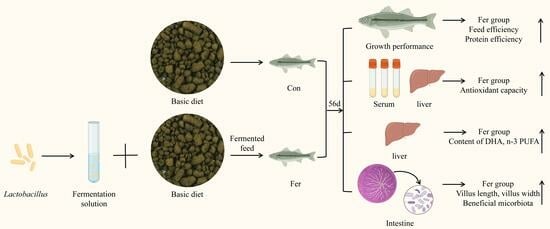
Effects of Co-Fermented Feed Using Lactobacillus acidophilus, Limosilactobacillus reuteri and Lactiplantibacillus plantarum on Growth, Antioxidant Capacity, Fatty Acids and Gut Microbiota of Largemouth Bass (Micropterus salmoides)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Feeding Trial
2.3. Sample Collection and Procession
2.4. Growth Assessment
2.5. Proximate Composition and Fatty Acid Profiles Analysis
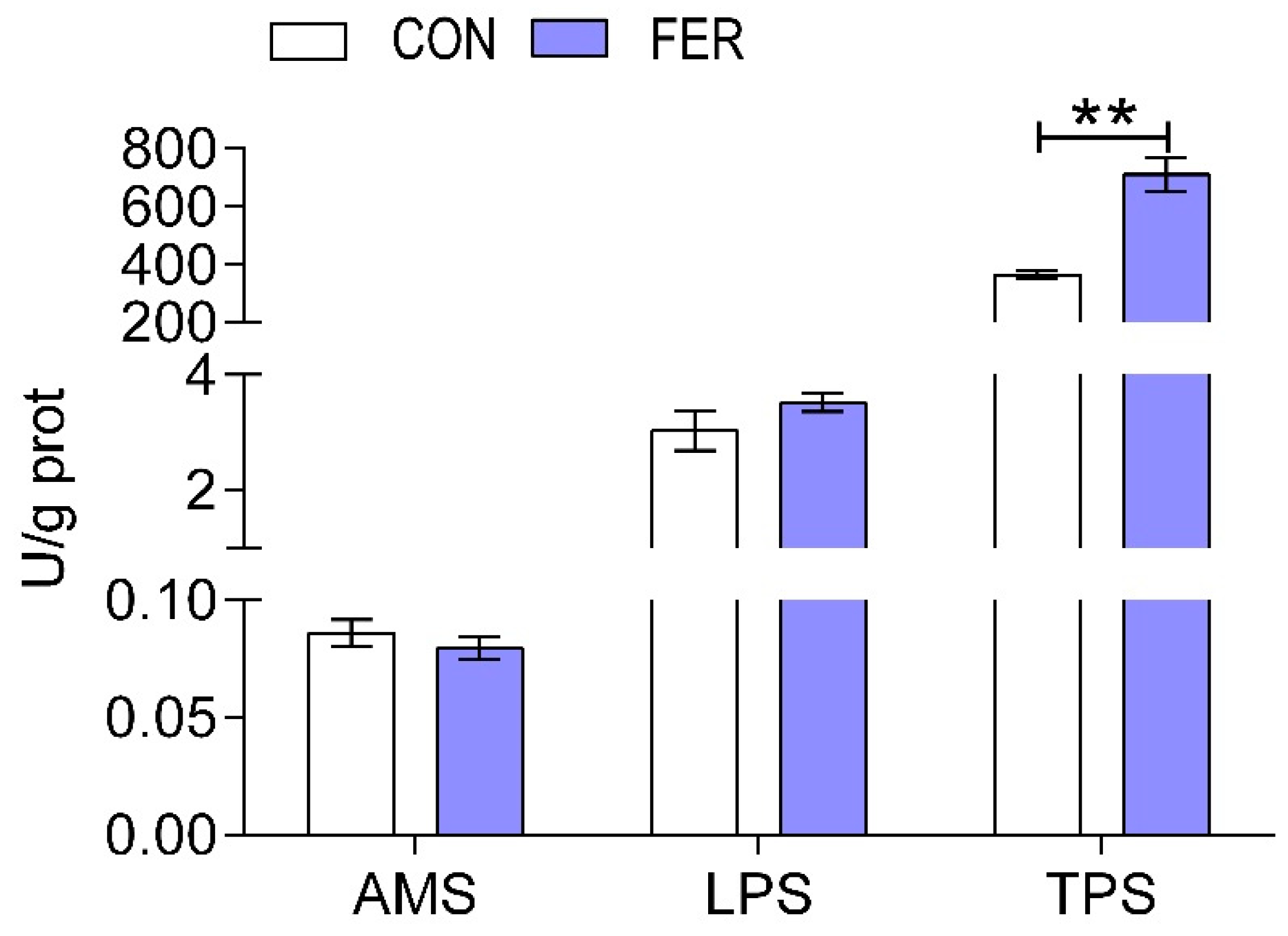
2.6. Biochemical Indices and Enzymatic Activities Analysis
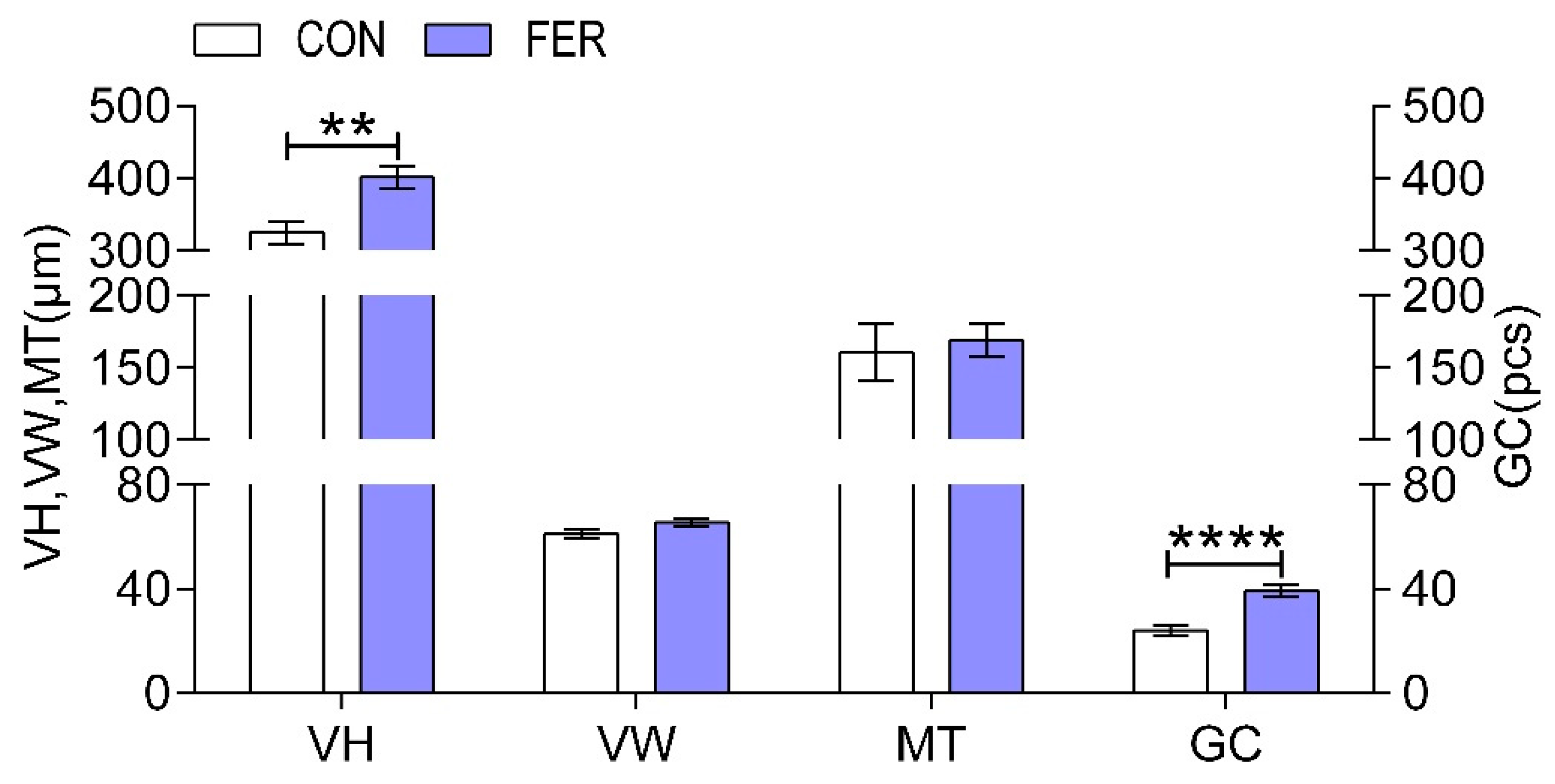
2.7. Liver and Intestine Histological Analysis
2.8. Intestinal Microbiota Analysis
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
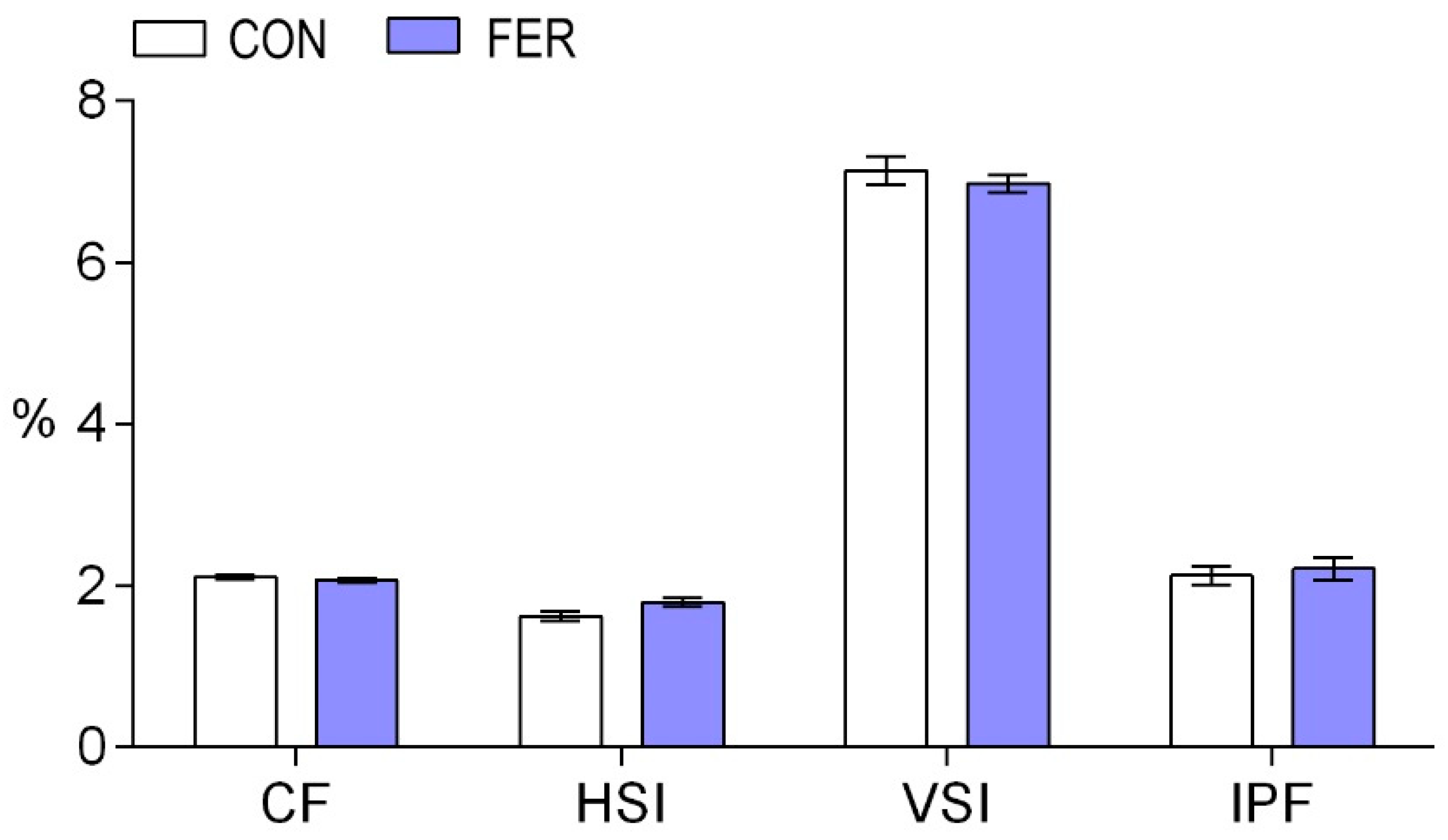
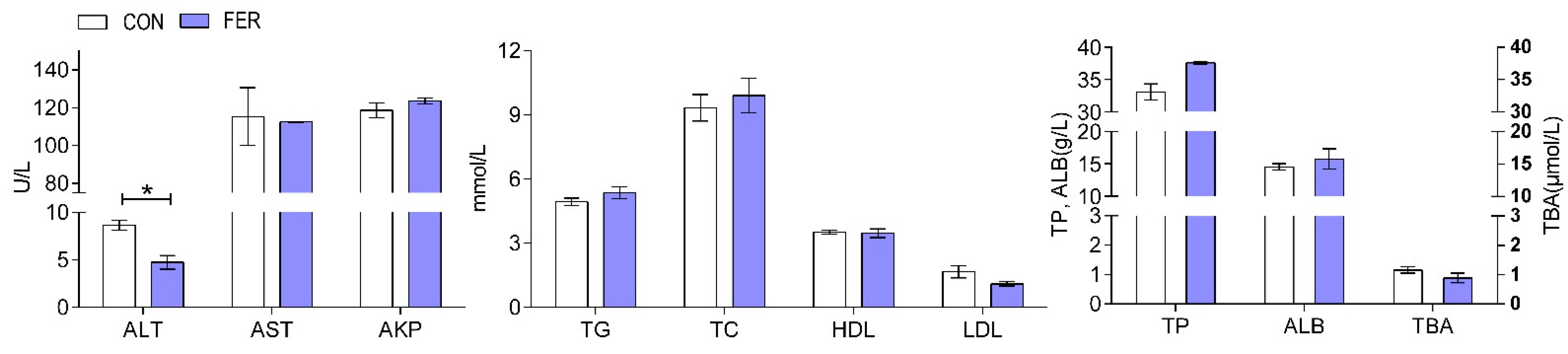
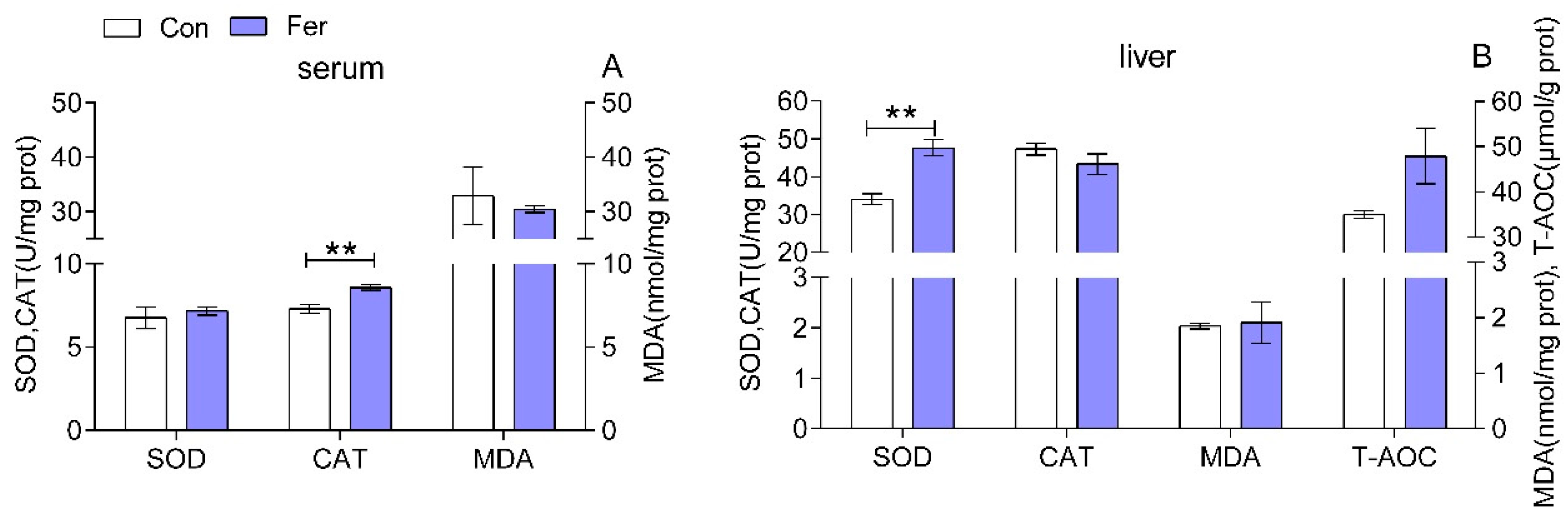
3.2. Biochemical Indices and Enzymatic Activities
3.3. Fatty Acids
3.3.1. Muscle Fatty Acids
3.3.2. Liver Fatty Acid Compositions
3.4. Histomorphology of the Intestine
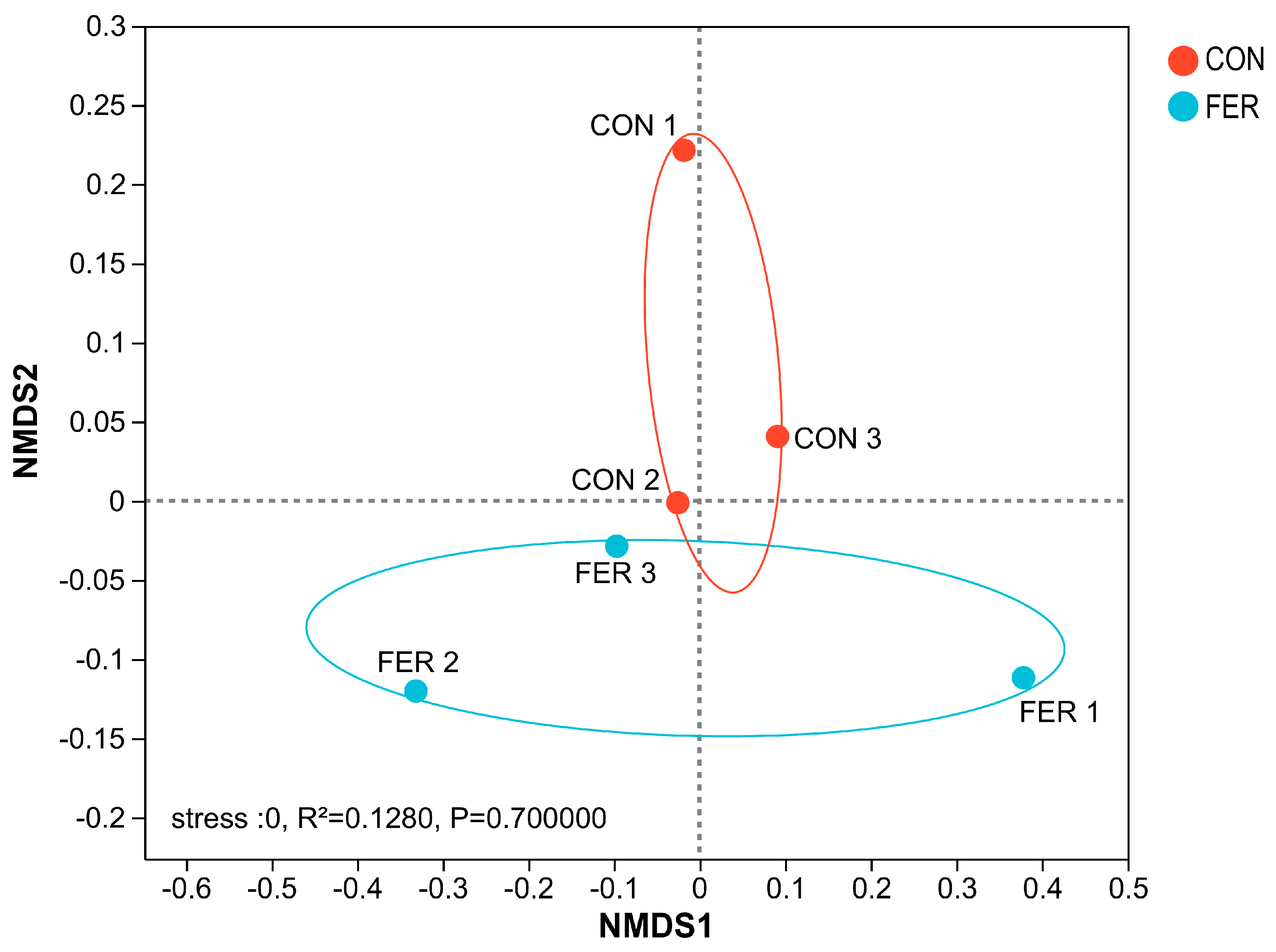
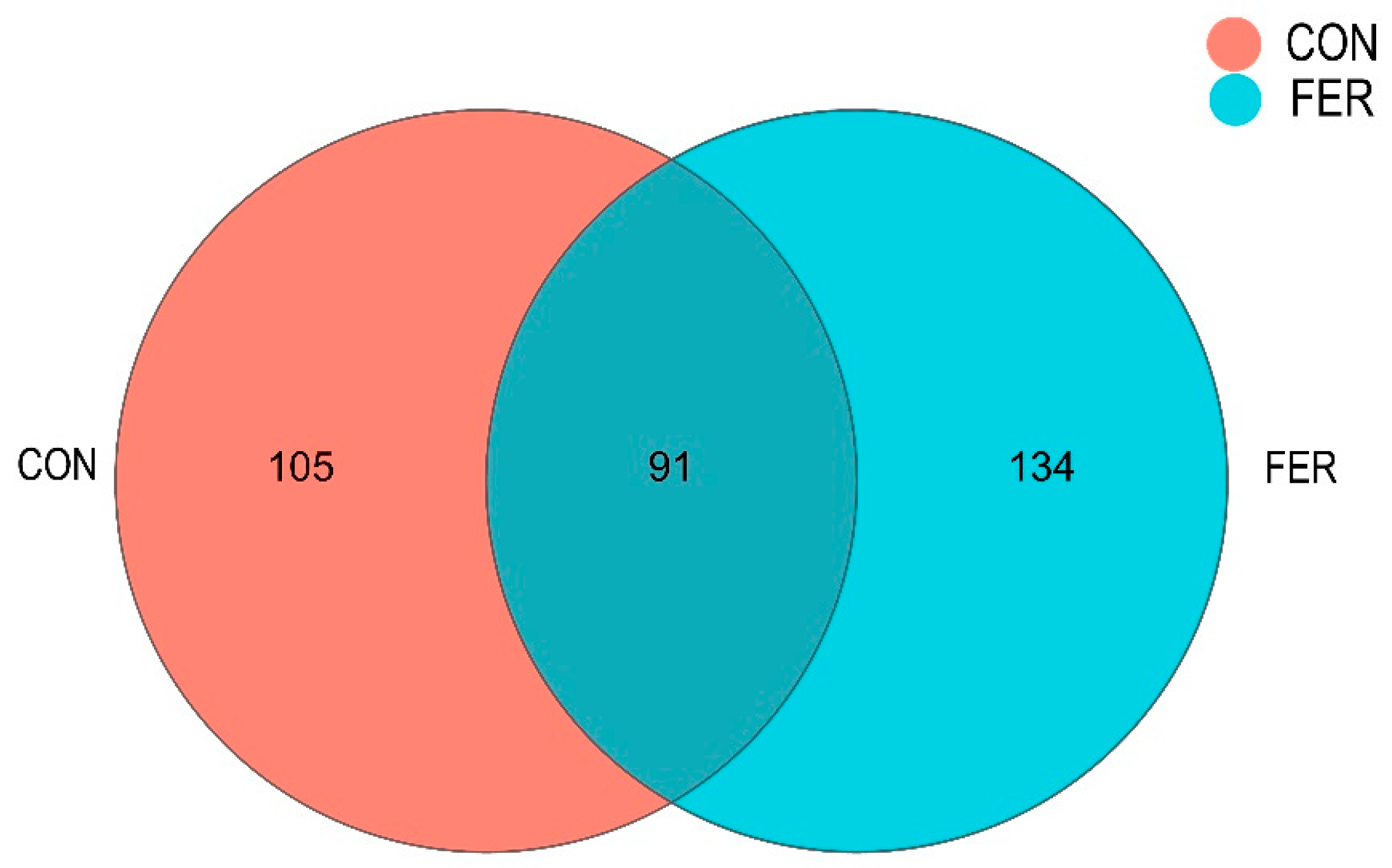
3.5. Intestinal Flora Analysis
3.5.1. Sequencing Data and Diversity Analysis
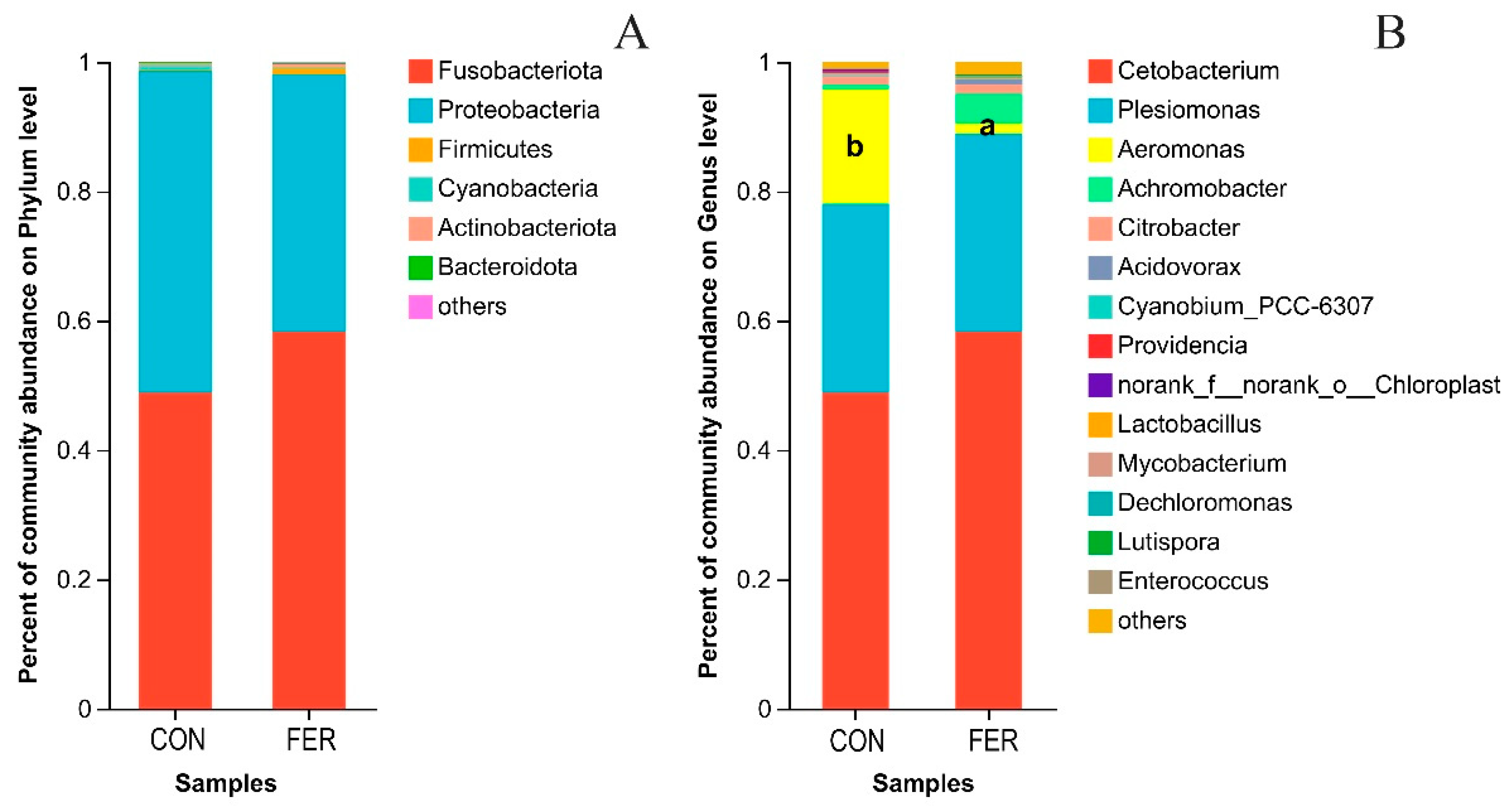
3.5.2. Intestinal Microbial Community Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| % | CON | FER |
|---|---|---|
| C:14 | 5.74 ± 0.12 | 5.62 ± 0.34 |
| C:15 | 0.45 ± 0.02 | 0.67 ± 0.10 |
| C:16 | 17.2 ± 0.46 | 17.16 ± 1.06 |
| C:16-1 | 6.17 ± 0.13 | 5.97 ± 0.23 |
| C:17 | 0.67 ± 0.00 | 0.65 ± 0.05 |
| C:17-1 | 1.03 ± 0.24 | 0.95 ± 0.17 |
| C:18 | 3.99 ± 0.15 | 3.87 ± 0.25 |
| C18:1n-9 | 14.6 ± 0.32 | 14.3 ± 0.95 |
| C18:2n-6 | 17.4 ± 0.03 | 16.0 ± 0.82 |
| C18:3n-3 | 0.79 ± 0.08 | 0.85 ± 0.06 |
| C18:3n-6 | 2.55 ± 0.03 | 2.44 ± 0.03 |
| C20:3n-3 | 1.49 ± 0.06 | 1.37 ± 0.04 |
| C20:5n-3(EPA) | 9.60 ± 0.26 | 9.07 ± 0.40 |
| C20:3n-6 | 1.89 ± 0.10 | 1.77 ± 0.10 |
| C22:6n-3(DHA) | 10.4 ± 0.16 | 10.7 ± 0.58 |
| C24:1n-9 | 0.67 ± 0.09 | 0.39 ± 0.08 |
| C24:6n-3 | 0.97 ± 0.17 | 1.02 ± 0.05 |
| SFA | 28.1 ± 0.51 | 28.0 ± 1.60 |
| MUFA | 22.5 ± 0.15 | 21.9 ± 1.43 |
| PUFA | 45.1 ± 0.11 | 43.2 ± 2.08 |
| n-3 PUFA | 23.3 ± 0.05 | 23.0 ± 1.13 |
| n-6 PUFA | 21.8 ± 0.16 | 20.2 ± 0.95 |
| n-3/n-6 PUFA | 1.07 ± 0.01 | 1.14 ± 0.00 |
References
- Romano, N.; Egnew, N.; Quintero, H.; Kelly, A.; Sinha, A.K. The Effects of Water Hardness on the Growth, Metabolic Indicators and Stress Resistance of Largemouth Bass Micropterus salmoides. Aquaculture 2020, 527, 735469. [Google Scholar] [CrossRef]
- Aguilar, G.L.; Sakmar, J.; Nicholls, A.; Litvak, M.K.; Hess, H.N.; Bruce, T.J.; Montague, H.R.; Kelly, A.M.; Roy, L.A.; Bernal, M.A.; et al. Effects of Temperature and Subspecies during Critical Early Life History Stages of Largemouth Bass (Micropterus salmoides). Aquaculture 2023, 570, 739350. [Google Scholar] [CrossRef]
- Guo, H.Y.; Chen, J.; Yuan, X.M.; Zhang, J.; Wang, J.Y.; Yao, J.Y.; Ge, H.X. The Combined Effect of a Novel Formula of Herbal Extracts on Bacterial Infection and Immune Response in Micropterus salmoides. Front. Microbiol. 2023, 14, 1185234. [Google Scholar] [CrossRef] [PubMed]
- Subhadra, B.; Lochmann, R.; Rawles, S.; Chen, R.G. Effect of Fish-Meal Replacement with Poultry by-Product Meal on the Growth, Tissue Composition and Hematological Parameters of Largemouth Bass (Micropterus salmoides) Fed Diets Containing Different Lipids. Aquaculture 2006, 260, 221–231. [Google Scholar] [CrossRef]
- Loh, J.Y.; Chan, H.K.; Yam, H.C.; In, L.L.A.; Lim, C.S.Y. An Overview of the Immunomodulatory Effects Exerted by Probiotics and Prebiotics in Grouper Fish. Aquacult. Int. 2020, 28, 729–750. [Google Scholar] [CrossRef]
- Wang, J.S.; Zhu, Z.Y.; Tian, S.H.; Fu, H.Y.; Leng, X.J.; Chen, L.M. Dietary Lactobacillus casei K17 Improves Lipid Metabolism, Antioxidant Response, and Fillet Quality of Micropterus salmoides. Animals 2021, 11, 2564. [Google Scholar] [CrossRef]
- Du, R.Y.; Zhang, H.Q.; Chen, J.X.; Zhu, J.; He, J.Y.; Luo, L.; Lin, S.M.; Chen, Y.J. Effects of Dietary Bacillus subtilis DSM 32315 Supplementation on the Growth, Immunity and Intestinal Morphology, Microbiota and Inflammatory Response of Juvenile Largemouth Bass Micropterus salmoides. Aquac. Nutr. 2021, 27, 2119–2131. [Google Scholar] [CrossRef]
- Sun, H.X.; Chen, D.; Cai, H.Y.; Chang, W.; Wang, Z.D.; Liu, G.H.; Deng, X.J.; Chen, Z.M. Effects of Fermenting the Plant Fraction of a Complete Feed on the Growth Performance, Nutrient Utilization, Antioxidant Functions, Meat Quality, and Intestinal Microbiota of Broilers. Animals 2022, 12, 2870. [Google Scholar] [CrossRef]
- Liu, Y.L.; Feng, J.; Wang, Y.M.; Lv, J.; Li, J.H.; Guo, L.J.; Min, Y.N. Fermented Corn–Soybean Meal Mixed Feed Modulates Intestinal Morphology, Barrier Functions and Cecal Microbiota in Laying Hens. Animals 2021, 11, 3059. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y.S. A Review on the Application of Bacillus as Probiotics in Aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Roosta, H.; Masoumi, H.; Farhadi, A.; Jeffs, A. Long-Term Effects of Three Probiotics, Singular or Combined, on Serum Innate Immune Parameters and Expressions of Cytokine Genes in Rainbow Trout during Grow-Out. Fish Shellfish Immunol. 2020, 98, 748–757. [Google Scholar] [CrossRef]
- Kos, B.; Šušković, J.; Vuković, S.A.; Šimpraga, M.; Frece, J.; Matošić, S. Adhesion and Aggregation Ability of Probiotic Strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.P.; Yang, W.T.; Jin, Y.B.; Huang, H.B.; Shi, C.W.; Jiang, Y.L.; Wang, J.Z.; Kang, Y.H.; Wang, C.F.; Yang, G.L. Lactobacillus reuteri Protects Mice against Salmonella typhimurium Challenge by Activating Macrophages to Produce Nitric Oxide. Microb. Pathog. 2019, 137, 103754. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Yao, L.; Su, J.; Fan, R.R.; Zheng, J.Q.; Han, Y.Z. Effects of Lactobacillus plantarum and Its Fermentation Products on Growth Performance, Immune Function, Intestinal PH, and Cecal Microorganisms of Lingnan Yellow Chicken. Poult. Sci. 2023, 102, 102610. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.S.; El-Sayed, A.M.I.; Mohammady, E.Y.; Zaki, M.A.A.; Elkhyat, M.M.; Jarmołowicz, S.; El-Haroun, E.R. Eubiotic Effect of a Dietary Potassium Diformate (KDF) and Probiotic (Lactobacillus acidophilus) on Growth, Hemato-Biochemical Indices, Antioxidant Status and Intestinal Functional Topography of Cultured Nile Tilapia Oreochromis niloticus Fed Diet Free Fishmeal. Aquaculture 2021, 533, 736147. [Google Scholar] [CrossRef]
- Ljubobratovic, U.; Kosanovic, D.; Vukotic, G.; Molnar, Z.; Stanisavljevic, N.; Ristovic, T.; Peter, G.; Lukic, J.; Jeney, G. Supplementation of Lactobacilli Improves Growth, Regulates Microbiota Composition and Suppresses Skeletal Anomalies in Juvenile Pike-Perch (Sander lucioperca) Reared in Recirculating Aquaculture System (RAS): A Pilot Study. Res. Vet. Sci. 2017, 115, 451–462. [Google Scholar] [CrossRef]
- Sagada, G.; Gray, N.; Wang, L.; Xu, B.Y.; Zheng, L.; Zhong, Z.W.; Ullah, S.; Tegomo, A.F.; Shao, Q.J. Effect of Dietary Inactivated Lactobacillus plantarum on Growth Performance, Antioxidative Capacity, and Intestinal Integrity of Black Sea Bream (Acanthopagrus schlegelii) Fingerlings. Aquaculture 2021, 535, 736370. [Google Scholar] [CrossRef]
- Andani, H.R.R.; Tukmechi, A.; Meshkini, S.; Sheikhzadeh, N. Antagonistic Activity of Two Potential Probiotic Bacteria from Fish Intestines and Investigation of Their Effects on Growth Performance and Immune Response in Rainbow Trout (Oncorhynchus mykiss). J. Appl. Ichthyol. 2012, 28, 728–734. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Chaklader, M.R.; Shukry, M.; Ahmed, H.A.; Khallaf, M.A. A Multispecies Probiotic Modulates Growth, Digestive Enzymes, Immunity, Hepatic Antioxidant Activity, and Disease Resistance of Pangasianodon Hypophthalmus Fingerlings. Aquaculture 2023, 563, 738948. [Google Scholar] [CrossRef]
- Liao, H.P.; Liu, P.Q.; Deng, Y.Y.; Zhang, W.Q.; Pan, C.G.; Jia, Y.M.; Long, F.P.; Tang, H.J. Feeding Effects of Low-Level Fish Meal Replacement by Algal Meals of Schizochytrium Limacinum and Nannochloropsis Salina on Largemouth Bass (Micropterus salmoides). Aquaculture 2022, 557, 738311. [Google Scholar] [CrossRef]
- Wang, S.D.; Zou, Z.H.; Wang, Q.J.; Zhou, A.G.; Wang, G.Q.; Xu, G.H.; Zou, J.X. Replacing Starch with Resistant Starch (Laminaria japonica) Improves Water Quality, Nitrogen and Phosphorus Budget and Microbial Community in Hybrid Snakehead (Channa maculata ♀ × Channa argus ♂). Water Environ. Res. 2023, 95, e10836. [Google Scholar] [CrossRef] [PubMed]
- Perez-Velazquez, M.; Gatlin, D.M.; González-Félix, M.L.; García-Ortega, A.; Cruz, C.R.D.; Juárez-Gómez, M.L.; Chen, K.Q. Effect of Fishmeal and Fish Oil Replacement by Algal Meals on Biological Performance and Fatty Acid Profile of Hybrid Striped Bass (Morone crhysops ♀ × M. saxatilis ♂). Aquaculture 2019, 507, 83–90. [Google Scholar] [CrossRef]
- Chen, S.W.; Jiang, X.L.; Liu, N.; Ren, M.C.; Wang, Z.J.; Li, M.K.; Chen, N.S.; Li, S.L. Effects of Dietary Berberine Hydrochloride Inclusion on Growth, Antioxidant Capacity, Glucose Metabolism and Intestinal Microbiome of Largemouth Bass (Micropterus salmoides). Aquaculture 2022, 552, 738023. [Google Scholar] [CrossRef]
- Yao, H.H.; Cha, X.R.; Yun, H.W.; Luo, K.; Fang, L.; Chen, Y.; Gao, W.H.; Tian, J.; Liu, Y.S. Effects of Fermented Feed on Growth Performance, Muscle Quality, Antioxidant Capacity and Intestinal Microflora of Red Swamp Crayfish (Procambarus clarkii). J. Fish. China 2022, 1–15. [Google Scholar]
- Zhu, F.H.; Zhang, B.B.; Li, J.; Zhu, L.Q. Effects of Fermented Feed on Growth Performance, Immune Response, and Antioxidant Capacity in Laying Hen Chicks and the Underlying Molecular Mechanism Involving Nuclear Factor-ΚB. Poult. Sci. 2020, 99, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Pichiah, P.B.T.; Cho, S.H.; Han, S.K.; Cha, Y.S. Fermented Barley Supplementation Modulates the Expression of Hypothalamic Genes and Reduces Energy Intake and Weight Gain in Rats. J. Med. Food 2016, 19, 418–426. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dossou, S.; Wang, W.L.; Zhang, X.X.; Ronick, S.S.; Mzengereza, K.; Zhu, K.H.; et al. Optimization of Soybean Meal Fermentation for Aqua-Feed with Bacillus Subtilis Natto Using the Response Surface Methodology. Fermentation 2021, 7, 306. [Google Scholar] [CrossRef]
- Buglione-Neto, C.; Mouriño, J.L.; Vieira, F.D.N.; Silva, B.C.D.; Jatobá, A.; Seiffert, W.; Fracalossi, D.M.; Andreatta, E. Métodos para determinação da digestibilidade aparente de dietas para camarão marinho suplementadas com probiótico. Pesq. Agropec. Bras. 2013, 48, 1021–1027. [Google Scholar] [CrossRef]
- Al-Dohail, M.A.; Hashim, R.; Aliyu-Paiko, M. Effects of the Probiotic, Lactobacillus Acidophilus, on the Growth Performance, Haematology Parameters and Immunoglobulin Concentration in African Catfish (Clarias gariepinus, Burchell 1822) Fingerling. Aquac. Res. 2009, 40, 1642–1652. [Google Scholar] [CrossRef]
- Anderson, F.H.; Zeng, L.; Rock, N.R.; Yoshida, E.M. An Assessment of the Clinical Utility of Serum ALT and AST in Chronic Hepatitis C. Hepatol. Res. 2000, 18, 63–71. [Google Scholar] [CrossRef]
- Fawole, F.J.; Sahu, N.P.; Shamna, N.; Phulia, V.; Emikpe, B.O.; Adeoye, A.A.; Aderolu, A.Z.; Popoola, O.M. Effects of Detoxified Jatropha Curcas Protein Isolate on Growth Performance, Nutrient Digestibility and Physio-Metabolic Response of Labeo Rohita Fingerlings. Aquac. Nutr. 2018, 24, 1223–1233. [Google Scholar] [CrossRef]
- Pan, W.J.; Miao, L.H.; Lin, Y.; Huang, X.; Ge, X.P.; Moosa, S.L.; Liu, B.; Ren, M.C.; Zhou, Q.L.; Liang, H.L.; et al. Regulation Mechanism of Oxidative Stress Induced by High Glucose through PI3K/Akt/Nrf2 Pathway in Juvenile Blunt Snout Bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2017, 70, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Liu, B.; Li, Z.H.; Sun, C.X.; Zhou, Q.L.; Zheng, X.C.; Sun, M.; Gao, L. Effect of Fermented Feed on Growth Performance, Antioxidant Capacity and Intestinal Microorganisms of Macrobrachium nipponense. Acta Hydrobiol. Sin. 2023, 47, 1435–1445. [Google Scholar] [CrossRef]
- Karbownik, M.; Lewiński, A. Melatonin Reduces Fenton Reaction-Induced Lipid Peroxidation in Porcine Thyroid Tissue. J. Cell. Biochem. 2003, 90, 806–811. [Google Scholar] [CrossRef]
- Wang, J.H.; Jia, R.; Liu, Y.J.; Du, J.L.; Cao, L.P.; Yin, G.J. Effects of Peptides from Swine Blood on Antioxidant Capacity and Lmmune Function of Jian Carp (Cyprinus carpio Var. Jian). Chin. J. Anim. Nutr. 2014, 26, 3689–3697. [Google Scholar] [CrossRef]
- Memudu, A.E.; Dongo, G.A. A Study to Demonstrate the Potential of Anabolic Androgen Steroid to Activate Oxidative Tissue Damage, Nephrotoxicity and Decline Endogenous Antioxidant System in Renal Tissue of Adult Wistar Rats. Toxicol. Rep. 2023, 10, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Pan, L.Q.; Fan, D.P.; He, J.J.; Su, C.; Gao, S.; Zhang, M.Y. Study of Fermented Feed by Mixed Strains and Their Effects on the Survival, Growth, Digestive Enzyme Activity and Intestinal Flora of Penaeus vannamei. Aquaculture 2021, 530, 735703. [Google Scholar] [CrossRef]
- Lin, Y.; Xiao, Q.Z. Dietary Glutamine Supplementation Improves Structure and Function of Intestine of Juvenile Jian Carp (Cyprinus carpio Var. Jian). Aquaculture 2006, 256, 389–394. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Y.M.; Yuan, M.Y.; Liu, Y.; Deng, J.M.; Tan, B.P. Effects of Different Types of Non-Starch Polysaccharides on Growth, Digestive Enzyme Activity, Intestinal Barrier Function and Antioxidant Activity of Tilapia (Oreochromis niloticus). Aquac. Rep. 2022, 25, 101198. [Google Scholar] [CrossRef]
- Solovyev, M.M.; Kashinskaya, E.N.; Izvekova, G.I.; Gisbert, E.; Glupov, V.V. Feeding Habits and Ontogenic Changes in Digestive Enzyme Patterns in Five Freshwater Teleosts: Fry Feeding Habits of Five Siberian Teleosts. J. Fish Biol. 2014, 85, 1395–1412. [Google Scholar] [CrossRef]
- Xu, H.G.; Turchini, G.M.; Francis, D.S.; Liang, M.Q.; Mock, T.S.; Rombenso, A.; Ai, Q.H. Are Fish What They Eat? A Fatty Acid’s Perspective. Prog. Lipid Res. 2020, 80, 101064. [Google Scholar] [CrossRef]
- Eryalçın, K.M.; Roo, J.; Saleh, R.; Atalah, E.; Benítez, T.; Betancor, M.; Hernandez-Cruz, M.D.C.; Izquierdo, M. Fish Oil Replacement by Different Microalgal Products in Microdiets for Early Weaning of Gilthead Sea Bream (Sparus aurata, L.). Aquac. Res. 2013, 44, 819–828. [Google Scholar] [CrossRef]
- Yadav, A.K.; Rossi, W.; Habte-Tsion, H.-M.; Kumar, V. Impacts of Dietary Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) Level and Ratio on the Growth, Fatty Acids Composition and Hepatic-Antioxidant Status of Largemouth Bass (Micropterus salmoides). Aquaculture 2020, 529, 735683. [Google Scholar] [CrossRef]
- Osmond, A.T.Y.; Arts, M.T.; Hall, J.R.; Rise, M.L.; Bazinet, R.P.; Armenta, R.E.; Colombo, S.M. Schizochytrium sp. (T18) Oil as a Fish Oil Replacement in Diets for Juvenile Rainbow Trout (Oncorhynchus mykiss): Effects on Growth Performance, Tissue Fatty Acid Content, and Lipid-Related Transcript Expression. Animals 2021, 11, 1185. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Sørensen, S.L.; Dahle, D.; Nadanasabesan, N.; Dias, J.; Valente, L.M.P.; Sørensen, M.; Kiron, V. Approaches to Improve Utilization of Nannochloropsis Oceanica in Plant-Based Feeds for Atlantic Salmon. Aquaculture 2020, 522, 735122. [Google Scholar] [CrossRef]
- Young, K.L. Omega-6 (n-6) and Omega-3 (n-3) Fatty Acids in Tilapia and Human Health: A Review. Int. J. Food Sci. Nutr. 2009, 60, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Artemis, P. Simopoulos Evolutionary Aspects of Diet: The Omega-6/Omega-3 Ratio and the Brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Liao, H.P. Evaluation of the Application Effect of Two Lactic Acid Bacteria in Nile Tilapia (Oreochromis niloticus) Feed. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2021. [Google Scholar]
- Zhou, Y.L.; He, G.L.; Jin, T.; Chen, Y.J.; Dai, F.Y.; Luo, L.; Lin, S.M. High Dietary Starch Impairs Intestinal Health and Microbiota of Largemouth Bass, Micropterus salmoides. Aquaculture 2021, 534, 736261. [Google Scholar] [CrossRef]
- Picoli, F.; Marques, S.D.O.; Oliveira, A.D.D.; Nunes, C.G.; Serafini, S.; Klein, B.; Oliveira, N.S.D.; Santos, N.N.O.D.; Zampar, A.; Lopes, D.L.D.A.; et al. Mixed Culture Microorganisms Fermented Soybean Meal Improves Productive Performance and Intestinal Health of Nile Tilapia (Oreochromis niloticus) Juveniles Fed Plant-Based Diets in a Biofloc System. Aquac. Res. 2022, 53, 4233–4245. [Google Scholar] [CrossRef]
- Wang, A.R.; Meng, L.; Hao, Q.; Xia, R.; Zhang, Q.S.; Ran, C.; Yang, Y.; Li, D.J.; Liu, W.S.; Zhang, Z.; et al. Effect of Supplementation of Solid-State Fermentation Product of Bacillus Subtilis HGcc-1 to High-Fat Diet on Growth, Hepatic Lipid Metabolism, Epidermal Mucus, Gut and Liver Health and Gut Microbiota of Zebrafish. Aquaculture 2022, 560, 738542. [Google Scholar] [CrossRef]
- Nayak, S.K. Role of Gastrointestinal Microbiota in Fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.J.; Fan, J.T.; Zhou, H.; Zhang, Y.M.; Cao, Y.X.; Jiang, W.; Zhang, W.; Deng, J.M.; Tan, B.P. Effects of Dietary Non-Starch Polysaccharides Level on the Growth, Intestinal Flora and Intestinal Health of Juvenile Largemouth Bass Micropterus salmoides. Aquaculture 2022, 557, 738343. [Google Scholar] [CrossRef]
- Xie, X.Z.; Wang, J.; Guan, Y.; Xing, S.; Liang, X.F.; Xue, M.; Wang, J.J.; Chang, Y.; Leclercq, E. Cottonseed Protein Concentrate as Fishmeal Alternative for Largemouth Bass (Micropterus salmoides) Supplemented a Yeast-Based Paraprobiotic: Effects on Growth Performance, Gut Health and Microbiome. Aquaculture 2022, 551, 737898. [Google Scholar] [CrossRef]
- Zhou, M.; Liang, R.S.; Mo, J.F.; Yang, S.; Na, G.; Wu, Z.H.; Babu, V.S.; Li, J.; Huang, Y.M.; Lin, L. Effects of Brewer’s Yeast Hydrolysate on the Growth Performance and the Intestinal Bacterial Diversity of Largemouth Bass (Micropterus salmoides). Aquaculture 2018, 484, 139–144. [Google Scholar] [CrossRef]
- Zhu, L.X.; Baker, S.S.; Gill, C.; Liu, W.S.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of Gut Microbiomes in Nonalcoholic Steatohepatitis (NASH) Patients: A Connection between Endogenous Alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel Ecological Niche of Cetobacterium Somerae, an Anaerobic Bacterium in the Intestinal Tracts of Freshwater Fish. Lett. Appl. Microbiol. 2007, 46, 282. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Monsalve, V.A.; Junca, H.; García-Bonilla, E.; Montoya-Campuzano, O.I.; Moreno-Herrera, C.X. Characterization of the Gastrointestinal Bacterial Microbiome of Farmed Juvenile and Adult White Cachama (Piaractus brachypomus). Aquaculture 2019, 512, 734325. [Google Scholar] [CrossRef]
- Behera, B.K.; Bera, A.K.; Paria, P.; Das, A.; Parida, P.K.; Kumari, S.; Bhowmick, S.; Das, B.K. Identification and Pathogenicity of Plesiomonas Shigelloides in Silver Carp. Aquaculture 2018, 493, 314–318. [Google Scholar] [CrossRef]
- Wang, E.L.; Yuan, Z.H.; Wang, K.Y.; Gao, D.Y.; Liu, Z.J.; Liles, M.R. Consumption of Florfenicol-Medicated Feed Alters the Composition of the Channel Catfish Intestinal Microbiota Including Enriching the Relative Abundance of Opportunistic Pathogens. Aquaculture 2019, 501, 111–118. [Google Scholar] [CrossRef]
- Liu, W.S.; Wang, W.W.; Ran, C.; He, S.X.; Yang, Y.L.; Zhou, Z.G. Effects of Dietary ScFOS and Lactobacilli on Survival, Growth, and Disease Resistance of Hybrid Tilapia. Aquaculture 2017, 470, 50–55. [Google Scholar] [CrossRef]

| Feed Ingredients | Basic Diets (%) | ||
|---|---|---|---|
| Fish meal | 50.0 | ||
| Soybean meal | 13.0 | ||
| Soybean isolate protein | 10.0 | ||
| Fish oil | 7.00 | ||
| High-gluten flour | 15.0 | ||
| Monocalcium phosphate | 1.00 | ||
| Choline chloride | 0.20 | ||
| Zeolite powder | 1.80 | ||
| Multi-dimensional multi-mineral premixes 1 | 2.00 | ||
| Total | 100 | ||
| Nutritional composition | Basic diets (CON) | Fermented feed (FER) | |
| Moisture/% | 3.80 ± 0.56 | 3.78 ± 0.34 | |
| Crude Protein/% | 51.5 ± 0.13 a | 54.5 ± 0.52 b | |
| Crude Lipid/% | 10.9 ± 1.74 | 9.58 ± 0.67 | |
| Crude Ash/% | 13.5 ± 0.57 | 12.6 ± 0.51 | |
| CON | FER | |
|---|---|---|
| Growth performance | ||
| IBW, g | 5.25 ± 0.04 | 5.34 ± 0.03 |
| FBW, g | 52.2 ± 1.12 | 56.0 ± 0.49 |
| WGR, % | 892.4 ± 39.6 | 949.5 ± 10.5 |
| SGR, % | 3.95 ± 0.17 | 4.04 ± 0.08 |
| FI, %/day | 2.82 ± 0.02 b | 2.14 ± 0.06 a |
| FE | 0.99 ± 0.03 a | 1.33 ± 0.04 b |
| SR, % | 92.7 ± 6.36 | 92.0 ± 4.62 |
| PER, % | 1.92 ± 0.06 a | 2.46 ± 0.12 b |
| Whole-body composition (%) | ||
| Moisture/% | 69.7 ± 1.00 | 70.0 ± 0.45 |
| Crude protein/% | 17.8 ± 0.16 | 18.0 ± 0.20 |
| Crude lipid/% | 3.64 ± 0.29 | 3.64 ± 0.11 |
| Crude ash/% | 6.81 ± 0.20 | 6.82 ± 0.11 |
| Muscle composition (%) | ||
| Moisture/% | 77.6 ± 0.38 | 77.2 ± 0.34 |
| Crude protein/% | 20.3 ± 0.09 | 20.3 ± 0.22 |
| Crude lipid/% | 1.33 ± 0.08 | 1.39 ± 0.19 |
| Crude ash/% | 1.14 ± 0.07 | 1.15 ± 0.02 |
| % | CON | FER |
|---|---|---|
| C:14 | 3.21 ± 0.12 | 3.39 ± 0.25 |
| C:15 | 0.32 ± 0.00 | 0.34 ± 0.02 |
| C:16 | 20.4 ± 0.25 | 20.7 ± 0.27 |
| C:16-1 | 5.31 ± 0.14 | 5.51 ± 0.31 |
| C:17 | 0.62 ± 0.05 | 0.47 ± 0.03 |
| C:17-1 | / | / |
| C:18 | 4.71 ± 0.09 | 4.28 ± 0.24 |
| C18:1n-9 (Oleic acid) | 16.9 ± 0.25 | 17.9 ± 0.39 |
| C18:2n-6 (linoleic acid) | 13.5 ± 0.24 | 13.6 ± 0.28 |
| C18:3n-3 (linolenic acid) | 0.82 ± 0.03 | 0.79 ± 0.01 |
| C18:3n-6 | 1.23 ± 0.07 | 1.38 ± 0.10 |
| C20:3n-3 | 1.65 ± 0.12 b | 1.23 ± 0.08 a |
| C20:5n-3 (EPA) | 5.17 ± 0.12 | 4.72 ± 0.17 |
| C20:3n-6 | 0.45 ± 0.04 | 0.43 ± 0.00 |
| C22:6n-3 (DHA) | 23.8 ± 0.31 | 23.3 ± 1.11 |
| C24:6n-3 | 2.57 ± 0.05 | 2.51 ± 0.07 |
| C24:1n-9 | 0.57 ± 0.12 | 0.61 ± 0.03 |
| SFA | 28.8 ± 0.19 | 28.7 ± 0.29 |
| MUFA | 22.6 ± 0.27 | 23.6 ± 0.68 |
| PUFA | 48.3 ± 0.22 | 46.9 ± 1.03 |
| n-3PUFAs | 33.5 ± 0.41 | 31.8 ± 1.12 |
| n-6PUFAs | 14.9 ± 0.26 | 15.2 ± 0.3 |
| n-3/n-6 PUFAs | 2.25 ± 0.07 | 2.11 ± 0.1 |
| % | CON | FER |
|---|---|---|
| C:14 | 3.07 ± 0.24 | 2.94 ± 0.33 |
| C:15 | 0.31 ± 0.02 | 0.24 ± 0.04 |
| C:16 | 18.7 ± 0.35 b | 17.4 ± 0.19 a |
| C:16-1 | 6.00 ± 0.28 | 5.56 ± 0.32 |
| C:17 | 0.58 ± 0.04 | 0.54 ± 0.04 |
| C:17-1 | 0.47 ± 0.14 | 0.34 ± 0.05 |
| C:18 | 5.6 ± 0.43 | 5.68 ± 0.16 |
| C18:1n-9 (Oleic acid) | 20.2 ± 1.07 | 19.8 ± 1.43 |
| C18:2n-6 (linoleic acid) | 9.25 ± 0.56 b | 7.71 ± 0.20 a |
| C18:3n-3 (linolenic acid) | 1.07 ± 0.05 | 1.10 ± 0.06 |
| C18:3n-6 | 1.07 ± 0.2 b | 0.63 ± 0.06 a |
| C20:3n-3 | 1.53 ± 0.19 a | 2.60 ± 0.15 b |
| C20:5n-3 (EPA) | 2.67 ± 0.14 | 2.60 ± 0.18 |
| C20:3n-6 | 0.55 ± 0.02 | 0.69 ± 0.07 |
| C22:6n-3 (DHA) | 21.4 ± 1.36 a | 27.1 ± 1.38 b |
| C24:6n-3 | 2.08 ± 0.10 | 1.85 ± 0.10 |
| C24:1n-9 | 0.52 ± 0.04 | 0.58 ± 0.02 |
| SFA | 26.7 ± 1.37 | 26.7 ± 0.51 |
| MUFA | 27.0 ± 1.12 | 26.2 ± 1.69 |
| PUFA | 37.1 ± 1.3 | 40.7 ± 1.46 |
| n-3 PUFAs | 29.1 ± 1.53 a | 35.2 ± 1.65 b |
| n-6 PUFAs | 11.0 ± 0.37 b | 9.04 ± 0.23 a |
| n-3/n-6 PUFAs | 2.49 ± 0.15 a | 3.76 ± 0.14 b |
| Diet | Read | Coverage (%) | Ace Index | Chao1 Index | Shannon Index | Simpson Index |
|---|---|---|---|---|---|---|
| CON | 38,657 ± 3799 | 0.999 ± 0.00 | 110.17 ± 40.17 | 107.14 ± 40.18 | 1.17 ± 0.15 | 0.39 ± 0.04 |
| FER | 38,567 ± 5283 | 0.999 ± 0.00 | 123.82 ± 23.73 | 125.11 ± 27.2 | 0.98 ± 0.09 | 0.56 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Liu, P.; Kong, Q.; Deng, Y.; Zhang, W.; Xu, G.; Tang, H. Effects of Co-Fermented Feed Using Lactobacillus acidophilus, Limosilactobacillus reuteri and Lactiplantibacillus plantarum on Growth, Antioxidant Capacity, Fatty Acids and Gut Microbiota of Largemouth Bass (Micropterus salmoides). Fishes 2023, 8, 433. https://doi.org/10.3390/fishes8090433
Yang Z, Liu P, Kong Q, Deng Y, Zhang W, Xu G, Tang H. Effects of Co-Fermented Feed Using Lactobacillus acidophilus, Limosilactobacillus reuteri and Lactiplantibacillus plantarum on Growth, Antioxidant Capacity, Fatty Acids and Gut Microbiota of Largemouth Bass (Micropterus salmoides). Fishes. 2023; 8(9):433. https://doi.org/10.3390/fishes8090433
Chicago/Turabian StyleYang, Zixin, Peiqin Liu, Qing Kong, Yongyan Deng, Wenqi Zhang, Guohuan Xu, and Huijuan Tang. 2023. "Effects of Co-Fermented Feed Using Lactobacillus acidophilus, Limosilactobacillus reuteri and Lactiplantibacillus plantarum on Growth, Antioxidant Capacity, Fatty Acids and Gut Microbiota of Largemouth Bass (Micropterus salmoides)" Fishes 8, no. 9: 433. https://doi.org/10.3390/fishes8090433
APA StyleYang, Z., Liu, P., Kong, Q., Deng, Y., Zhang, W., Xu, G., & Tang, H. (2023). Effects of Co-Fermented Feed Using Lactobacillus acidophilus, Limosilactobacillus reuteri and Lactiplantibacillus plantarum on Growth, Antioxidant Capacity, Fatty Acids and Gut Microbiota of Largemouth Bass (Micropterus salmoides). Fishes, 8(9), 433. https://doi.org/10.3390/fishes8090433