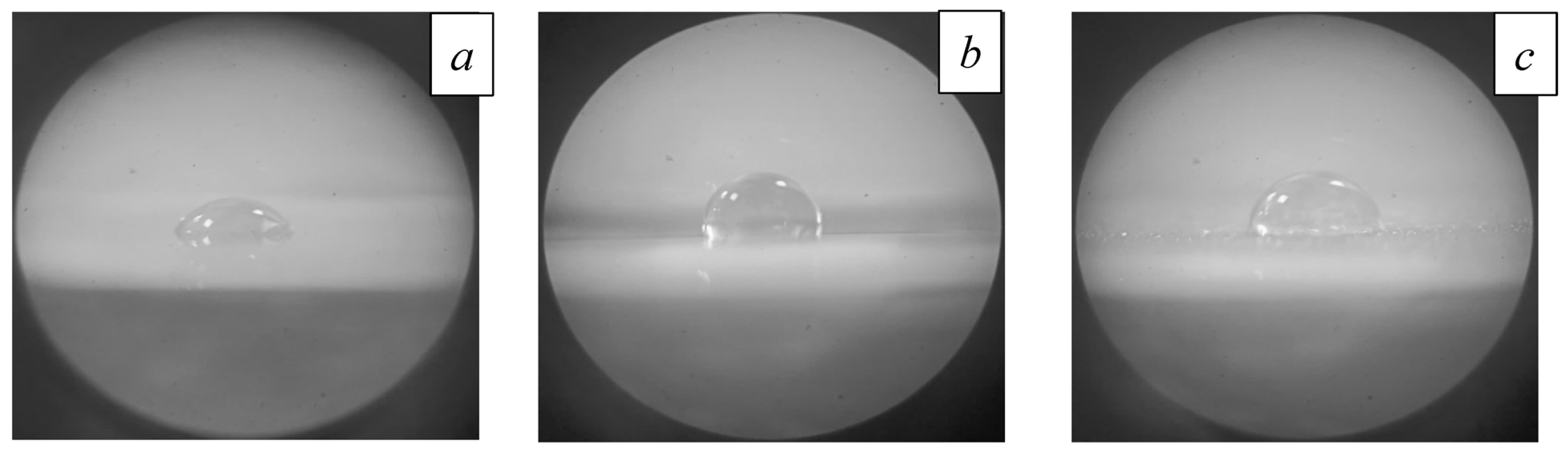
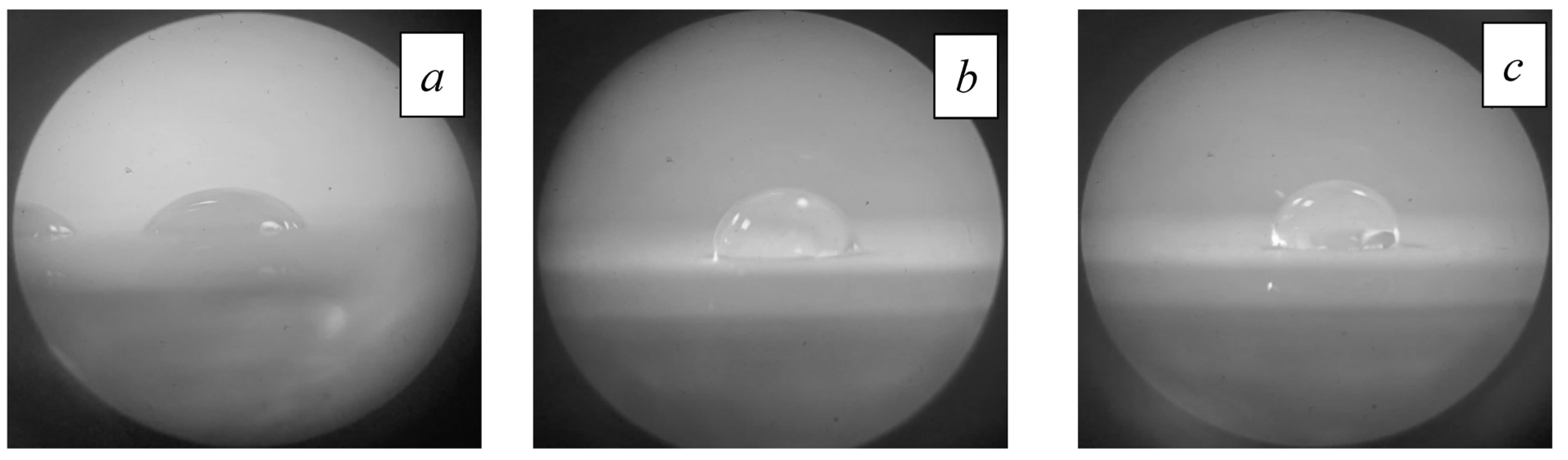
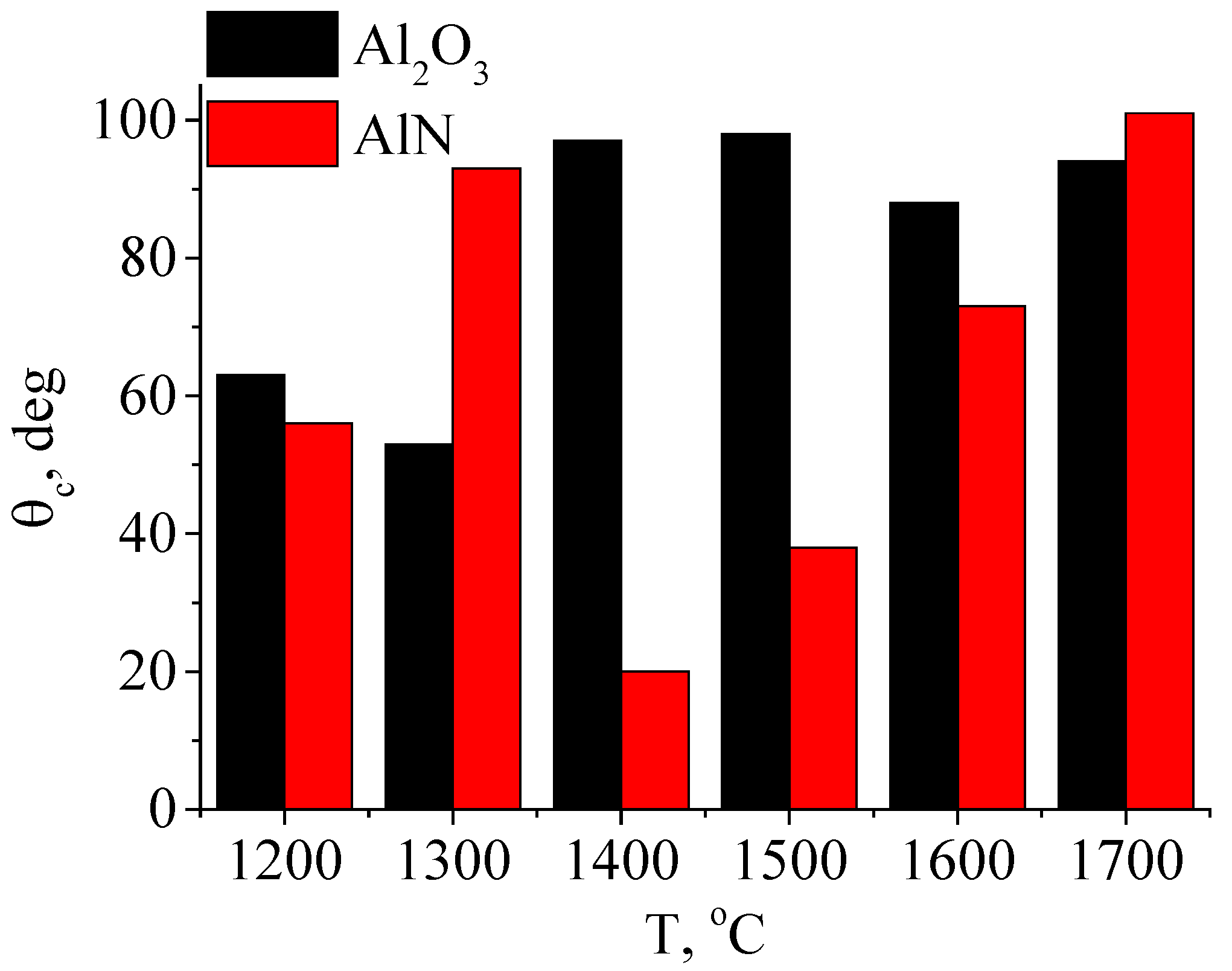
4.5. Effect of Electron-Beam Processing on Ceramic Wettability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Baik, Y.; Drew, R.A. Aluminum nitride: Processing and applications. Key Eng. Mater. 1996, 122, 553. [Google Scholar] [CrossRef]
- Werdecker, W.; Aldinger, F. Aluminum nitride-an alternative ceramic substrate for high power applications in microcircuits. IEEE Trans. Compon. Hybrids Manuf. Technol. 1984, 7, 399–404. [Google Scholar] [CrossRef]
- Taylor, K.M.; Lenie, C. Some properties of aluminum nitride. J. Electrochem. Soc. 1960, 107, 308. [Google Scholar] [CrossRef]
- Ivanov, S.N.; Popov, P.A.; Egorov, G.V. Thermophysical properties of ceramic aluminum nitride. Phys. Solids 1997, 39, 93–96. [Google Scholar] [CrossRef]
- Abyzov, A.M. Aluminum oxide and alumina ceramics (review). Part 1. Properties of Al2O3 and commercial production of dispersed Al2O3. Refract. Ind. Ceram. 2019, 60, 24–32. [Google Scholar] [CrossRef]
- Figiel, P.; Rozmus, M.; Smuk, B. Properties of alumina ceramics obtained by conventional and non-conventional methods for sintering ceramics. J. Achiev. Mater. Manuf. Eng. 2011, 48, 29–34. [Google Scholar]
- Parikh, P.B. Alumina ceramics: Engineering applications and domestic market potential. Trans. Indian Ceram. Soc. 1995, 54, 179–184. [Google Scholar] [CrossRef]
- Dalla Pria, P. Evolution and new application of the alumina ceramics in joint replacement. Eur. J. Orthop. Surg. Traumatol. 2007, 17, 253–256. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, J.; Lee, S.M.; Jeong, H.; Ryu, S.S. Low-temperature hot-press sintering of AlN ceramics with MgO–CaO–Al2O3–SiO2 glass additives for ceramic heater applications. Ceram. Int. 2022, 48, 26022–26027. [Google Scholar] [CrossRef]
- Fiedler, H.; Leveneur, J.; Kennedy, J. Advanced AlN ceramic materials for energy-efficient communication devices. In Advanced Ceramics for Energy Storage, Thermoelectrics and Photonics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 237–255. [Google Scholar]
- Ruppi, S.; Larsson, A. Chemical vapour deposition of k-Al2O3. Thin Solid Film 2001, 388, 50–61. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Reucroft, P.J.; Ko, Y. Crystallinity of Al2O3 films deposited by metalorganic chemical vapor deposition. Surf. Coat. Technol. 2004, 176, 382–384. [Google Scholar] [CrossRef]
- Kawakami, N.; Yokota, Y.; Tachibana, T.; Hayashi, K.; Kobashi, K. Atomic layer deposition of Al2O3 thin films on diamond. Diam. Relat. Mater. 2005, 14, 2015–2018. [Google Scholar] [CrossRef]
- Zhang, Y.; Bertrand, J.A.; Yang, R.; George, S.M.; Lee, Y.C. Electroplating to visualize defects in Al2O3 thin films grown using atomic layer deposition. Thin Solid Film. 2009, 517, 3269–3272. [Google Scholar] [CrossRef]
- Guo, J.; Sun, Y.; Qiu, T.; Xue, W.; Yang, H. Gelating and drying process of aqueous gelcasting aluminum nitride ceramics. Int. J. Appl. Ceram. Technol. 2015, 12, E23–E32. [Google Scholar] [CrossRef]
- Gottmann, J.; Kreutz, E.W. Pulse laser deposition of alumina and zirconia thin films on polymers and glass as optical and protective coating. Surf. Coat. Technol. 1999, 116, 1189–1194. [Google Scholar] [CrossRef]
- Jing, C.; Zhao, X.; Zhang, Y. Sol–gel fabrication of compact, crack-free alumina film. Mater. Res. Bull. 2007, 42, 600–608. [Google Scholar] [CrossRef]
- Masalski, J.; Gluszek, J.; Zabrzeski, J.; Nitsch, K.; Gluszek, P. Improvement in corrosion resistance of the 316l stainless steel by means of Al2O3 coatings deposited by the sol-gel method. Thin Solid Film 1999, 349, 186–190. [Google Scholar] [CrossRef]
- Sun, R.; Yang, X.; Arima, K.; Kawai, K.; Yamamura, K. High-quality plasma-assisted polishing of aluminum nitride ceramic. CIRP Ann. 2020, 69, 301–304. [Google Scholar] [CrossRef]
- Dukenbayev, K.; Kozlovskiy, A.; Korolkov, I.; Zdorovets, M. Investigation of radiation resistance of AlN ceramics. Vacuum 2019, 159, 144–151. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Vlasov, V.A.; Malyshev, A.V.; Surzhikov, A.P. Structural and magnetic properties of lithium-substituted ferrite ceramics sintered by continuous electron beam heating. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 470, 28–31. [Google Scholar] [CrossRef]
- Karansky, V.V.; Klimov, A.S.; Smirnov, S.V. Structural transformations in Mn–Zn ferrite under low-energy electron beam treatment. Vacuum 2020, 173, 109115. [Google Scholar] [CrossRef]
- Mirzayev, M.N.; Popov, E.; Demir, E.; Abdurakhimov, B.A.; Mirzayeva, D.M.; Sukratov, V.A.; Kristavchuk, O. Thermophysical behavior of boron nitride and boron trioxide ceramics compounds with high energy electron fluence and swift heavy ion irradiated. J. Alloys Compd. 2020, 834, 155119. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, N.; Wu, J.; Wei, J.; Yan, B.; Li, L.; He, N. Rapid fabrication of surface microstructures on AlN HTCC substrate by chemically assisted laser ablation. Ceram. Int. 2021, 47, 27598–27608. [Google Scholar] [CrossRef]
- Stolz, B.; Poprawe, R. Surface conductivity modification of ceramics with laser radiation. Surf. Coat. Technol. 1999, 112, 394–400. [Google Scholar] [CrossRef]
- Egashira, Y.; Kim, H.; Komiyama, H. Cluster Size Determination in the Chemical Vapor Deposition of Aluminum Nitride. J. Am. Ceram. Soc. 1994, 8, 2009–2016. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Ivanov, K.V.; Chesnokov, A.E.; Smirnov, A.V. Application of high current pulsed electron beam irradiation to smoothing of cold spray aluminum bronze coating. Vacuum 2022, 197, 110780. [Google Scholar] [CrossRef]
- Sabet, M.; Hassan, A.; Ratnam, C.T. Mechanical, electrical, and thermal properties of irradiated low-density polyethylene by electron beam. Polym. Bull. 2020, 68, 2323–2339. [Google Scholar] [CrossRef]
- Stankova, N.E.; Atanasov, P.A.; Nikov, R.G.; Nikov, R.G.; Nedyalkov, N.N.; Stoyanchov, T.R.; Fukata, N.; Kolev, K.N.; Valova, E.I.; Georgieva, J.S.; et al. Optical properties of polydimethylsiloxane (PDMS) during nanosecond laser processing. Surf. Sci. 2016, 374, 96–103. [Google Scholar] [CrossRef]
- Kumar, R.; Khan, M.W.; Srivastava, J.P.; Arora, S.K.; Sofin, R.G.S.; Choudhary, R.J.; Shvets, I.V. Swift heavy ion irradiation-induced modifications in structural. magnetic and electrical transport properties of epitaxial magnetic thin films. J. Appl. Phys. 2006, 100, 033703. [Google Scholar] [CrossRef]
- Sreedevi, A.; Priyanka, K.P.; Babitha, K.K.; Ganesh, S.; Varghese, T. Influence of electron beam irradiation on structural and optical properties of α-Ag2WO4 nanoparticles. Micron 2016, 88, 1–6. [Google Scholar]
- Onoe, J.; Nakayama, T.; Aono, M.; Hara, T. Structural and electrical properties of an electron-beam-irradiated C60 film. Appl. Phys. Lett. 2003, 4, 595–597. [Google Scholar] [CrossRef]
- Yasuda, K.; Kinoshita, C. Electron-beam induced dissociation of dislocation loops in magnesia–alumina ceramics. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2002, 191, 559–564. [Google Scholar] [CrossRef]
- Burdovitsin, V.A.; Dvilis, E.S.; Medovnik, A.V.; Oks, E.M.; Khasanov, O.L.; Yushkov, Y.G. Surface structure of alumina ceramics during irradiation by a pulsed electron beam. Tech. Phys. 2013, 58, 111–113. [Google Scholar] [CrossRef]
- Bakeev, I.Y.; Dvilis, E.S.; Klimov, A.S.; Oks, E.M.; Zenin, A.A. The possibilities of dimensional electron-beam processing as applied to selective sintering of oxide ceramics in the forevacuum pressure range. J. Phys. Conf. Ser. 2018, 945, 012016. [Google Scholar] [CrossRef]
- Surzhikov, A.P.; Frangulyan, T.S.; Ghyngazov, S.A.; Vasiliev, I.P. Electron microscopy studies of near-surface layers of ZrO2(Y)-Al2O3 composite ceramic modified by high-current beam of low-energy electrons. Inorg. Mater. Appl. Res. 2014, 5, 536–539. [Google Scholar] [CrossRef]
- Burdovitsin, V.A.; Klimov, A.S.; Medovnik, A.V.; Oks, E.M. Electron beam treatment of non-conducting materials by a fore-pump-pressure plasma-cathode electron beam source. Plasma Sources Sci. Technol. 2010, 19, 055003. [Google Scholar] [CrossRef]
- Klimov, A.S.; Bakeev, I.Y.; Dvilis, E.S.; Oks, E.M.; Zenin, A.A. Electron beam sintering of ceramics for additive manufacturing. Vacuum 2019, 169, 108933. [Google Scholar] [CrossRef]
- Klimov, A.S.; Bakeev, I.Y.; Oks, E.M.; Zenin, A.A. Electron-beam sintering of an Al2O3/Ti composite using a forevacuum plasma-cathode electron source. Ceram. Int. 2020, 46, 22276–22281. [Google Scholar] [CrossRef]
- Klimov, A.S.; Zenin, A.A.; Bakeev, I.Y.; Oks, E.M. Formation of gradient metalloceramic materials using electron-beam irradiation in the forevacuum. Russ. Phys. J. 2019, 62, 1123–1129. [Google Scholar] [CrossRef]
- Ivanova, A.S. Aluminum oxide and systems based on it: Properties and applications. Kinet. Catal. 2012, 53, 425–439. [Google Scholar] [CrossRef]
- Miyashiro, F.; Iwase, N.; Tsuge, A.; Ueno, F.; Nakahashi, M.; Takahashi, T. High thermal conductivity aluminum nitride ceramic substrates and packages. IEEE Transactions on Components. Hybrids Manuf. Technol. 1990, 13, 313–319. [Google Scholar] [CrossRef]
- Jackson, T.B.; Virkar, A.V.; More, K.L.; Dinwiddie, R.B., Jr.; Cutler, R.A. High-thermal-conductivity aluminum nitride ceramics: The effect of thermodynamic, Kinetic, and microstructural factors. J. Am. Ceram. Soc. 1997, 80, 1421–1435. [Google Scholar] [CrossRef]
- Miroshkin, V.P.; Panova, Y.I.; Pasynkov, V.V. Dielectric relaxation in polycrystalline ferrites. Phys. Stat. Sol. A 1981, 66, 779–782. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Klimov, A.; Bakeev, I.; Oks, E.; Zenin, A. Forevacuum plasma source of continuous electron beam. Laser Part. Beams 2019, 37, 203–208. [Google Scholar] [CrossRef]
- Sarkar, R. Refractory Technology: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Ansell, S.; Krishnan, S.; Weber, J.R.; Felten, J.J.; Nordine, P.C.; Beno, M.A.; Saboungi, M.L. Structure of liquid aluminum oxide. Phys. Rev. Lett. 1997, 78, 464. [Google Scholar] [CrossRef]
- San Miguel, M.A.; Sanz, J.F.; Alvarez, L.J.; Odriozola, J.A. Molecular-dynamics simulations of liquid aluminum oxide. Phys. Rev. B 1998, 58, 2369. [Google Scholar] [CrossRef]
- Bityukov, V.K.; Petrov, V.A. Absorption coefficient of molten aluminum oxide in semitransparent spectral range. Appl. Phys. Res. 2013, 5, 51. [Google Scholar] [CrossRef]
- Maslyaev, S.A.; Morozov, E.V.; Romakhin, P.A.; Pimenov, V.N.; Gribkov, V.A.; Tikhonov, A.N.; Bondarenko, G.G.; Dubrovsky, A.V.; Kazilin, E.E.; Sasinovskaya, I.P.; et al. Damage of Al2O3 ceramics under the action of pulsed ion and plasma fluxes and laser irradiation. Inorg. Mater. Appl. Res. 2016, 7, 330–339. [Google Scholar] [CrossRef]
- Lee, H.M.; Bharathi, K.; Kim, D.K. Processing and characterization of aluminum nitride ceramics for high thermal conductivity. Adv. Eng. Mater. 2014, 16, 655–669. [Google Scholar] [CrossRef]
- Harris, J.H. Sintered aluminum nitride ceramics for high-power electronic applications. JOM 1998, 50, 56–60. [Google Scholar] [CrossRef]
- Muradyan, G.N.; Dolukhanyan, S.K.; Aleksanyan, A.G.; Ter-Galstyan, O.P.; Mnatsakanyan, N.L. Regularities and Mechanism of Formation of Aluminides in the TiH2-ZrH2-Al System. Russ. J. Phys. Chem. B 2019, 13, 86–95. [Google Scholar] [CrossRef]
- Hlavac, J. Melting temperatures of refractory oxides: Part I. Pure Appl. Chem. 1982, 54, 681–688. [Google Scholar] [CrossRef]
- Paigin, V.D.; Deulina, D.E.; Ilela, A.E.; Lyamina, G.V.; Dvilis, E.S.; Valiev, D.T.; Stepanov, S.A.; Khasanov, O.L.; Ditts, A.A. Synthesis and Investigation of the Properties of Al2O3–Y2O3 Powders Using Nanospray Drying. Rev. Adv. Chem. 2022, 12, 270–276. [Google Scholar] [CrossRef]
- Pu, G.; Zou, J.; Lin, L.; Zhang, K.; Liu, B.; Ma, F.; Wang, Q.; Li, Q. Effects of He ion irradiation on the microstructures and mechanical properties of t’phase yttria-stabilized zirconia ceramics. J. Alloys Compd. 2019, 771, 777–783. [Google Scholar] [CrossRef]
- Ghyngazov, S.A. Zirconia ceramics processing by intense electron and ion beams. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 435, 190–193. [Google Scholar] [CrossRef]
- Slack, G.A.; Tanzilli, R.A.; Pohl, R.O.; Vandersande, J.W. The intrinsic thermal conductivity of AIN. J. Phys. Chem. Solids 1987, 48, 641–647. [Google Scholar] [CrossRef]
- Slack, G.A.; McNelly, T.F. Growth of high purity AlN crystals. J. Cryst. Growth 1976, 34, 263–279. [Google Scholar] [CrossRef]
- Slack, G.A.; Tanzilli, R.A. Pohl RO and Vandersande JW. J. Phys. Chem. Solids 1987, 1987, 48. [Google Scholar]
- Slack, G.A. Nonmetallic crystals with high thermal conductivity. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Virkar, A.V.; Jackson, T.B.; Cutler, R.A. Thermodynamic and kinetic effects of oxygen removal on the thermal conductivity of aluminum nitride. J. Am. Ceram. Soc. 1989, 72, 2031–2042. [Google Scholar] [CrossRef]
- Yano, T.; Iseki, T. Thermal and mechanical properties of neutron-irradiated aluminum nitride. J. Nucl. Mater. 1991, 179, 387–390. [Google Scholar] [CrossRef]
- Watari, K.; Kawamoto, M.; Ishizaki, K. Sintering chemical reactions to increase thermal conductivity of aluminium nitride. J. Mater. Sci. 1991, 26, 4727–4732. [Google Scholar] [CrossRef]
- Fujimoto, M.; Ueda, S. Effect of annealing in CF4 gas atmosphere on thermal conductivity of AlN ceramics. J. Ceram. Soc. Jpn. 1988, 96, 1210–1213. [Google Scholar] [CrossRef][Green Version]
- Kasori, M.; Ueno, F.; Tsuge, A. Effects of Transition-Metal Additions on the Properties of AlN. J. Am. Ceram. Soc. 1994, 77, 1991–2000. [Google Scholar] [CrossRef]
- Hirano, M.; Kato, K.; Isobe, T.; Hirano, T. Sintering and characterization of fully dense aluminium nitride ceramics. J. Mater. Sci. 1993, 28, 4725–4730. [Google Scholar] [CrossRef]
- Zhao, Y.-P. Characterization of Amorphous and Crystalline Rough Surface: Principles and Applications; Elsevier: San Diego, CA, USA, 2000. [Google Scholar]
- Foadi, F.; Brink, G.H.T.; Mohammadizadeh, M.R.; Palasantzas, G. Roughness dependent wettability of sputtered copper thin films: The effect of the local surface slope. J. Appl. Phys. 2019, 125, 244307. [Google Scholar] [CrossRef]
- Zhang, Y. The Effect of Surface Roughness Parameters on Contact and Wettability of Solid Surfaces. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2007. [Google Scholar]
- Lai, L.; Irene, E.A. Area evaluation of microscopically rough surfaces. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1999, 17, 33–39. [Google Scholar] [CrossRef]
- Croll, S.G. Surface roughness profile and its effect on coating adhesion and corrosion protection: A review. Prog. Org. Coat. 2020, 148, 105847. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic surfaces: Insights from theory and experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 2002, 28, 988–994. [Google Scholar] [CrossRef]
- Yang, Q.; Lv, Z.; Cai, Y.; Cheng, J.; Lou, D.; Chen, L.; Liu, D. Femtosecond laser processing of AlN ceramics for gradient wettability control. ECS J. Solid State Sci. Technol. 2020, 9, 123010. [Google Scholar] [CrossRef]
- Niu, C.N.; Han, J.Y.; Hu, S.P.; Chao, D.Y.; Song, X.G.; Howlader, M.M.R.; Cao, J. Fast and environmentally friendly fabrication of superhydrophilic-superhydrophobic patterned aluminum surfaces. Surf. Interfaces 2021, 22, 100830. [Google Scholar] [CrossRef]
- Vidhya, Y.E.B.; Pattamatta, A.; Manivannan, A.; Vasa, N.J. Influence of fluence. beam overlap and aging on the wettability of pulsed Nd3+: YAG nanosecond laser-textured Cu and Al sheets. Appl. Surf. Sci. 2021, 548, 149259. [Google Scholar] [CrossRef]
- Cardoso, J.T.; Garcia-Girón, A.; Huerta-Murillo, D.; Jagdheesh, R.; Walker, M.; Dimov, S.S.; Ocaña, J.L. Influence of ambient conditions on the evolution of wettability properties of an IR-, ns-laser textured aluminium alloy. RSC Adv. 2017, 7, 39617. [Google Scholar] [CrossRef]




| Parameter | Policor (Al2O3) | INC-AN180 (AlN) |
|---|---|---|
| Content, % | 99.7 | 96 |
| Density, g/cm3 | 3.96 | 3.3 |
| TCLE, 10−6/°C | 8 | 4.8 |
| Thermal conductivity, W/m·K | 30 | 160–180 |
| Dielectric constant (at 20 °C) | 9.45–9.95 | 9 |
| tan δ, 10−4 | 1 | 5 |
| Melting point, °C | 2072 | 2200 |
| Nitrogen | Oxygen | Aluminum | Yttrium | |
|---|---|---|---|---|
| AlN | 48.2 | 0.9 | 49.4 | 1.5 |
| Al2O3 | - | 59.7 | 40.3 | - |
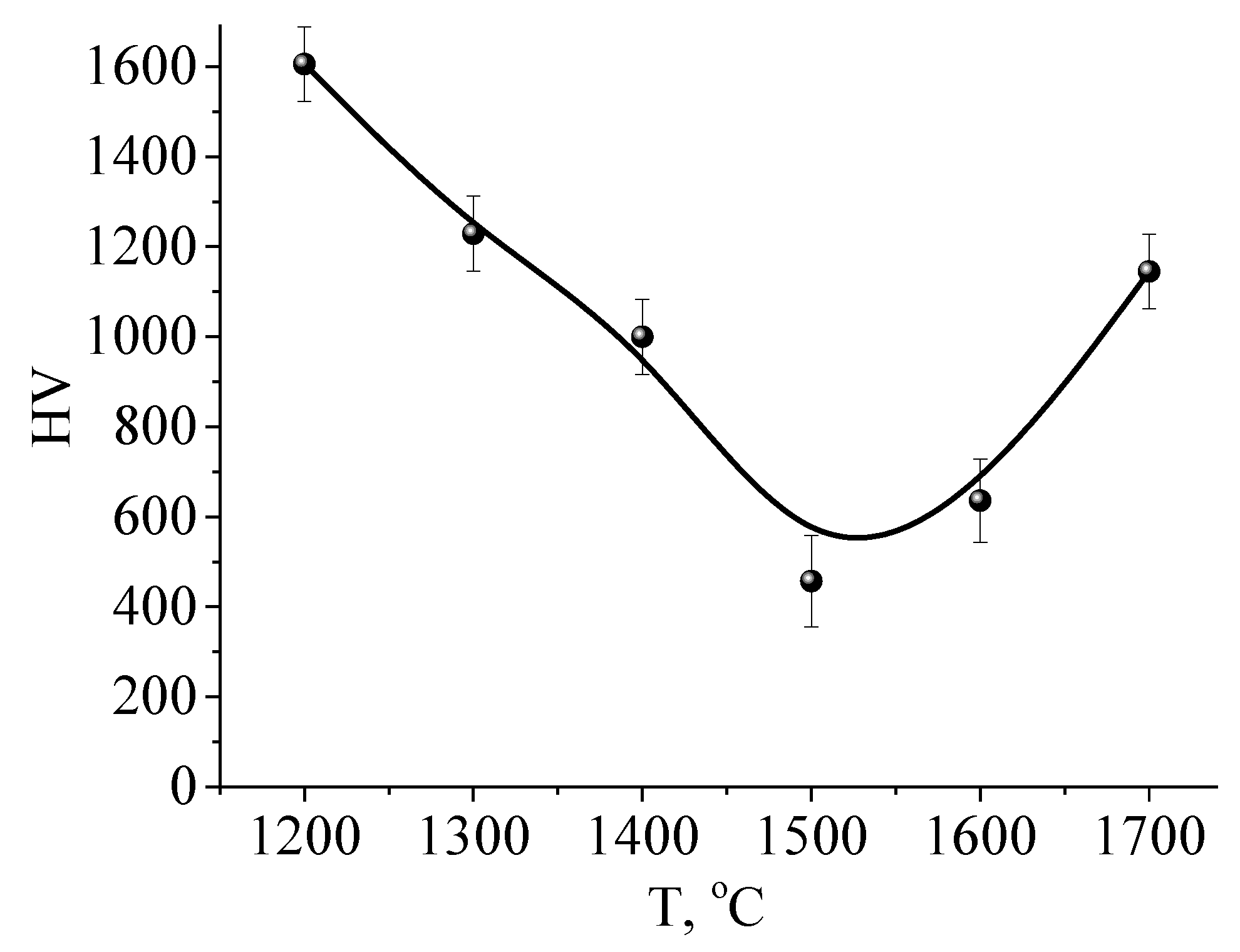
| Processing Regime | HV5 |
|---|---|
| Original | 1700 |
| 1300 °C | 1620 |
| 1400 °C | 1600 |
| 1500 °C | 1580 |
| 1600 °C | 1510 |
| 1700 °C | - |
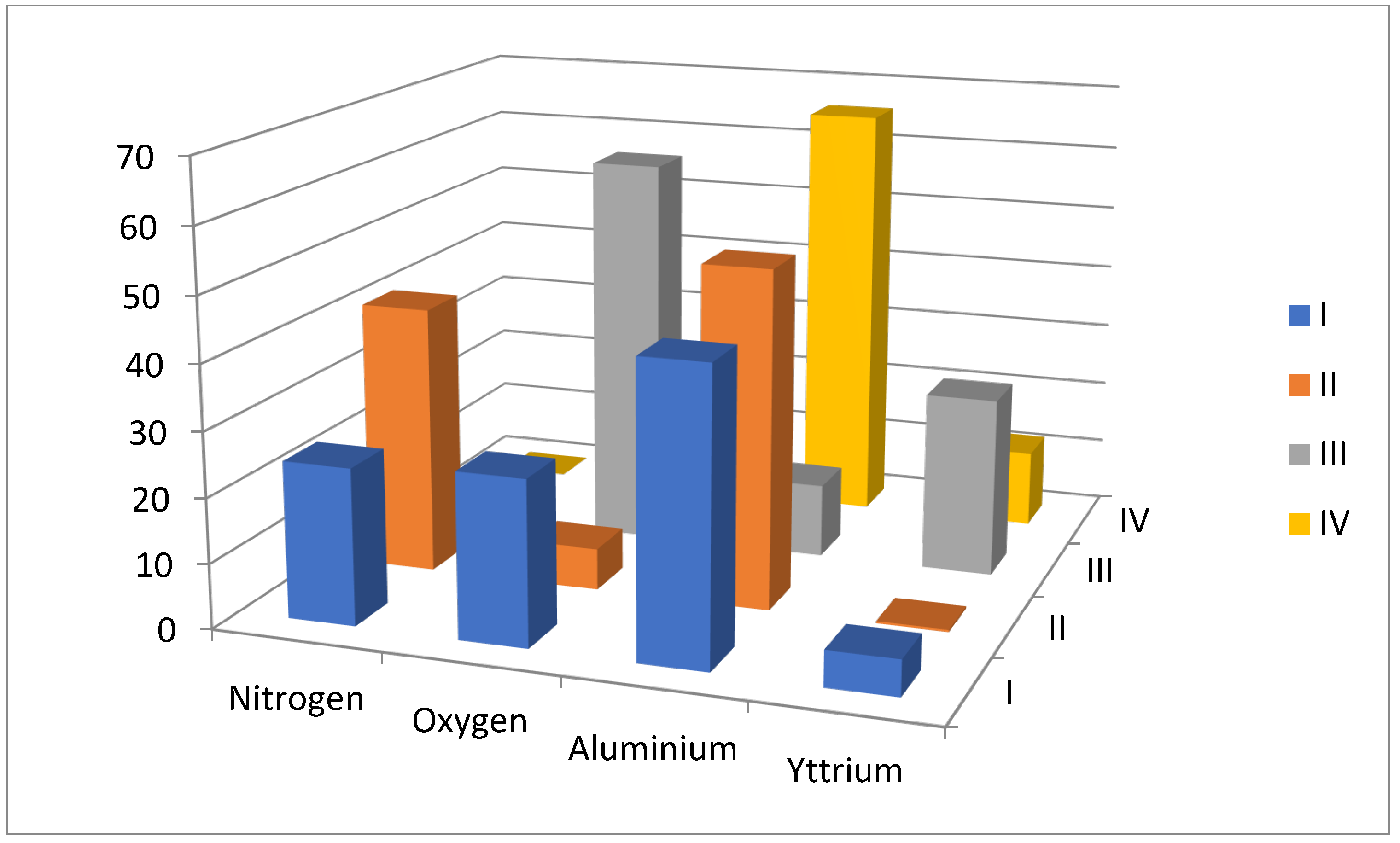
| Nitrogen | Oxygen | Aluminum | Yttrium | |
|---|---|---|---|---|
| I | 24.2 | 25.5 | 44.8 | 5.6 |
| II | 41.6 | 6.6 | 52.0 | 0.3 |
| III | 0 | 60.6 | 11.5 | 27.9 |
| IV | 0 | 21.4 | 65.8 | 12.0 |
| Processing Regime | Ra. µm |
|---|---|
| Original | 0.671 |
| 1300 °C | 0.754 |
| 1400 °C | 0.679 |
| 1500 °C | 2.656 |
| 1600 °C | 2.581 |
| 1700 °C | 3.795 |
| Processing Regime | Al2O3 | INC-AN180 |
|---|---|---|
| Original | 63° | 56° |
| 1300 °C | 53° | 93° |
| 1400 °C | 97° | 20° |
| 1500 °C | 98° | 38° |
| 1600 °C | 88° | 73° |
| 1700 °C | 94° | 101° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimov, A.; Bakeev, I.; Zenin, A. Electron-Beam Processing of Aluminum-Containing Ceramics in the Forevacuum Pressure Range. Ceramics 2023, 6, 2098-2116. https://doi.org/10.3390/ceramics6040129
Klimov A, Bakeev I, Zenin A. Electron-Beam Processing of Aluminum-Containing Ceramics in the Forevacuum Pressure Range. Ceramics. 2023; 6(4):2098-2116. https://doi.org/10.3390/ceramics6040129
Chicago/Turabian StyleKlimov, Aleksandr, Ilya Bakeev, and Aleksey Zenin. 2023. "Electron-Beam Processing of Aluminum-Containing Ceramics in the Forevacuum Pressure Range" Ceramics 6, no. 4: 2098-2116. https://doi.org/10.3390/ceramics6040129
APA StyleKlimov, A., Bakeev, I., & Zenin, A. (2023). Electron-Beam Processing of Aluminum-Containing Ceramics in the Forevacuum Pressure Range. Ceramics, 6(4), 2098-2116. https://doi.org/10.3390/ceramics6040129






