Abstract
Rare-earth oxycarbonates have been proposed as promising chemoresistive materials for CO2 sensors. In this contribution we present the results of a broad investigation focused on selecting the best candidates in the rare-earth compounds and, in the case of the best performing material, preliminary results dealing with the understanding of sensing by the operando methods.
1. Introduction
CO2 sensing is of paramount importance for monitoring the state of the atmosphere, controlling indoor air quality, and cultivating crops in greenhouses or plant factories. Obtaining low cost, simple and good performance chemoresistive CO2 gas sensors has the potential to be a game changer. Rare-earth oxycarbonates Ln2O2CO3 (Ln = La and Nd) have been proposed as promising chemoresistive materials for CO2 sensors [1,2]. We have been exploring new rare-earth based CO2 sensitive materials and investigating into the conduction and sensing mechanism by using operando methods [3].
2. Material Synthesis and Sensor Fabrication
Rare-earth oxycarbonates and rare-earth oxides (rare-earth element = La, Ce, Nd, Sm, Gd, Dy, Er, Yb) were produced by the heat treatments of the oxalate hydrate or the acetate hydrate in a flow of ambient air at temperatures between 450 °C and 550 °C for 18 or 72 h. The powders after the heat treatment were mixed with propane-1,2-diol. The resulting pastes were screen printed onto alumina sensor substrates (provided with Pt interdigitated electrodes and Pt heater). The substrates were dried and then heated at the same temperature as its heat treatment.
3. Results and Discussion
3.1. DC Resistance Measurements
Figure 1 shows the comparison of sensor signals at 1000 ppm CO2 under standard humidity and operation temperature conditions (20 °C 50% rh, 300 °C) for all (10) sensors. The sensor signal is defined as the relative change of the resistance with respect to the resistance in air (CO2 = 0 ppm). Every sensor, excepting the CeO2 and Nd2O3 based, was sensitive to CO2.
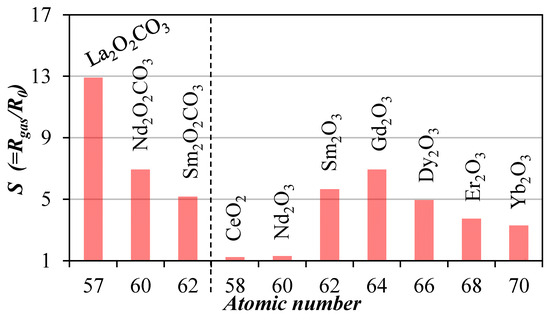
Figure 1.
Comparison of sensor signal at 1000 ppm CO2.
Additional investigations of selectivity and stability indicated that hexagonal La2O2CO3 possesses the best properties for a CO2 sensor so far. The detailed performance is shown in Figure 2.
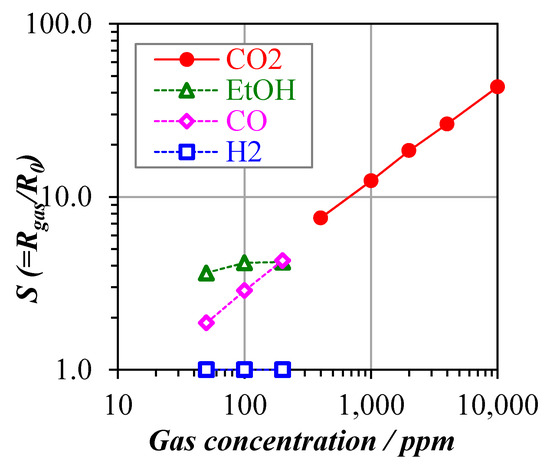
Figure 2.
Sensing performance of hexagonal La2O2CO3.
3.2. Operando Investigations
To reveal the sensing mechanism, we started by investigating the transduction by focusing on the conduction through the sensitive layer, with the help of operando AC impedance spectroscopy, and the effect of humidity, with the help of operando work function changes measurements; these investigations will be complemented by operando DRIFTS (Diffuse reflectance infrared Fourier transform spectroscopy) experiments; the operando stands for actual gas sensing conditions (e.g., at an operation temperature of 300 °C, with or without gas exposure, humid or dry atmosphere)
Out of the results of AC impedance spectroscopy, presented in Figure 3 as Cole-Cole plots, one can derive an equivalent circuit, see Figure 4. In it, there are two contributions that describe space charge regions–comprising parallel resistive and capacitive contributions. They can either describe electrode contact and intergranular contributions or heterogeneous intergranular contributions. In series, one finds an additional resistive contribution, which could describe the grains bulk. In DC conditions, the resistive contributions that are describing space charge regions, dominate and will show an exponential dependency on the surface barriers, which vary with ambient conditions. The changes of resistive contributions (Rc + Rgb) are correlated with the changes in the surface barrier height ΔVs as in Equation (1).
(Rc + Rgb)0/(Rc + Rgb)gas = exp (−qΔVs/kT)

Figure 3.
Cole-Cole plots from AC Impedance spectroscopy of hexagonal La2O2CO3. (20 °C 10% rh, Operation temperature = 300 °C).
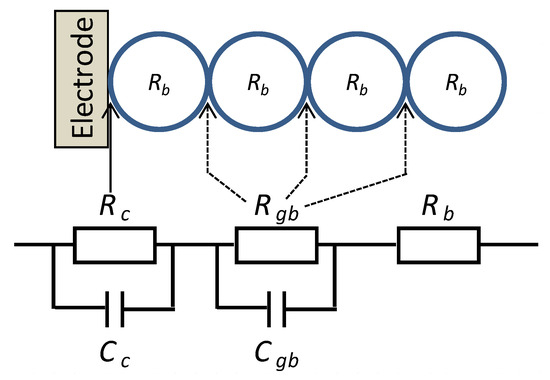
Figure 4.
Equivalent circuit composed of the electrode contact (RcCc), the inter-granular contact (RgbCgb), and the bulk (Rb).
where (Rc + Rgb)0 and (Rc + Rgb)gas are the values at 0 ppm and at a certain concentration of CO2, and q is elementary charge respectively.
The inputs from the AC impedance spectroscopy are allowing to separate the contribution of electron affinity Δχ and band bending qΔVs to the work function changes ΔΦ as in (2).
ΔΦ = qΔVs + Δχ
Figure 5 show the preliminary results in the case of the hexagonal La2O2CO3 based sensor operated at 300 °C in 20 °C 10% rh. In this case, the work function changes more than 0.6 eV at 4000 ppm CO2 and the contribution of electron affinity Δχ is larger than that of band bending qΔVs.
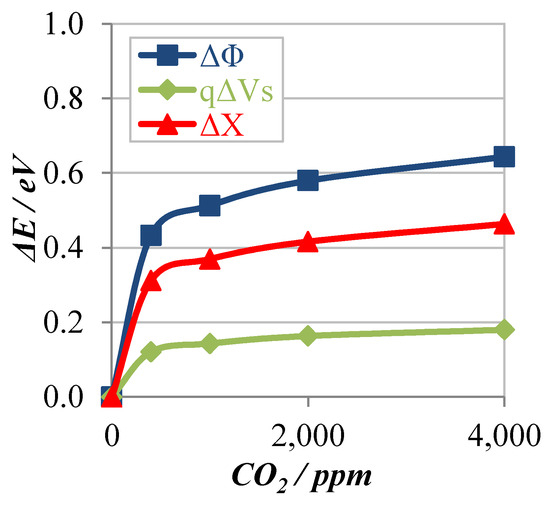
Figure 5.
Variation of work function, band bending, and electron affinity with CO2 concentration.
The electron affinity mainly depends on the surface dipoles which are caused by surface adsorbents such as hydroxyl groups. We will identify the surface adsorbents by operando DRIFTS experiments.
References
- Djerdj, I.; Haensch, A.; Koziej, D.; Pokhrel, S.; Barsan, N.; Weimar, U.; Niederberger, M. Neodymium Dioxide Carbonate as a Sensing Layer for Chemoresistive CO2 Sensing. Chem. Mater. 2009, 21, 5375–5381. [Google Scholar] [CrossRef]
- Haensch, A.; Djerj, I.; Niederberger, M.; Barsan, N.; Weimar, U. CO2 sensing with chemoresistive Nd2O2CO3 sensors-Operando insights. Procedia Chem. 2009, 1, 650–653. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).