Abstract
The determination of L-glutamate in biological media is very important, as it is the most common excitatory neurotransmitter related to some neurological diseases, such as Parkinson’s, communication dysfunction, stroke, epilepsy and schizophrenia. When aiming to study the pathways of these diseases, as well as for the evaluation of medical treatments, it is very important to have rapid and reliable methods for the determination of L-glutamate. This study presents the new approach of an enzyme-based biosensor operating at −0.1 V, which ensures its good sensitivity and selectivity. The reduced graphene oxide used in the biosensor allowed for the monitoring of L-glutamate via the electro-oxidation of the NH3 released during the reaction catalyzed by Glutamate oxidase.
1. Introduction
Neurological diseases such as Parkinson’s, communication dysfunction, stroke, epilepsy and schizophrenia may be caused by the abnormal transmission of L-glutamate, as it is the most common excitatory neurotransmitter. When aiming to control these diseases, as well as for research purposes, it is very important to have reliable, simple methods for the determination of glutamate. At present, the most commonly used glutamate methods are slow, requiring complex equipment and the special training of service personnel [1,2]. Methods based on electrochemical biosensors are recognized as the most optimal in terms of their price, simplicity and reliability. Almost all of them are based on the oxidation reaction of hydrogen peroxide at Pt electrodes, which is carried out at high electrode potentials, ca. +0.6 V vs. Ag/AgCl. In this study, an amperometric enzyme-based biosensor operating at −0.1 V vs. Ag/AgCl is proposed, which ensures its good sensitivity and selectivity. After applying thermally reduced graphene oxide (RGO) in the design of the biosensor, the concentration of L-glutamate was monitored according to the electro-oxidation of the ammonia (NH3) released during the reaction, which was catalyzed by Glutamate oxidase (GluOx). The main characteristics and advantages of the proposed biosensor have been compared with a conventional Pt-based amperometric biosensor.
2. Materials and Methods
2.1. Materials
GluOx was from Streptomyces sp. (EC 1.4.3.11), Merck KGaA, Germany. Other chemical reagents were obtained from Sigma–Aldrich and were of analytical grade. RGO was synthesized according to the protocol reported by Ieva Sakinyte’s doctoral dissertation (Vilnius University).
2.2. Construction of the L-Glutamate Biosensor
The biosensor consists of a semipermeable membrane with immobilized GluOx, a working graphite electrode as the contact zone and isolating corps. Aiming to design enzymatic membranes, a mixture containing GluOx, bovine serum albumin in PBS, glutaraldehyde and RGO paste, which was prepared by mixing RGO powder with a pasting liquid consisting of 10% polyvinyl dichloride in acetone, was deposited on the inner surface of the ring-fixed membrane (working area Ø 2.4 mm) and then left at 4 °C overnight. After that, the membrane was mechanically attached to the surface of the graphite electrode. In a control Pt-based version, GluOx was immobilized in the semipermeable membrane and the Pt, at +0.6 V, served as the working electrode.
3. Discussion
After applying RGO and GluOx to the biosensor’s design, the concentration of L-glutamate could be monitored according to the release of NH3 from the reaction catalyzed by GluOx (Figure 1).
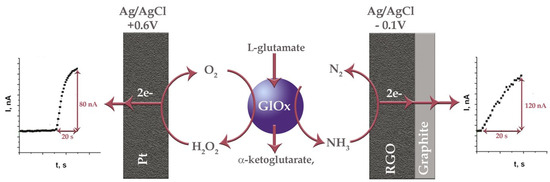
Figure 1.
Principal scheme of two different pathways of amperometric biosensing of L-glutamate when Pt and RGO are utilized as electrode surfaces. Responses of biosensors to 0.5 mM of L-glutamate, obtained at potentials of +0.6 V (left) and −0.1 V (right).
The dependence of the biosensor’s response to L-glutamate vs. the applied electrode potential allowed us to determine that the most optimal working potential was −0.1 V vs. Ag/AgCl. The conducted studies confirmed that, at −0.1 V, the biosensor registers the NH3 oxidation current. The influence of oxygen reduction at this potential on the response of the biosensor was also evaluated. Finally, the reliability of the biosensor was confirmed in real brain extract samples (Table S1), and its performance was compared with that of a traditional biosensor based on GluOx acting on a Pt electrode at +0.6 V (Table S2).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/proceedings2024097005/s1, Table S1: concentrations of L-glutamate in brain samples obtained using RGO-based biosensor, Table S2: comparison of analytical performances of Pt- and RGO-based biosensors.
Author Contributions
Conceptualization, J.R.; methodology, J.R. and V.G.; software, I.S.-U.; validation, J.R., formal analysis, I.S.-U. and V.G.; investigation, V.G.; resources, J.R.; data curation, J.R. and I.S.-U.; writing—original draft preparation, J.R. and I.S.-U.; writing—review and editing, J.R. and I.S.-U.; visualization, I.S.-U.; supervision, J.R.; project administration, J.R.; funding acquisition, J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Joint Programme on Rare Diseases (EJP-RD JTC2019) under Measure No. S-EJPRD-20-1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zieminska, E.; Toczylowska, B.; Diamandakis, D.; Hilgier, W.; Filipkowski, R.K.; Polowy, R.; Orzel, J.; Gorka, M.; Lazarewicz, W. Glutamate, glutamine and GABA levels in rat brain measured using MRS, HPLC and NMR methods in study of two models of autism. Front. Mol. Neurosci. 2018, 11, 418. [Google Scholar] [CrossRef]
- Chapmanustin, J.; Zhou, M. Microplate-based fluorometric methods for the enzymatic determination of l- glutamate: Application in measuring l-glutamate in food samples. Anal. Chim. Acta 1999, 402, 47–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).