First Records with Biological Notes of Umbrina ronchus, Valenciennes, 1843 (Osteichthyes, Sciaenidae) in the Strait of Sicily (Central Mediterranean Sea)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Schematic Overview of the Experimental Study
2.2. Sample Collection and Identification
2.3. Morphometric Measurements
2.4. Otolithometry and Age Estimation
2.5. Geographical Distribution
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Froese, R.; Pauly, D. (Eds.) FishBase. Sciaenidae Cuvier, 1829. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=125558 (accessed on 15 February 2023).
- Nelson, J.S. Fishes of the World, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; p. 372. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) ECoF. In Eschmeyer’s Catalog of Fishes: Genera, Species, References; California Academy of Sciences: San Francisco, CA, USA; Available online: http://researcharchive.calacademy.org/research/Ichthyology/catalog/fishcatmain.asp (accessed on 21 February 2023).
- Heemstra, P.C. Sciaenidae. In Smiths’ Sea Fishes; Smith, M.M., Heemstra, P.C., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 1986; pp. 616–619. [Google Scholar]
- Sasaki, K. Phylogeny of the family Sciaenidae, with notes on its zoogeography (Teleostei, Perciformes). Mem. Fac. Fish. Hokkaido Univ. 1989, 36, 1–137. [Google Scholar]
- Parenti, P. An annotated checklist of fishes of the family Sciaenidae. J. Animal Divers. 2020, 2, 1–92. [Google Scholar] [CrossRef]
- Chao, L.N. Sciaenidae. In Fishes of the Northern-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, C., Nielsen, J., Tortonese, Eds.; Unesco: Paris, France, 1986; pp. 865–874. [Google Scholar]
- Tortonese, E. Fauna d’Italia. Osteichthyes; Edizioni Calderini: Bologna, Italy, 1975; p. 636. [Google Scholar]
- Harmelin, J.G. Statut du corb (Sciaena umbra) en Méditerranée. In Les Espèces Marines à Protéger en Méditerranée; Boudouresque, C.F., Avon, M., Gravez, V., Eds.; GIS Posidonie: Paris, France, 1991; pp. 219–227. [Google Scholar]
- Chakroun, N.; Ktari, M.H.; Kamoun, M.N. Production des Sciaenidae (Poissons, Teleostéens) des côtes tunisiennes. Bull. L’institut Natl. Sci. Tech. D’oceanographie Peche Salammbo 1982, 9, 121–126. [Google Scholar]
- Walker, H.J., Jr.; Radford, K.W. Eastern Pacific species of the genus Umbrina (Pisces: Sciaenidae) with a description of a new species. Especies del Pacífico oriental del género Umbrina (Pisces: Sciaenidae) con la descripción de una nueva especie. Fish. Bull. 1992, 90, 574–587. [Google Scholar]
- Randall, J.E. Coastal Fishes of Oman; Crawford House Publishing Pty Ltd.: Bathurst, Australia, 1995; p. 439. [Google Scholar]
- Sasaki, K. Sciaenid fishes of the Indian Ocean (Teleostei, Perciformes). Mem. Fac. Sci. Kochi Univ. Ser. D 1996, 16, 83–95. [Google Scholar]
- Bianchi, G.; Carpenter, K.E.; Roux, J.P.; Molloy, F.J.; Boyer, D.; Boyer, H.J. FAO species identification guide for fishery purposes. In Field Guide to the Living Marine Resources of Namibia; FAO: Rome, Italy, 1999; p. 265. [Google Scholar]
- Garofalo, G.; Gristina, M.; Toccaceli, M.; Giusto, G.B.; Rizzo, P.; Sinacori, G. Geostatistical modelling of biocenosis distribution in the Strait of Sicily. GIS/Spatial Analyses in Fishery and Acquatic Science. In Proceeding of the Second International Symposium on GIS/Spatial Analyses in Fishery and Aquatic Sciences, University of Sussex, Brighton, UK, 3–6 September 2004. [Google Scholar]
- Tortonese, E. Fauna d’Italia. Osteichthyes, 2nd ed.; Calderini: Bologna, Italy, 1970; p. 636. [Google Scholar]
- Chao, L.N.; Trewavas, E. Sciaenidae. In FAO Species Identification Sheets for Fishery Purposes. Eastern Central Atlantic (Fishing Areas 34 and 47 in Part); Fischer, W., Bianchi, G., Scott, W.B., Eds.; FAO: Rome, Italy, 1981; pp. 1–7. [Google Scholar]
- Dardignac, J. Les ombrines des côtes atlantiques du Maroc (avec remarques sur les types conservés au Muséum national d’Histoire naturelle). Rev. Trav. Inst. Peches Marit. 1961, 25, 263–279. [Google Scholar]
- Palmer, G. Additional notes on some Umbrinine fishes (Family Sciaenidae). Ann. Mag. Nat. 1966, 9, 423–427. [Google Scholar] [CrossRef]
- Hutchings, K.; Griffiths, M.H. Identity and distribution of southern African sciaenid fish species of the genus Umbrina. Afr. J. Mar. Sci. 2005, 27, 1–21. [Google Scholar] [CrossRef]
- Follesa, M.C.; Carbonara, P. Atlas of the Maturity Stages of Mediterranean Fishery Resources; FAO Studies and Reviews: Rome, Italy, 2019; p. 268. [Google Scholar]
- Secor, D.H.; Dean, J.M.; Laban, E.H. Otolith removal and preparation for microstructural examination. Otolith microstructure examination and analysis. Can. J. Fish. Aquat. Sci. 1992, 117, 19–57. [Google Scholar]
- Sardo, G.; Geraci, M.L.; Falsone, F.; Gancitano, S.; Gancitano, V.; Scannella, D.; Okpala, C.O.R.; Titone, A.; Vitale, S. First recordand otolith morphometric description of an adult lightfish, Ichthyococcus ovatus (Actinopterygii: Stomiiformes: Phosichthyidae), caught in the Strait of Sicily (central Mediterranean Sea). Acta Ichthyol. Piscat. 2022, 52, 159–166. [Google Scholar] [CrossRef]
- Harvey, J.T.; Oughlin, T.R.; Perez, M.A.; Oxman, D.S. Relationship between Fish Size and Otolith Length for 63 Species of Fishes from the Eastern North Pacific Ocean; NOAA Technical Report; U.S. Department of Commerce: Seattle, WA, USA, 2000; p. 36. [Google Scholar]
- Russ, J.C. Computer-Assisted Microscopy: The Measurement and Analysis of Images; Plenum Press: New York, NY, USA, 1990; p. 453. [Google Scholar]
- Tuset, V.M.; Lozano, I.J.; González, J.A.; Pertusa, J.F.; García-Díaz, M.M. Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L., 1758). J. Appl. Ichthyol. 2003, 19, 88–93. [Google Scholar] [CrossRef]
- Pavlov, D.A. Differentiation of three species of the genus Upeneus (Mullidae) based on otolith shape analysis. J. Ichthyol. 2016, 56, 37–51. [Google Scholar] [CrossRef]
- Lombarte, A.; Cruz, A. Otolith size trends in marine communities from different depth strata. J. Fish Biol. 2007, 71, 53–76. [Google Scholar] [CrossRef]
- Lombarte, A.; Chic, V.; Parisi-Baradad, R.; Olivella, J.; Garca-Ladona, P.; Garca-Ladona, E. A web-based environment from shape analysis of fish otoliths. The AFORO database. Sci. Mar. 2006, 70, 147–152. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2022. Available online: https://www.R-project.org/ (accessed on 20 July 2022).
- Colloca, F.; Scannella, D.; Geraci, M.L.; Falsone, F.; Giusto, B.; Vitale, S.; Di Lorenzo, M.; Βοnο, G. British sharks in Sicily: Records of long distance migration of tope shark (Galeorhinus galeus) from North-eastern Atlantic to Mediterranean Sea. Med. Mar. Sci. 2019, 20, 309. [Google Scholar] [CrossRef]
- Geraci, M.L.; Di Lorenzo, M.; Falsone, F.; Scannella, D.; Di Maio, F.; Colloca, F.; Vitale, S.; Serena, F. The occurrence of Norwegian skate, Dipturus nidarosiensis (Elasmobranchii: Rajiformes: Rajidae), in the Strait of Sicily, central Mediterranean. Acta Ichthyol. Piscat. 2019, 49, 203–208. [Google Scholar] [CrossRef]
- Geraci, M.L.; Scannella, D.; Falsone, F.; Gancitano, V.; Gancitano, S.; Sardo, G.; Vitale, S. First record of Trachipterus trachypterus Gmelin 1789 (Lampriformes) in the Strait of Sicily. Acta Adriat. 2022, 63, 231–240. [Google Scholar] [CrossRef]
- GBIF. Global Biodiversity Information Facility. 2023. Available online: https://www.gbif.org (accessed on 5 January 2023).
- Smale, M.J.; Watson, G.; Hecht, T. Otolith Atlas of Southern African Marine Fishes; Ichthyological Monographs of the J.L.B. Smith Institute of Ichthyology: Southern, Africa, 1995; p. 253. [Google Scholar]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Bauchot, M.L.; Desoutter, M. Catalogue critique des types de poissons du Muséum national d’Histoire naturelle. (Suite) (Famille des Sciaenidae). In Bulletin du MUSÉUM NATIOnal D’histoire Naturelle; Series 4, Section A, Zoologie, biologie et écologie animals; Paris Muséum National D’histoire Naturelle: Paris, France, 1987; Volume 9, pp. 1–43. [Google Scholar]
- Trewavas, E. The Sciaenid Fishes with a Single Mental Barbel. Copeia 1964, 1964, 107–117. [Google Scholar] [CrossRef]
- Schwarzhans, W. A Comparative Morphological Treatise of Recent and Fossil Otoliths of the Family Sciaenidae (Perciformes); Verlag Dr. Friedrich Pfeil: Munich, Germany, 1993; p. 245. [Google Scholar]
- Morat, F.; Letourneur, Y.; Nérini, D.; Banaru, D.; Batjakas, I.E. Discrimination of red mullet populations (Teleostean, Mullidae) along multi-spatial and ontogenetic scales within the Mediterranean basin on the basis of otolith shape analysis. Aquat. Living Resour. 2012, 25, 27–39. [Google Scholar] [CrossRef]
- Lombarte, A.; Lleonart, J. Otolith size changes related with body growth, habitat depth and temperature. Environ. Biol. Fishes 1993, 37, 297–306. [Google Scholar] [CrossRef]
- Torres, G.J.; Lombarte, A.; Morales-Nin, B. Saggital otolith size and shape variability to identify geographical intraspecific differences in three species of genus Merluccius. J. Mar. Biol. Assoc. U.K. 2000, 80, 333–342. [Google Scholar] [CrossRef]
- Özpiçak, M.; Saygin, S.; Aydin, A.; Hancer, E.; Yilmaz, S.; Polat, N. Otolith shape analyses of Squalius cephalus (Linnaeus, 1758) (Actinopterygii: Cyprinidae) inhabiting four inland water bodies of the middle Black Sea region, Turkey. Iran. J. Ichthyol. 2018, 5, 293–302. [Google Scholar]
- Campana, S.E. Otolith elemental composition as a natural marker of fish stocks. In Stock Identification Methods; Cadrin, S.X., Friedland, K.D., Waldman, J.R., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2005; pp. 227–245. [Google Scholar]
- Sieli, G.; Badalucco, C.; Di Stefano, G.; Rizzo, P.; D’Anna, G.; Fiorentino, F. Biology of Red mullet, Mullus barbatus (L. 1758), in the Gulf of Castellammare (NW Sicily, Mediterranean Sea) subjected to a trawling ban. J. Appl. Ichthyol. 2011, 27, 1218–1225. [Google Scholar] [CrossRef]
- Jones, C.M.; Wells, B. Age, growth, and mortality of black drum, Pogonias cromis, in the Chesapeake Bay region. Fish. Bull. 1998, 96, 451–461. [Google Scholar]
- Artüz, M.L. Abundance and growth observations of Sciaena umbra Linnaeus, 1758 in Sea of Marmara. Hydrobiologica 2006, 1a, 124–128. [Google Scholar]
- Chater, I.; Romdhani-Dhahri, A.; Dufour, J.L.; Mahé, K.; Chakroun-Marzouk, N. Age, growth and mortality of Sciaena umbra (Sciaenidae) in the Gulf of Tunis. Sci. Mar. 2018, 82, 17–25. [Google Scholar] [CrossRef]
- Fiorentino, F.; Camilleri, M.; Bono, G.; Gancitano, S.; Giusto, G.B.; Ragonese, S.; Rizzo, P.; Rosso, B. On a spawning aggregation of the brown meagre Sciaena umbra L. 1758 (Sciaenidae, Osteichthyes) in the Maltese waters (Sicilian Channel—Central Mediterranean). Rapp. Commun. Int. Mer Médit. 2001, 36, 266. [Google Scholar]
- La Mesa, M.; Colella, S.; Giannetti, G.; Arneri, E. Age and growth of brown meagre Sciaena umbra (Sciaenidae) in the Adriatic Sea. Aquat. Living Resour. 2008, 21, 153–161. [Google Scholar] [CrossRef]
- Fischer, W.; Bauchot, M.L.; Schneider, M. Fiches FAO d’ Identification des Espèces pour les Besoins de la Pêche. (Revision 1). Méditerranée et mer Noire. Zone de Pêche 37; Végëtaux et Invertébrés. Publication préparée par la FAO, résultant d’un accord entre la FAO et la Commission des Communautés européennes (Project GCP/INT/422/EEC) financëe conjointement par ces deux organisations; FAO: Rome, Italy, 1987; Volume 1, p. 760. [Google Scholar]
- Chao, L.N.; Trewavas, E. Sciaenidae. In Check-List of the Fishes the Eastern Tropical Atlantic (CLOFETA); Quero, J.C., Hureau, J.C., Karrer, C., Post, A., Saldanha, L., Eds.; JNICT: Lisbon, Portugal; Madrid, Spain; Paris, France; UNESCO: Paris, France, 1990; Volume 2, pp. 813–826. [Google Scholar]
- Bertrand, J.; Relini, G. Demersal resources in the Mediterranean. In Proceedings of the Symposium Actes Colloq, Pisa, Italy, 18–21 March 1998. [Google Scholar]
- Zagami, G.; Badalamenti, F.; Guglielmo, L.; Manganaro, A. Short-term variations of the zooplankton community near the Straits of Messina (North-eastern Sicily): Relationships with the hydrodynamic regime. Estuar. Coast. Shelf Sci. 1996, 42, 667–681. [Google Scholar] [CrossRef]
- Montenat, C.; Barrier, P.; Di Geronimo, S.I. The Messina Strait, Past and Present: A Review.; Documents et Travaux Institut Geologique Albert De Lapparent: Paris, France, 1987; pp. 7–13. [Google Scholar]
- Fredj, G.; Giaccone, G. Particularités des peuplements benthiques du détroit de Messine. In The Strait of Messina Ecosystem, Guglielmo, L., Manganaro, A., De Domenico, E., Eds.; Dipartimento di Biologia Animale ed Ecologia Marina, University of Messina: Messina, Italy, 1995; pp. 119–128. [Google Scholar]
- De Domenico, F.; Giacobbe, S.; Rinelli, P. The genus Antedon (Crinoidea, Echinodermata) in the Strait of Messina and the nearby Tyrrhenian Sea (Central Mediterranean). Ital. J. Zool. 2009, 76, 70–75. [Google Scholar] [CrossRef]
- Robinson, A.R.; Golnaraghi, M.; Leslie, W.G.; Artegiani, A.; Hecht, A.; Lazzoni, E.; Michelato, A.; Sansone, E.; Theocharis, A.; Ünlüata, Ü. The eastern Mediterranean general circulation: Features, structure and variability. Dyn. Atmos. 1991, 15, 215–240. [Google Scholar] [CrossRef]
- Lermusiaux, P.F.J.; Robinson, A.R. Features of dominant mesoscale variability, circulation patterns and dynamics in the Strait of Sicily. Deep Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 1953–1997. [Google Scholar] [CrossRef]
- Agostini, V.N.; Bakun, A. Ocean triads’ in the Mediterranean Sea: Physical mechanisms potentially structuring reproductive habitat suitability (with example application to European anchovy, Engraulis encrasicolus). Fish. Oceanogr. 2002, 11, 129–142. [Google Scholar] [CrossRef]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar] [CrossRef]
- Scannella, D.; Falsone, F.; Geraci, M.L.; Froglia, C.; Fiorentino, F.; Giusto, G.B.; Zava, B.; Insacco, G.; Colloca, F. First report of Northern brown shrimp Penaeus aztecus Ives, 1891 in Strait of Sicily. BioInvasions Rec. 2017, 6, 67–72. [Google Scholar] [CrossRef]
- Geraci, M.L.; Falsone, F.; Scannella, D.; Vitale, S. An additional record of the non-indigenous species (NIS) Seriola fasciata from the southern coast of Sicily (Central Mediterranean Sea. Acta Adriat. 2020, 61, 223–230. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In Life in the Mediterranean Sea: A Look at Habitat Changes; Stambler, N., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 1–55. [Google Scholar]
- Geraci, M.L.; Scannella, D.; Falsone, F.; Colloca, F.; Vitale, S.; Rizzo, P.; Di Maio, F.; Milisenda, G.; Fiorentino, F. Preliminary study on the biological traits of the Por’s goatfish Upeneus pori (Chordata: Actinopterygii) off the southern coast of Lampedusa Island (Central Mediterranean). Eur. Zool. J. 2018, 85, 231–241. [Google Scholar] [CrossRef]


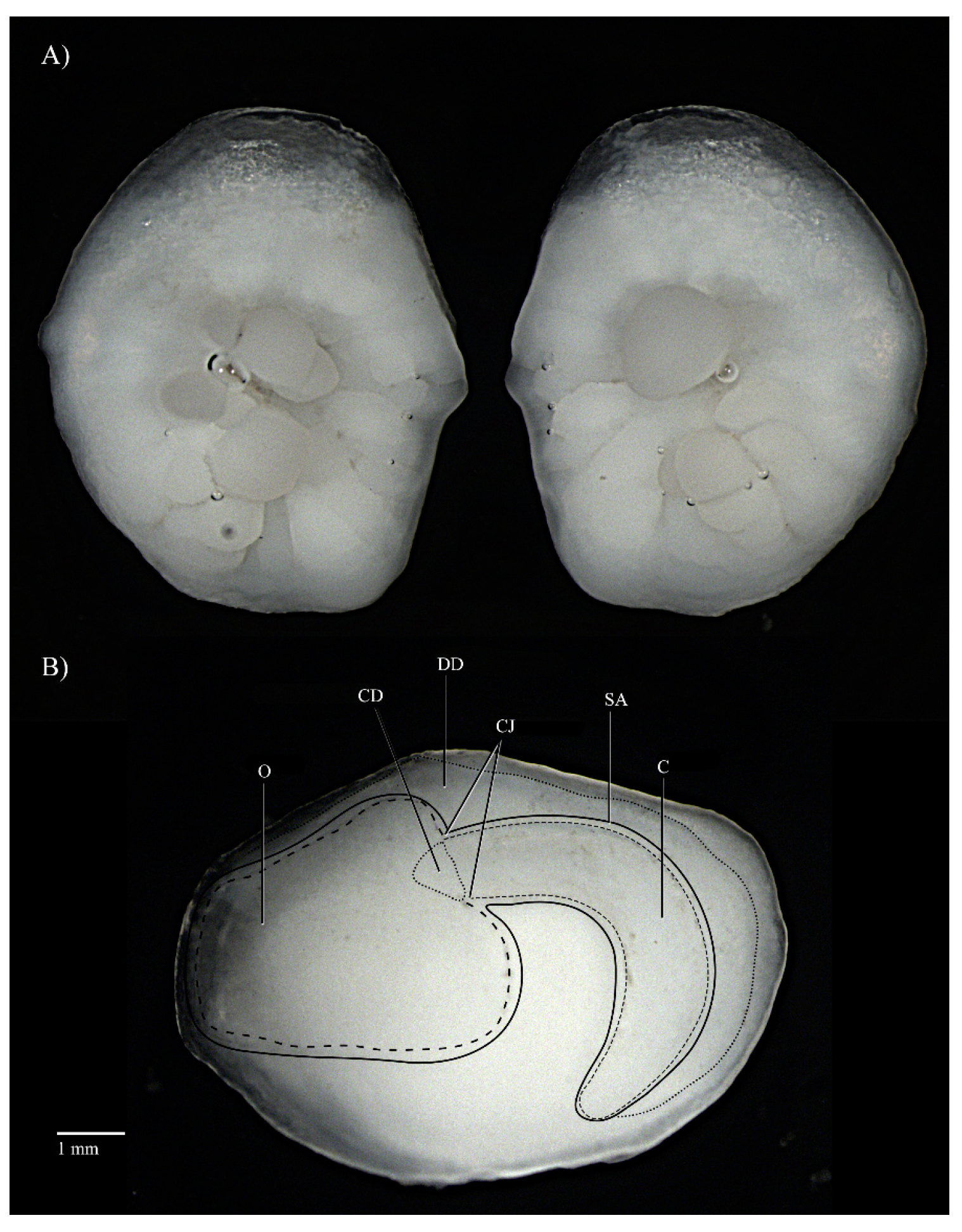
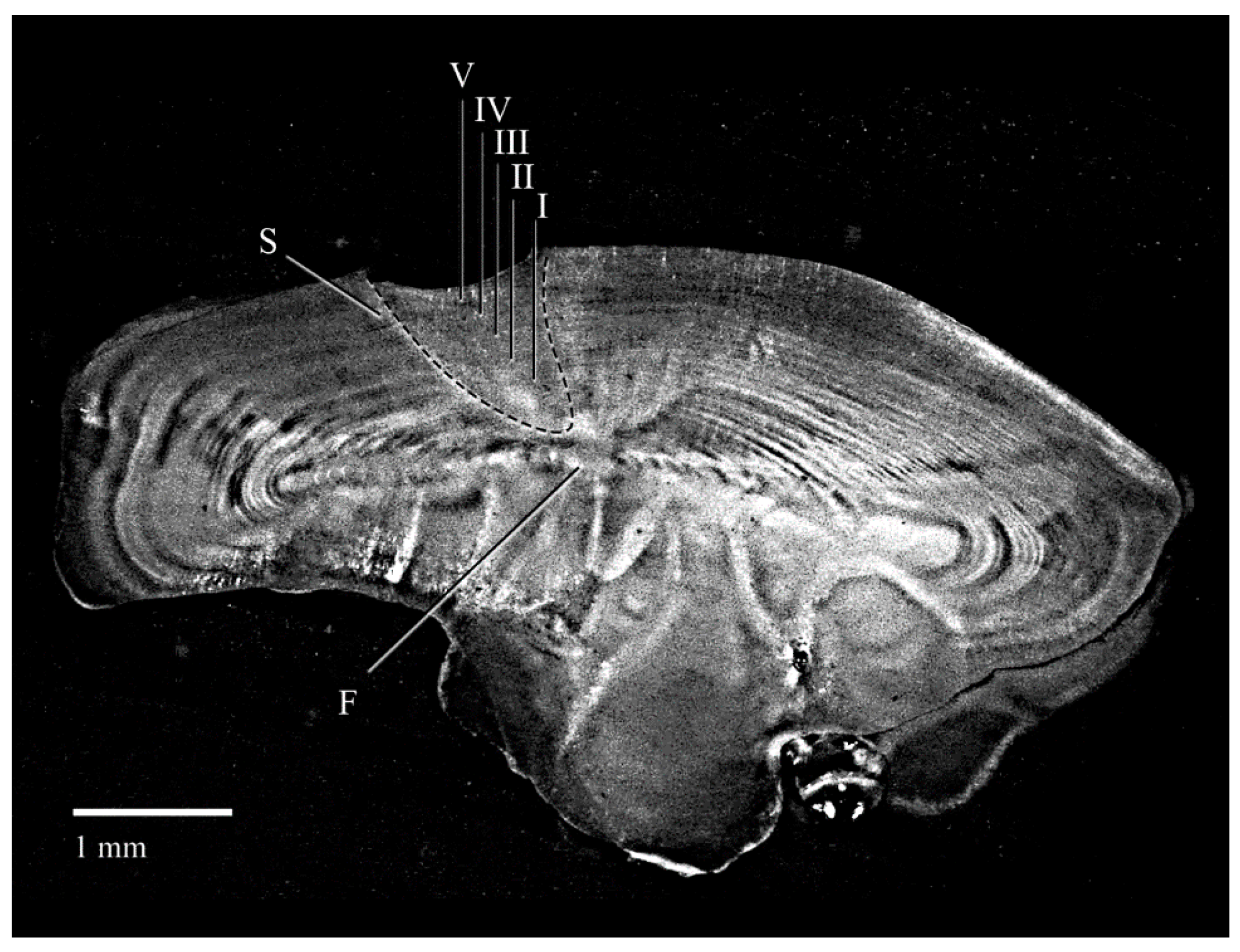
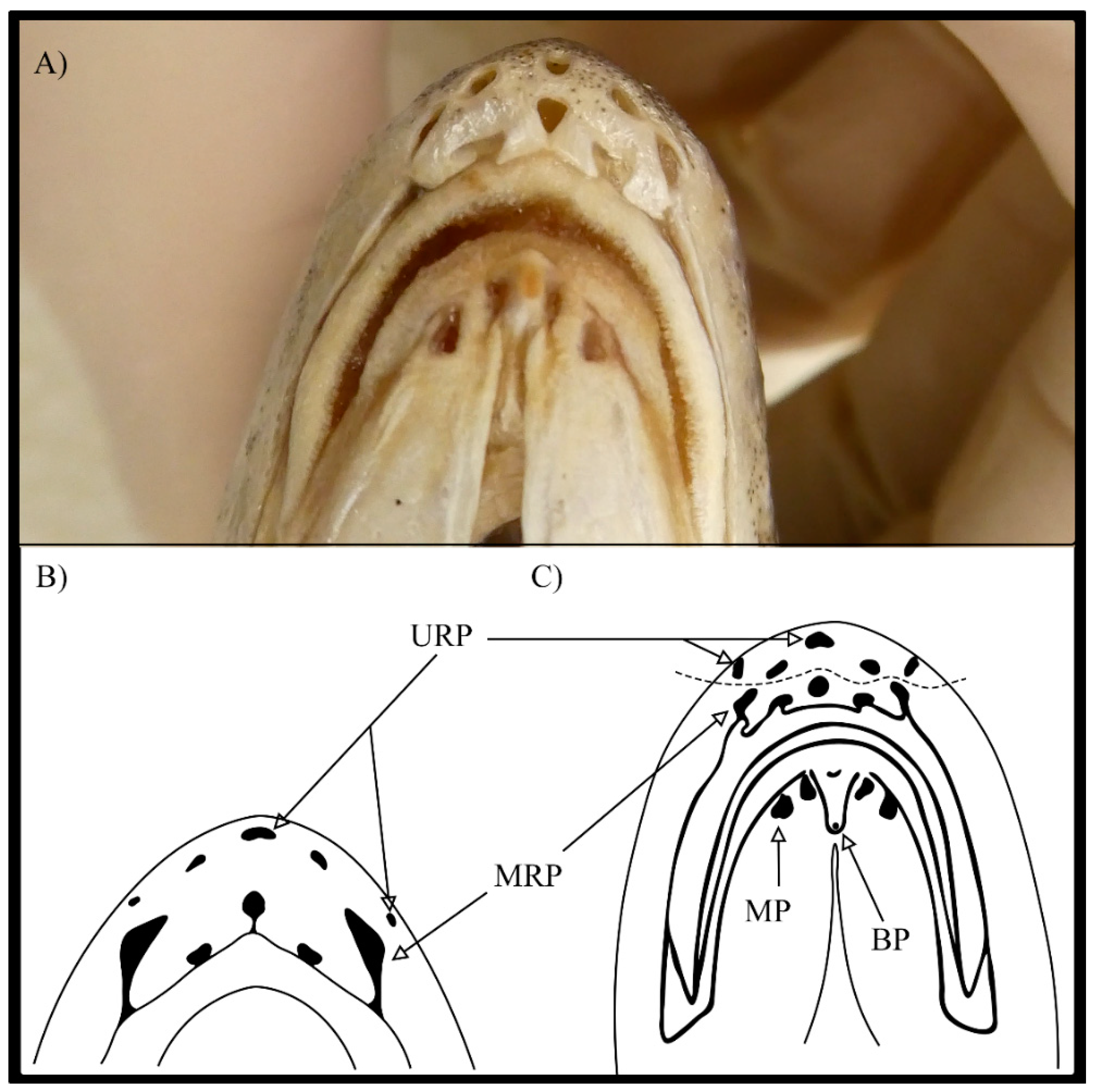
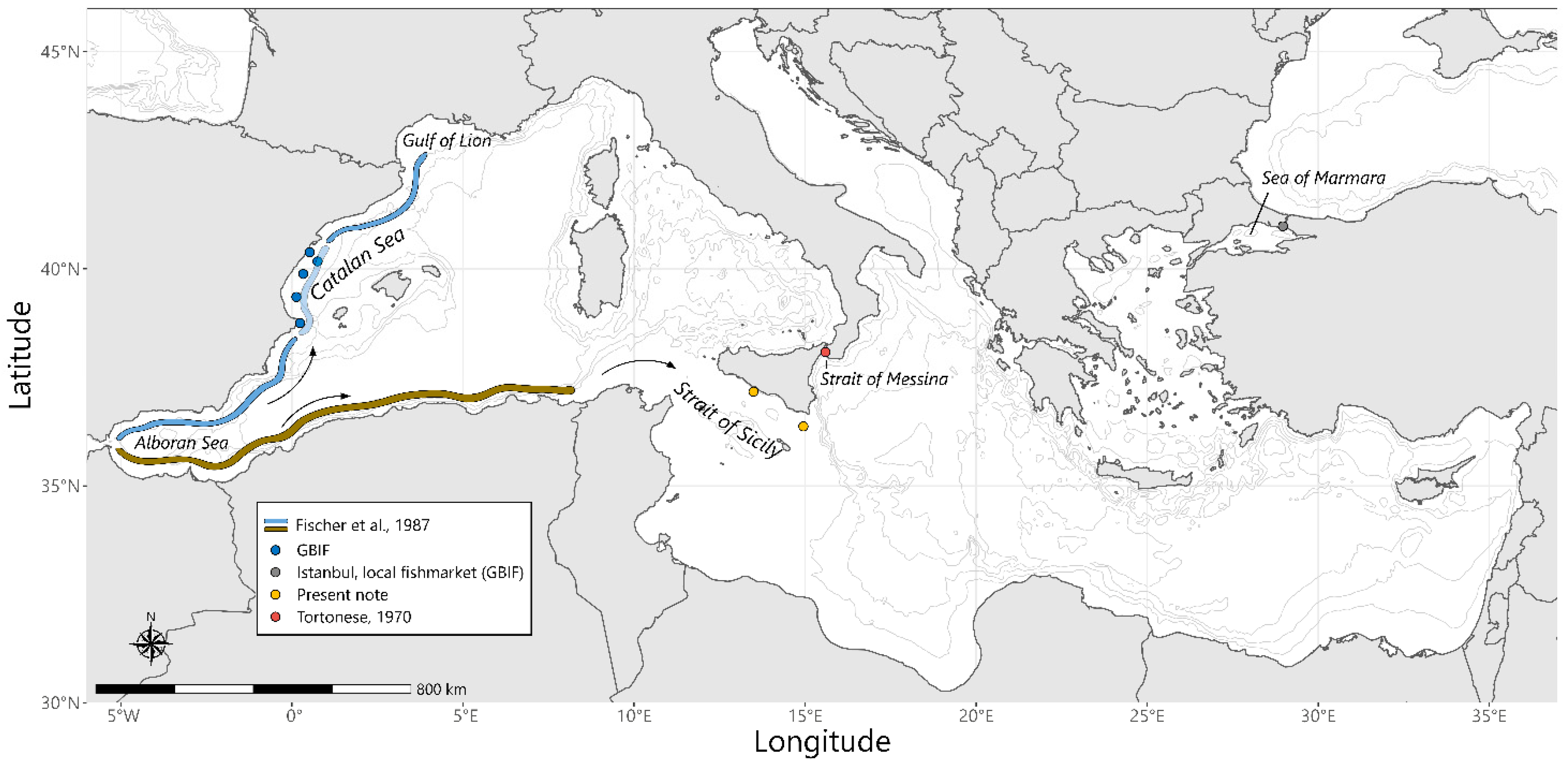
| Specimen | TL (mm) | SL (mm) | W (g) | Sex | Maturity Stage | Age |
|---|---|---|---|---|---|---|
| 1 | 225 | 180 | 123.67 | F | 2c | 5 |
| 2 | 180 | 140 | 65.96 | M | 4a | 4 |
| 3 | 205 | 165 | 96.37 | F | 3 | 4 |
| 4 | 240 | 195 | 149.51 | F | 3 | 5 |
| 5 | 210 | 170 | 102.19 | F | 3 | 5 |
| 6 | 185 | 155 | 69.44 | M | 2c | 3 |
| 7 * | 215 | 175 | 119.48 | F | 3 | 3 |
| Present Study n = 7 | Palmer et al. [19] | Dardignac [18] | ||||
|---|---|---|---|---|---|---|
| BMNH 1964.12.30.1 | BMNH 1964.12.30. 2 | n = na | ||||
| Morphometric Parameters | Min–Max | Mean ± sd | Value * | Value * | Min–Max | Mean |
| Total length [TL, mm] | 180–240 | 517 | 575 | - | - | |
| Standard length [SL, mm] | 140–195 | 444 (436) | 484 (465) | - | - | |
| Measurement [% SL] | ||||||
| Head length | 27.8–30.4 | 29.1 ± 1.0 | 31 | 31 | - | 22.6 [%TL] |
| Pre-dorsal length | 34.0–37.4 | 35.4 ± 1.1 | 38 | 39 | - | - |
| Maximal body height | 31.3–33.1 | 32.2 ± 0.9 | 31 | 31 | 24.1–27.3 | 26.0 [%TL] |
| Pre-anal length | 62.3–68.8 | 65.0 ± 2.2 | - | - | - | - |
| Caudal peduncle height | 8.9–10.3 | 9.8 ± 0.5 | 8.7 | 9.5 | - | - |
| First dorsal fin length | 15.2–19.8 | 17.2 ± 1.6 | - | - | - | - |
| Second dorsal fin length | 40.6–48.1 | 43.9 ± 2.6 | - | - | - | - |
| Pectoral fin length | 19.7–23.0 | 21.9 ± 1.2 | 18 | 18 | - | - |
| Ventral fin length | 21.0–23.2 | 22.2 ± 0.9 | 20 | 19 | - | - |
| Anal fin length | 17.1–22.4 | 19.4 ± 1.6 | 19 | 19 | 18.0–19.9 | - |
| Measurement [% HL] | ||||||
| Pre-orbital length | 20.4–28.4 | 25.7 ± 3.6 | 21 | 21 | 33.6–40.0 | 36.4 |
| Sub-orbital length | 15.2–20.7 | 17.5 ± 1.6 | - | - | 21.0–24.5 | 22.2 |
| Eye horizontal diameter | 25.9–28.8 | 27.5 ± 1.1 | 15 | 15 | 14.0–25.0 | 20.5 |
| Interorbital space | 23.2–25.8 | 25.2 ± 1.3 | 29 | 29 | - | - |
| Meristic Data | Counts | Counts | Counts | Counts | ||
| 1st dorsal fin spine | 10 | 10 | 10 | 10 | ||
| 2nd dorsal fin spine | 1 | 1 | 1 | 1 | ||
| 2nd dorsal fin soft ray | 25–27 (25 1) | 25 | 25 | 23–27 (25 1) | ||
| Pectoral fin rays | 16–18 | 16 | 16 | 17–18 | ||
| Anal fin spine | 2 | 2 | 2 | 1 | ||
| Anal fin soft ray | 7 | 7 | 7 | 7 | ||
| Upper gill-rakers | 3 | 5 (3 *) | 5 (3 *) | - | ||
| Lower gill-rakers | 8 | 7 (8 *) | 7 (9 *) | - | ||
| Rostral pores | 10 | 10 | 10 | - | ||
| Mental pores | 4 | 4 | 4 | - | ||
| Otolith Characters | Description |
|---|---|
| Shape: | Approximately oval, dorsal margin angled and irregular, ventral margin rounded and slightly irregular |
| Thickness: | Very thick |
| Form: | Mesial convex, lateral concave with cluster of nodules in centre |
| Sulcus acusticus: | Pseudo-ostial, supramedian, heterosulcoid |
| Ostium: | Mushroom shaped, lateral, occupying the anterior part of the otolith |
| Cauda: | Tubular, strongly flexed, narrows at tip |
| Ostio-cauda differentiation: | Dorso-ventral constriction |
| Dorsal depression: | Shallow, elongated from ostium to caudal tip |
| Anterior region: | Round, rostrum long, antirostrum absent, excisura absent |
| Posterior region: | Round |
| Measurement | n | Otolith | Mean ± sd | Min | Max |
|---|---|---|---|---|---|
| Lo | 7 | Dx | 8.34 ± 0.66 | 7.40 | 8.96 |
| Sx | 8.29 ± 0.58 | 7.46 | 8.93 | ||
| Ho | 7 | Dx | 6.43 ± 0.38 | 5.98 | 6.95 |
| Sx | 6.43 ± 0.39 | 5.94 | 6.91 | ||
| Wo | 7 | Dx | 0.192 ± 0.04 | 0.13 | 0.24 |
| Sx | 0.191 ± 0.04 | 0.13 | 0.24 |
| Present Study | Lombarte et al. [29] | ||
|---|---|---|---|
| Shape Parameters | Mean ± sd | Value 1 | Value 2 |
| Area (Ao) [mm2] | 41.20 ± 5.37 | 121.78 | 113.13 |
| Perimeter (Po) [mm] | 28.30 ± 3.61 | 44.68 | 41.83 |
| Weight (Wo) [mg] | 0.192 ± 0.04 | - | - |
| Length (Lo) [mm] | 8.34 ± 0.66 | 14.75 | 13.91 |
| Height (Ho) [mm] | 6.43 ± 0.38 | 10.75 | 10.24 |
| Shape Indices | |||
| Otolith relative length (TL) | 4.01 ± 0.13 | 4.03 | 4.16 |
| Otolith relative length (SL) | 4.96 ± 0.22 | - | - |
| Otolith relative size (OR) | 0.95 ± 0.07 | 0.91 | 1.01 |
| Aspect ratio (Ar) | 0.77 ± 0.03 | 0.73 | 0.74 |
| Form factor (Ff) | 0.65 ± 0.09 | 0.77 | 0.81 |
| Ellipticity (El) | 0.13 ± 0.02 | 0.16 | 0.15 |
| Roundness (Ro) | 0.75 ± 0.03 | 0.71 | 0.74 |
| Rectangularity (Re) | 0.77 ± 0.01 | 0.77 | 0.79 |
| Circularity (Ci) | 19.53 ± 2.82 | 16.39 | 15.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardo, G.; Geraci, M.L.; Falsone, F.; Gancitano, S.; Gancitano, V.; Massi, D.; Okpala, C.O.R.; Scannella, D.; Titone, A.; Vitale, S.; et al. First Records with Biological Notes of Umbrina ronchus, Valenciennes, 1843 (Osteichthyes, Sciaenidae) in the Strait of Sicily (Central Mediterranean Sea). Fishes 2023, 8, 434. https://doi.org/10.3390/fishes8090434
Sardo G, Geraci ML, Falsone F, Gancitano S, Gancitano V, Massi D, Okpala COR, Scannella D, Titone A, Vitale S, et al. First Records with Biological Notes of Umbrina ronchus, Valenciennes, 1843 (Osteichthyes, Sciaenidae) in the Strait of Sicily (Central Mediterranean Sea). Fishes. 2023; 8(9):434. https://doi.org/10.3390/fishes8090434
Chicago/Turabian StyleSardo, Giacomo, Michele Luca Geraci, Fabio Falsone, Salvatore Gancitano, Vita Gancitano, Daniela Massi, Charles Odilichukwu R. Okpala, Danilo Scannella, Antonino Titone, Sergio Vitale, and et al. 2023. "First Records with Biological Notes of Umbrina ronchus, Valenciennes, 1843 (Osteichthyes, Sciaenidae) in the Strait of Sicily (Central Mediterranean Sea)" Fishes 8, no. 9: 434. https://doi.org/10.3390/fishes8090434
APA StyleSardo, G., Geraci, M. L., Falsone, F., Gancitano, S., Gancitano, V., Massi, D., Okpala, C. O. R., Scannella, D., Titone, A., Vitale, S., & Fiorentino, F. (2023). First Records with Biological Notes of Umbrina ronchus, Valenciennes, 1843 (Osteichthyes, Sciaenidae) in the Strait of Sicily (Central Mediterranean Sea). Fishes, 8(9), 434. https://doi.org/10.3390/fishes8090434








