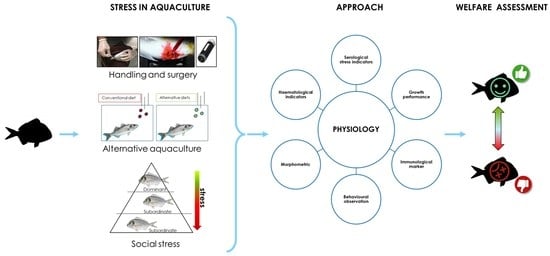
Fish Welfare in Aquaculture: Physiological and Immunological Activities for Diets, Social and Spatial Stress on Mediterranean Aqua Cultured Species
Abstract
:1. Introduction
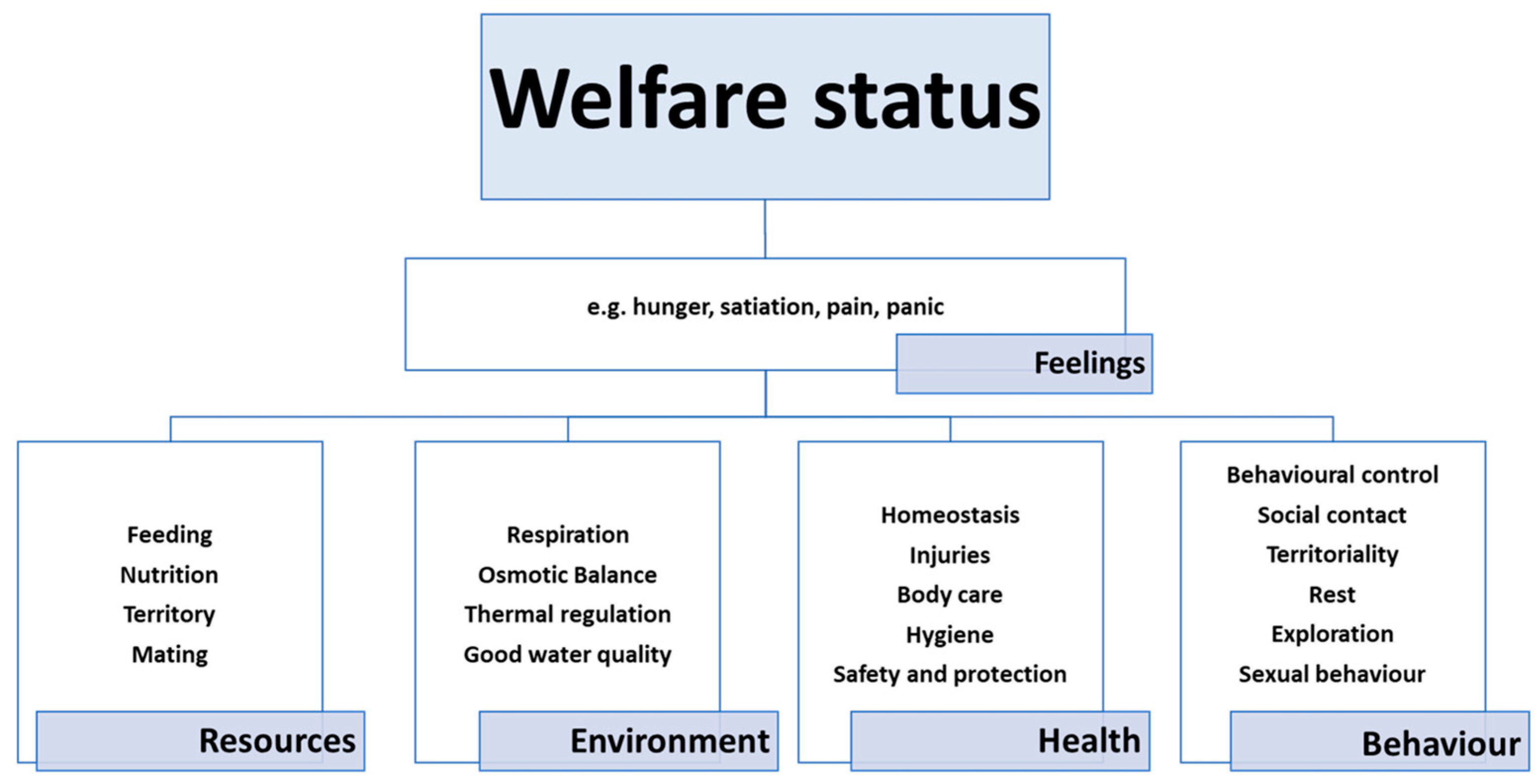
1.1. Welfare
- The animal is free from hunger, thirst and malnutrition, because it has ready access to drinking water and a suitable diet.
- The animal is free from physical and thermal discomfort, because it has access to shelter from the elements and a comfortable resting area.
- The animal is free from pain, injury and disease, thanks to suitable prevention and/or rapid diagnosis and treatment.
- The animal is able to express most of its normal behavioural patterns, because it has sufficient space, proper facilities and the company of other animals of its kind.
- The animal does not experience fear or distress, because the conditions needed to prevent mental suffering have been ensured.
1.2. Current Knowledge Gaps Related to Fish Welfare
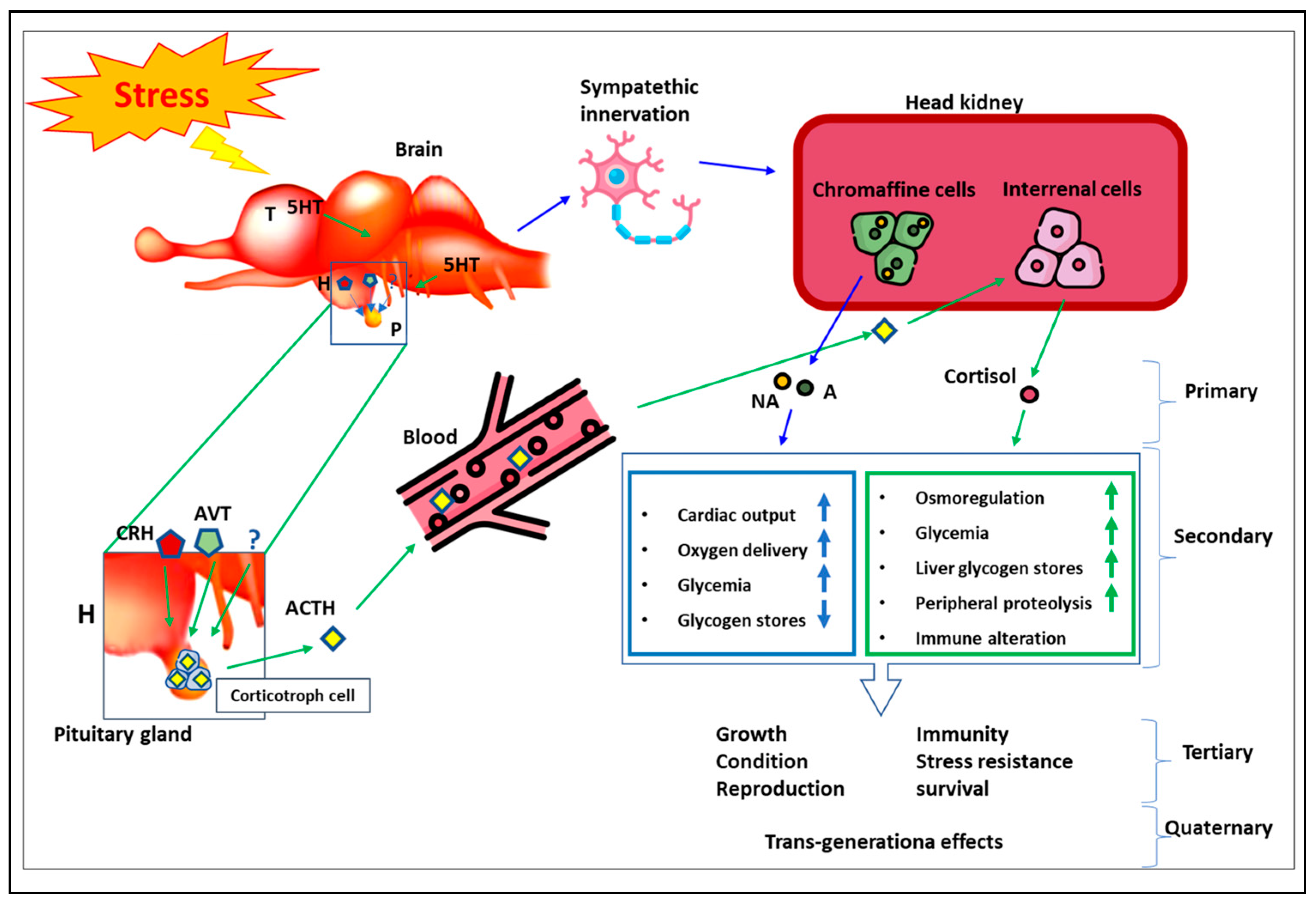
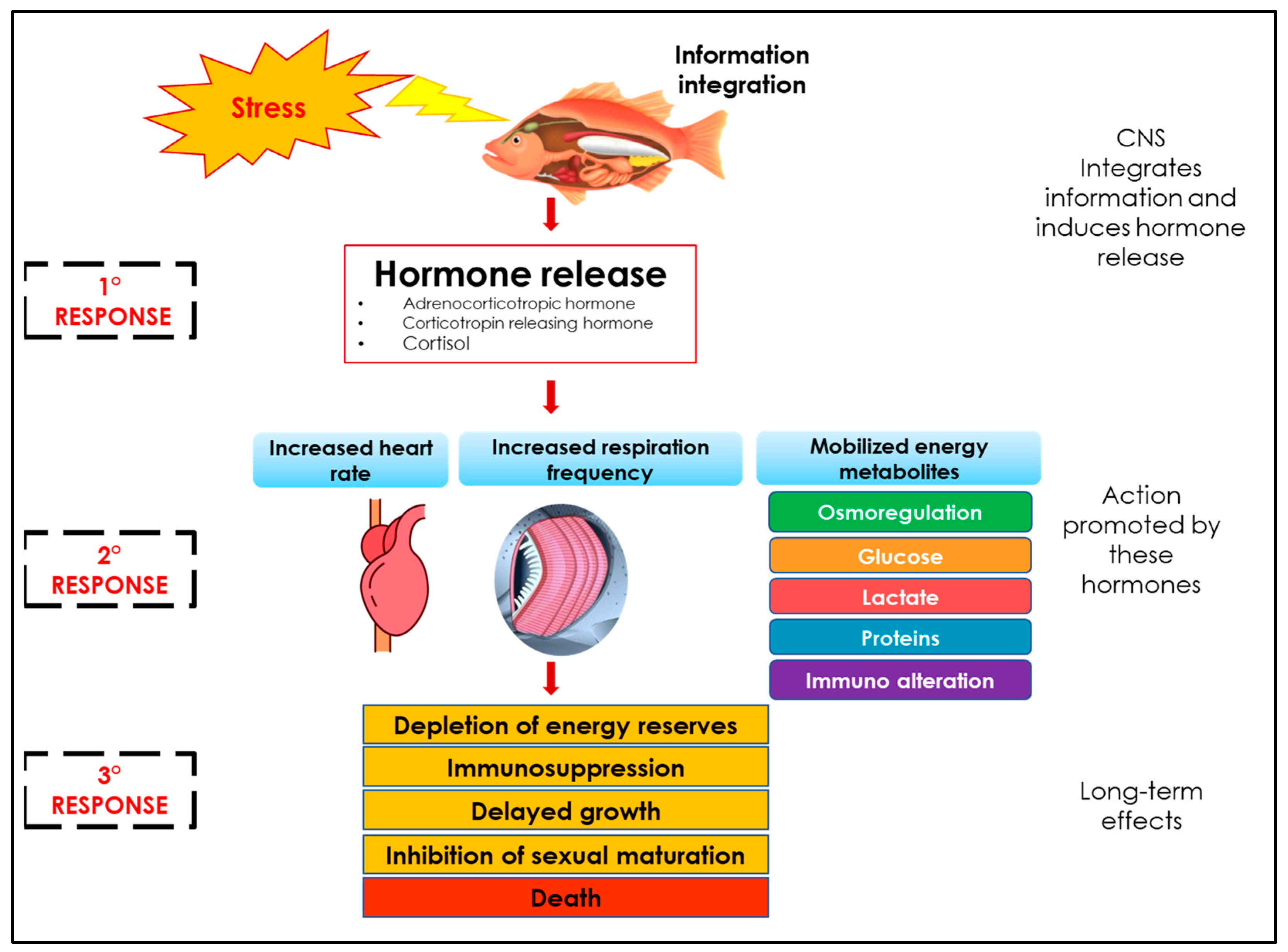
1.3. Stress Physiology
1.4. Physiological Indicators
| System | Parameters | References |
|---|---|---|
| Acid-base balance | H+, OH−, HCO3−, PO42−, SO42− | [75,76,77] |
| Hydric-ionic balance | H2O, osmolality, Na+, Cl−, K+, Ca2+, Mg2+, others | [78,79,80,81] |
| O2 (CO2) transport | Haemoglobin/haemocyanin, haematocrit | [82,83,84] |
| Energy management | Glucose, lactate, amino acids, triglycerides, free fatty acids, etc. | [77,85,86] |
| Immune system (innate) | Physical barriers, cell-cell mediated defence (phagocytosis), humoral defence (antimicrobial enzymes, non-specific proteins, complement system), inflammation | [31,33,34,35,87,88,89,90,91,92,93,94,95] |
| Immune system (adaptative) | Cell-mediated defence (B and T lymphocytes) | [92] |
| Free radical balance | Oxidative stress system | [96,97,98,99,100] |
| Others | Hormones, temperature, etc. | [97,100] |
1.5. Stress Assessment via Molecular Approach
1.6. Stress Assessment via Histological Approach
1.7. Social Stress and Adaptative Stress Coping Styles
1.8. Aquaculture: The State of the Art
2. Fish Welfare Assessment
2.1. Telemetry as a New Tool in the Field of Fish Welfare Assessment
2.2. Application of Telemetry for Fish Welfare Assessment in Aquaculture Farms
2.3. Multi-Parametric Approach Applied to Organic Aquaculture for the Evaluation of the Effects of an Enriched-Organic Diet Composition
2.4. Social and Spatial Stress Effects on Sea Bream Welfare
3. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Broom, D.M. A History of Animal Welfare Science. Acta Biotheor. 2011, 59, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Benn, A.L.; McLelland, D.J.; Whittaker, A.L. A review of welfare assessment methods in reptiles, and preliminary application of the welfare quality® protocol to the pygmy blue-tongue skink, tiliqua adelaidensis, using animal-based measures. Animals 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carere, C.; Mather, J. (Eds.) The Welfare of Invertebrate Animals; Springer International Publishing: Cham, Switzerland, 2019; Volume 18, ISBN 978-3-030-13946-9. [Google Scholar]
- Diggles, B.K. Review of some scientific issues related to crustacean welfare. ICES J. Mar. Sci. 2019, 76, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Branson, E.J. Fish Welfare; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Huntingford, F.A.; Adams, C.; Braithwaite, V.A.; Kadri, S.; Pottinger, T.G.; Sandøe, P.; Turnbull, J.F. Current issues in fish welfare. J. Fish Biol. 2006, 68, 332–372. [Google Scholar] [CrossRef] [Green Version]
- Kristiansen, T.S.; Bracke, M.B.M. A Brief Look into the Origins of Fish Welfare Science. In The Welfare of Fish; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–17. ISBN 978-3-030-41674-4. [Google Scholar]
- Webster, J. Animal Welfare: Limping Towards Eden: A Practical Approach to Redressing the Problem of Our Dominion over the Animals; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 9781405118774. [Google Scholar]
- Manteca, X.; Mainau, X.; Temple, D. The Farm Animal Welfare Fact Sheet. Farm Anim. Welf. Fact Sheet 2012. Available online: https://www.fawec.org/media/com_lazypdf/pdf/Fact_sheet_FAWEC_2_en.pdf (accessed on 6 August 2023).
- Mellor, D.; Patterson-Kane, E.; Stafford, K.J. The science of animal welfare. Proc. Br. Soc. Anim. Prod. (1972) 2009, 1990, 222. [Google Scholar]
- The Council of the European Union Treaty of Amsterdam amending the treaty on European Union, the treaties establishing the European Communities and certain related acts, 10 November 1997. Off. J. Eur. Communities 1997, 340, 1–71.
- Algers, B.; Blokhuis, H.J.; Bøtner, A.; Broom, D.M.; Costa, P.; Domingo, M.; Greiner, M.; Hartung, J.; Koenen, F.; Müller-Graf, C.; et al. General approach to fish welfare and to the concept of sentience in fish Scientific Opinion of the Panel on Animal Health and Welfare. EFSA J. 2009, 954, 1–27. [Google Scholar]
- Browman, H.I.; Cooke, S.J.; Cowx, I.G.; Derbyshire, S.W.G.; Kasumyan, A.; Key, B.; Rose, J.D.; Schwab, A.; Skiftesvik, A.B.; Don Stevens, E.; et al. Welfare of aquatic animals: Where things are, where they are going, and what it means for research, aquaculture, recreational angling, and commercial fishing. ICES J. Mar. Sci. 2019, 76, 82–92. [Google Scholar] [CrossRef] [Green Version]
- 2010/63/EU Directive 2010/63/EU of the European parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 1–61. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 6 August 2023).
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; de Girolamo, P.; D’angelo, L.; Dickel, L.; Gestal, C.; Grasso, F.; Kuba, M.; et al. Guidelines for the Care and Welfare of Cephalopods in Research–A consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab. Anim. 2015, 49, 1–90. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L.; Farrell, A.P.; Brauner, C.J. Biology of Stress in Fish; Elsevier Science: Amsterdam, The Netherlands, 2016; ISBN 9780128027370. [Google Scholar]
- Schreck, C.B.; Tort, L.; Farrell, A.P.; Brauner, C.J. Biology of Stress in Fish Fish Physiology, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128027288. [Google Scholar]
- Martins, C.I.M.; Galhardo, L.; Noble, C.; Damsgård, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.C.; Carter, T.; et al. Behavioural indicators of welfare in farmed fish. Fish Physiol. Biochem. 2012, 38, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Broom, D.M. Animal welfare concepts and measurement. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar]
- Arlinghaus, R.; Schwab, A.; Cooke, S.J.; Cowx, I.G. Contrasting pragmatic and suffering-centred approaches to fish welfare in recreational angling. J. Fish Biol. 2009, 75, 2448–2463. [Google Scholar] [CrossRef]
- Bøtner, A.; Broom, D.; Doherr, M.G.; Domingo, M.; Hartung, J.; Keeling, L.; Koenen, F.; More, S.; Morton, D.; Oltenacu, P.; et al. Statement on the use of animal-based measures to assess the welfare of animals; Statement on the use of animal-based measures to assess the welfare of animals. EFSA J. 2012, 10, 2767. [Google Scholar] [CrossRef]
- Seibel, H.; Weirup, L.; Schulz, C. Fish Welfare–Between Regulations, Scientific Facts and Human Perception. Food Ethics 2020, 5, 4. [Google Scholar] [CrossRef]
- Olivotto, I.; Joan Holt, G.; Carnevali, O. Advances in marine ornamental aquaculture: Breeding and rearing studies. Coral Reefs Biol. Threat. Restor. 2011, 42, 1–40. [Google Scholar]
- Sneddon, L.U.; Wolfenden, D.C.C.; Thomson, J.S. Stress Management and Welfare; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, ISBN 9780128027288. [Google Scholar]
- Barragán-Méndez, C.; Sánchez-García, F.; Sobrino, I.; Mancera, J.M.; Ruiz-Jarabo, I. Air exposure in catshark (Scyliorhinus canicula) modify muscle texture properties: A pilot study. Fishes 2018, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, Q.; Lyu, J.; Kong, C.; Song, S.; Luo, Y. The impact of stunning methods on stress conditions and quality of silver carp (Hypophthalmichthys molitrix) fillets stored at 4 °C during 72 h postmortem. Food Chem. 2017, 216, 130–137. [Google Scholar] [CrossRef]
- Daskalova, A. Farmed fish welfare: Stress, post-mortem muscle metabolism, and stress-related meat quality changes. Int. Aquat. Res. 2019, 11, 113–124. [Google Scholar] [CrossRef] [Green Version]
- European Commission Wealfare of Farmed Fish: Common Practices during Transport and at Slaughter Executive Summary; 2017; ISBN 9789279753367. Available online: http://publications.europa.eu/resource/cellar/facddd32-cda6-11e7-a5d5-01aa75ed71a1.0001.01/DOC_1 (accessed on 6 August 2023).
- Falaise, M. Animal Welfare: From Science to Law; 2019; ISBN 9782951216754. Available online: https://www.fondation-droit-animal.org/documents/7-FALAISE-AnimalWelfare2019.v1.pdf (accessed on 6 August 2023).
- Gräns, A.; Niklasson, L.; Sandblom, E.; Sundell, K.; Algers, B.; Berg, C.; Lundh, T.; Axelsson, M.; Sundh, H.; Kiessling, A. Stunning fish with CO2 or electricity: Contradictory results on behavioural and physiological stress responses. Animal 2015, 10, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Alfonso, S.; Zupa, W.; Manfrin, A.; Fiocchi, E.; Dioguardi, M.; Dara, M.; Lembo, G.; Carbonara, P.; Cammarata, M.; Cammarata, M. Surgical implantation of electronic tags does not induce medium-term effect: Insights from growth and stress physiological profile in two marine fish species. Anim. Biotelemetry 2020, 8, 4–9. [Google Scholar] [CrossRef]
- Carbonara, P.; Alfonso, S.; Dioguardi, M.; Zupa, W.; Vazzana, M.; Dara, M.; Spedicato, M.T.; Lembo, G.; Cammarata, M. Calibrating accelerometer data, as a promising tool for health and welfare monitoring in aquaculture: Case study in European sea bass (Dicentrarchus labrax) in conventional or organic aquaculture. Aquac. Rep. 2021, 21, 4–13. [Google Scholar] [CrossRef]
- Carbonara, P.; Zupa, W.; Bitetto, I.; Alfonso, S.; Dara, M.; Cammarata, M. Evaluation of the effects of the enriched-organic diets composition on european sea bass welfare through a multi-parametric approach. J. Mar. Sci. Eng. 2020, 8, 934. [Google Scholar] [CrossRef]
- Dara, M.; Dioguardi, M.; Vazzana, M.; Vazzana, I.; Accardi, D.; Carbonara, P.; Alfonso, S.; Cammarata, M. Effects of Social Hierarchy Establishment on Stress Response and Cell Phagocytosis in Gilt-Head Sea Bream (Sparus aurata). Fishes 2022, 7, 75. [Google Scholar] [CrossRef]
- Dara, M.; Dioguardi, M.; Vazzana, M.; Vazzana, I.; Carbonara, P.; Alfonso, S.; Cammarata, M. The Role of Spatial Exploration and Territoriality in Establishing Gilthead Seabream (Sparus aurata) Hierarchies, and Their Effects upon Underlying Stress Physiology. Fishes 2023, 8, 132. [Google Scholar] [CrossRef]
- Noble, C.; Gismervik, K.; Iversen, M.H.; Kolarevic, J.; Nilsson, J.; Stien, L.H.; Turnbull, J.F. Welfare Indicators for Farmed Atlantic Salmon: Tools for Assessing Fish Welfare; 2018; ISBN 9788282965569. Available online: https://core.ac.uk/download/pdf/225907892.pdf (accessed on 6 August 2023).
- Noble, C.; Gismervik, K.; Iversen, M.H.; Kolarevic, J.; Nilsson, J.; Stien, L.H.; Turnbull, J.F. Welfare Indicators for Farmed Rainbow Trout: Tools for Assessing Fish Welfare; 2020; ISBN 978-1-405-13495-8. Available online: https://nofima.no/wp-content/uploads/2020/05/Welfare-Indicators-for-farmed-rainbow-trout-Noble-et-al.-2020.pdf (accessed on 6 August 2023).
- RSPCA. RSPCA welfare standards for farmed rainbow trout. RSPCA Welf. Stand. 2020, 42. Available online: https://science.rspca.org.uk/documents/1494935/9042554/RSPCA+Trout+Welfare+Standards+2020.pdf/3f74881f-577b-d4bb-22f0-a9792a298db6?t=1618819287216 (accessed on 6 August 2023).
- Saraiva, J.L.; Arechavala-Lopez, P.; Sneddon, L.U. Farming fish. In Routledge Handbook of Animal Welfare; Routledge: London, UK, 2022; pp. 115–127. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Cabrera-Álvarez, M.J.; Maia, C.M.; Saraiva, J.L. Environmental enrichment in fish aquaculture: A review of fundamental and practical aspects. Rev. Aquac. 2022, 14, 704–728. [Google Scholar] [CrossRef]
- Saraiva, J.L.; Rachinas-Lopes, P.; Arechavala-Lopez, P. Finding the “golden stocking density”: A balance between fish welfare and farmers’ perspectives. Front. Vet. Sci. 2022, 9, 1099. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The Concept of Stress in Fish, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, ISBN 9780128027288. [Google Scholar]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Gorissen, M.; Flik, G. The Endocrinology of the Stress Response in Fish: An Adaptation-Physiological View; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, ISBN 9780128027288. [Google Scholar]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Gesto, M. Chapter 9-Characterization of the neuroendocrine stress status as part of the multiparametric assessment of welfare in fish. In Cellular and Molecular Approaches in Fish Biology; Fernandez, I., Fernandes, J., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 285–308. ISBN 978-0-12-822273-7. [Google Scholar]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Malham, S.K.; Lacoste, A.; Gélébart, F.; Cueff, A.; Poulet, S.A. A first insight into stress-induced neuroendocrine and immune changes in the octopus Eledone cirrhosa. Aquat. Living Resour. 2002, 15, 187–192. [Google Scholar] [CrossRef]
- Reid, S.G.; Bernier, N.J.; Perry, S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. Comp. Biochem. Physiol.-C Pharmacol. Toxicol. Endocrinol. 1998, 120, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Jarabo, I.; Barragán-Méndez, C.; Jerez-Cepa, I.; Fernández-Castro, M.; Sobrino, I.; Mancera, J.M.; Aerts, J. Plasma 1α-Hydroxycorticosterone as Biomarker for Acute Stress in Catsharks (Scyliorhinus canicula). Front. Physiol. 2019, 10, 1217. [Google Scholar] [CrossRef] [Green Version]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Costas, B.; Conceição, L.E.C.; Aragão, C.; Martos, J.A.; Ruiz-Jarabo, I.; Mancera, J.M.; Afonso, A. Physiological responses of Senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: Effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture 2011, 316, 68–76. [Google Scholar] [CrossRef]
- Barragán-Méndez, C.; González-Duarte, M.M.; Sobrino, I.; Vila, Y.; Mancera, J.M.; Ruiz-Jarabo, I. Physiological recovery after bottom trawling as a method to manage discards: The case study of Nephrops norvegicus and Squilla mantis. Mar. Policy 2020, 116, 103895. [Google Scholar] [CrossRef]
- Wedemeyer, G.; Barton, B.B.; McLeay, D.J. Stress and acclimation. In Methods for Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 451–489. [Google Scholar]
- Arjona, F.J.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Gonçalves, O.; Páscoa, I.; Martín del Río, M.P.; Mancera, J.M. Tertiary stress responses in Senegalese sole (Solea senegalensis Kaup, 1858) to osmotic challenge: Implications for osmoregulation, energy metabolism and growth. Aquaculture 2009, 287, 419–426. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Ruiz-Jarabo, I. Physiology: An important tool to assess the welfare of aquatic animals. Biology 2021, 10, 61. [Google Scholar] [CrossRef]
- Pottinger, T.G. The Stress Response in Fish-Mechanisms, Effects and Measurement. Fish Welf. 2008, 32–48. [Google Scholar] [CrossRef]
- Romero, L.M.; Reed, J.M. Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2005, 140, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Vamvakopoulos, N.C.; Chrousos, G.P. Hormonal regulation of human corticotropin-releasing hormone gene expression: Implications for the stress response and immune/inflammatory reaction. Endocr. Rev. 1994, 15, 409–420. [Google Scholar] [CrossRef]
- Kassahn, K.S.; Crozier, R.H.; Pörtner, H.O.; Caley, M.J. Animal performance and stress: Responses and tolerance limits at different levels of biological organisation. Biol. Rev. 2009, 84, 277–292. [Google Scholar] [CrossRef]
- Basu, N.; Nakano, T.; Grau, E.G.; Iwama, G.K. The effects of cortisol on heat shock protein 70 levels in two fish species. Gen. Comp. Endocrinol. 2001, 124, 97–105. [Google Scholar] [CrossRef]
- Vijayan, M.M.; Raptis, S.; Sathiyaa, R. Cortisol treatment affects glucocorticoid receptor and glucocorticoid-responsive genes in the liver of rainbow trout. Gen. Comp. Endocrinol. 2003, 132, 256–263. [Google Scholar] [CrossRef]
- Wood, C.; Turner, J.; Graham, M. Why do Fish die after Severe Exercise. J. Fish Biol. 2006, 22, 189–201. [Google Scholar] [CrossRef]
- Sopinka, N.M.; Donaldson, M.R.; O’Connor, C.M.; Suski, C.D.; Cooke, S.J. Stress Indicators in Fish; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, ISBN 9780128027288. [Google Scholar]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Sadoul, B.; Vijayan, M.M. Stress and Growth; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, ISBN 9780128027288. [Google Scholar]
- Adams, S.M.; Brown, A.M.; Goede, R.W. A Quantitative Health Assessment Index for Rapid Evaluation of Fish Condition in the Field. Trans. Am. Fish. Soc. 1993, 122, 63–73. [Google Scholar] [CrossRef]
- Jain, K.E.; Birtwell, I.K.; Farrell, A.P. Repeat swimming performance of mature sockeye salmon following a brief recovery period: A proposed measure of fish health and water quality. Can. J. Zool. 1998, 76, 1488–1496. [Google Scholar] [CrossRef]
- Brodeur, J.C.; Dixon, D.G.; McKinly, R.S. Assessment of cardiac output as a predictor of metabolic rate in rainbow trout. J. Fish Biol. 2001, 58, 439–452. [Google Scholar] [CrossRef]
- Carbonara, P.; Corsi, I.; Focardi, S.; Lembo, G.; Rochira, S.; Scolamacchia, M.; Spedicato, M.T.; Mckinley, R.S. The effects of stress induced by cortisol administration on the repeatability of swimming performance tests in the European sea bass (Dicentrarchus labrax L.). Mar. Freshw. Behav. Physiol. 2010, 43, 283–296. [Google Scholar] [CrossRef]
- Faught, E.; Hernandez-Perez, J.; Wilson, J.; Vijayan, M. Stress in response to environmental changes. In Climate Change and Non-Infectious Fish Disorders; Cabi Digital Library: Wallingford, UK, 2020; pp. 136–162. ISBN 9781786393982. [Google Scholar]
- Ryu, T.; Veilleux, H.D.; Donelson, J.M.; Munday, P.L.; Ravasi, T. The epigenetic landscape of transgenerational acclimation to ocean warming. Nat. Clim. Chang. 2018, 8, 504–509. [Google Scholar] [CrossRef]
- Vera-Chang, M.N.; St-Jacques, A.D.; Gagné, R.; Martyniuk, C.J.; Yauk, C.L.; Moon, T.W.; Trudeau, V.L. Transgenerational hypocortisolism and behavioral disruption are induced by the antidepressant fluoxetine in male zebrafish Danio rerio. Proc. Natl. Acad. Sci. USA 2018, 115, E12435–E12442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, M.; Hamilton, T.J. Acid-base physiology, neurobiology and behaviour in relation to CO2-induced ocean acidification. J. Exp. Biol. 2017, 220, 2136–2148. [Google Scholar] [CrossRef] [Green Version]
- Falco, F.; Bono, G.; Cammarata, M.; Cavalca, J.; Vazzana, I.; Dara, M.; Scannella, D.; Guicciardi, S.; Faggio, C.; Ragonese, S. Stress related blood values in Scyliorhinus canicula as live-indicators of physiological status after bottom trawling capture activity. Comp. Biochem. Physiol. Part B 2023, 263, 110802. [Google Scholar] [CrossRef]
- Hwang, P.P.; Lee, T.H.; Lin, L.Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, 28–47. [Google Scholar] [CrossRef]
- Foster, C.; Amado, E.M.; Souza, M.M.; Freire, C.A. Do osmoregulators have lower capacity of muscle water regulation than osmoconformers? A study on decapod crustaceans. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2010, 313, 80–94. [Google Scholar] [CrossRef]
- Freire, C.A.; Onken, H.; McNamara, J.C. A structure-function analysis of ion transport in crustacean gills and excretory organs. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2008, 151, 272–304. [Google Scholar] [CrossRef] [PubMed]
- Mccormick, S.D. Hormonal Control of Metabolism and Ionic Regulation; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 2, ISBN 9780123745538. [Google Scholar]
- Wells, R.M.G. Chapter 6 Blood-Gas Transport and Hemoglobin Function: Adaptations for Functional and Environmental Hypoxia, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 27. [Google Scholar]
- Barragán-Méndez, C.; Sobrino, I.; Marín-Rincón, A.; Fernández-Boo, S.; Costas, B.; Mancera, J.M.; Ruiz-Jarabo, I. Acute-stress biomarkers in three octopodidae species after bottom trawling. Front. Physiol. 2019, 10, 784. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.E.; Hiney, M.P.; Shields, D.C.; Uhlar, C.M.; Lindsay, A.J.; Whitehead, A.S. Acute phase proteins in salmonids: Evolutionary analyses and acute phase response. J. Immunol. 1997, 158, 384–392. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Carbohydrate Metabolism in Cephalopod Molluscs. In Metabolic Biochemistry and Molecular Biomechanics; Hochachka, P.W., Ed.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 91–136. ISBN 978-0-12-751401-7. [Google Scholar]
- Speers-Roesch, B.; Treberg, J.R. The unusual energy metabolism of elasmobranch fishes. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2010, 155, 417–434. [Google Scholar] [CrossRef]
- Cammarata, M.; Vazzana, M.; Accardi, D.; Parrinello, N. Seabream (Sparus aurata) long-term dominant-subordinate interplay affects phagocytosis by peritoneal cavity cells. Brain Behav. Immun. 2012, 26, 580–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbonara, P.; Dioguardi, M.; Cammarata, M.; Zupa, W.; Vazzana, M.; Spedicato, M.T.; Lembo, G. Basic knowledge of social hierarchies and physiological profile of reared sea bass Dicentrarchus labrax (L.). PLoS ONE 2019, 14, e0208688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dara, M.; Giulianini, P.G.; Manfrin, C.; Parisi, M.G.; Parrinello, D.; La Corte, C.; Vasta, G.R.; Cammarata, M. F-type lectin from serum of the Antarctic teleost fish Trematomus bernacchii (Boulenger, 1902): Purification, structural characterization, and bacterial agglutinating activity. Comp. Biochem. Physiol. Part-B Biochem. Mol. Biol. 2021, 256, 110633. [Google Scholar] [CrossRef]
- Decker, H.; Jaenicke, E. Recent findings on phenoloxidase activity and antimicrobial activity of hemocyanins. Dev. Comp. Immunol. 2004, 28, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Hirata, T.; Nishioka, T.; Sakaguchi, M. Hemocyte components in crustaceans convert hemocyanin into a phenoloxidase-like enzyme. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2003, 134, 135–141. [Google Scholar] [CrossRef]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef] [Green Version]
- Gestal, C.; Castellanos-Martínez, S. Understanding the cephalopod immune system based on functional and molecular evidence. Fish Shellfish. Immunol. 2015, 46, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Loker, E.S.; Adema, C.M.; Zhang, S.M.; Kepler, T.B. Invertebrate immune systems-Not homogeneous, not simple, not well understood. Immunol. Rev. 2004, 198, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, L.; Alpuche, J.; Maldonado, G.; Agundis, C.; Pereyra-Morales, A.; Zenteno, E. Review: Immunity mechanisms in crustaceans. Innate Immun. 2009, 15, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Porte, C.; Janer, G.; Lorusso, L.C.; Ortiz-Zarragoitia, M.; Cajaraville, M.P.; Fossi, M.C.; Canesi, L. Endocrine disruptors in marine organisms: Approaches and perspectives. Comp. Biochem. Physiol.-C Toxicol. Pharmacol. 2006, 143, 303–315. [Google Scholar] [CrossRef]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- McCormick, S.D.; Bradshaw, D. Hormonal control of salt and water balance in vertebrates. Gen. Comp. Endocrinol. 2006, 147, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.; Wolf, J.C. Morphologic effects of the stress response in fish. ILAR J. 2009, 50, 387–396. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [Green Version]
- Forgati, M.; Kandalski, P.K.; Herrerias, T.; Zaleski, T.; Machado, C.; Souza, M.R.D.P.; Donatti, L. Effects of heat stress on the renal and branchial carbohydrate metabolism and antioxidant system of Antarctic fish. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2017, 187, 1137–1154. [Google Scholar] [CrossRef]
- Johansson, L.H.; Timmerhaus, G.; Afanasyev, S.; Jørgensen, S.M.; Krasnov, A. Smoltification and seawater transfer of Atlantic salmon (Salmo salar L.) is associated with systemic repression of the immune transcriptome. Fish Shellfish. Immunol. 2016, 58, 33–41. [Google Scholar] [CrossRef]
- Miao, L.H.; Lin, Y.; Pan, W.J.; Huang, X.; Ge, X.P.; Zhou, Q.L.; Liu, B.; Ren, M.C.; Zhang, W.X.; Liang, H.L.; et al. Comparative transcriptome analysis reveals the gene expression profiling in bighead carp (Aristichthys nobilis) in response to acute nitrite toxicity. Fish Shellfish. Immunol. 2018, 79, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Narum, S.R.; Campbell, N.R. Transcriptomic response to heat stress among ecologically divergent populations of redband trout. BMC Genom. 2015, 16, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebl, A.; Verleih, M.; Köbis, J.M.; Kühn, C.; Wimmers, K.; Köllner, B.; Goldammer, T. Transcriptome Profiling of Gill Tissue in Regionally Bred and Globally Farmed Rainbow Trout Strains Reveals Different Strategies for Coping with Thermal Stress. Mar. Biotechnol. 2013, 15, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Iwama, G.K. Stress in fish. Ann. N. Y. Acad. Sci. 1998, 851, 304–310. [Google Scholar] [CrossRef]
- Prunet, P.; Sturm, A.; Milla, S. Multiple corticosteroid receptors in fish: From old ideas to new concepts. Gen. Comp. Endocrinol. 2006, 147, 17–23. [Google Scholar] [CrossRef]
- Alsop, D.; Vijayan, M.M. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Sathiyaa, R.; Vijayan, M.M. Autoregulation of glucocorticoid receptor by cortisol in rainbow trout hepatocytes. Am. J. Physiol.-Cell Physiol. 2003, 284, 1508–1515. [Google Scholar] [CrossRef] [Green Version]
- Aluru, N.; Vijayan, M.M. Hepatic transcriptome response to glucocorticoid receptor activation in rainbow trout. Physiol. Genom. 2007, 31, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Wiseman, S.; Osachoff, H.; Bassett, E.; Malhotra, J.; Bruno, J.; VanAggelen, G.; Mommsen, T.P.; Vijayan, M.M. Gene expression pattern in the liver during recovery from an acute stressor in rainbow trout. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2007, 2, 234–244. [Google Scholar] [CrossRef]
- Momoda, T.S.; Schwindt, A.R.; Feist, G.W.; Gerwick, L.; Bayne, C.J.; Schreck, C.B. Gene expression in the liver of rainbow trout, Oncorhynchus mykiss, during the stress response. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2007, 2, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Bortoletti, M.; Maccatrozzo, L.; Radaelli, G.; Caberlotto, S.; Bertotto, D. Muscle cortisol levels, expression of glucocorticoid receptor and oxidative stress markers in the teleost fish argyrosomus regius exposed to transport stress. Animals 2021, 11, 1160. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Mechanisms of cortisol action in fish hepatocytes. Comp. Biochem. Physiol. Part-B Biochem. Mol. Biol. 2016, 199, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Schena, M.; Heller, R.A.; Theriault, T.P.; Konrad, K.; Lachenmeier, E.; Davis, R.W. Microarrays: Biotechnology’s discovery platform for functional genomics. Trends Biotechnol. 1998, 16, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.O.; Botstein, D. Exploring the new world of the genome with DNA microarrays. Nat. Genet. 1999, 21, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sipe, C.W.; Saha, M.S. The use of microarray technology in nonmammalian vertebrate systems. Methods Mol. Biol. 2007, 382, 1–16. [Google Scholar] [CrossRef]
- Miller, K.M.; Maclean, N. Teleost microarrays: Development in a broad phylogenetic range reflecting diverse applications. J. Fish Biol. 2008, 72, 2039–2050. [Google Scholar] [CrossRef]
- Aluru, N.; Vijayan, M.M. Stress transcriptomics in fish: A role for genomic cortisol signaling. Gen. Comp. Endocrinol. 2009, 164, 142–150. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. Nihms229948. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Morozova, O.; Marra, M.A. Applications of next-generation sequencing technologies in functional genomics. Genomics 2008, 92, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Parma, L.; Candela, M.; Soverini, M.; Turroni, S.; Consolandi, C.; Brigidi, P.; Mandrioli, L.; Sirri, R.; Fontanillas, R.; Gatta, P.P.; et al. Next-generation sequencing characterization of the gut bacterial community of gilthead sea bream (Sparus aurata, L.) fed low fishmeal based diets with increasing soybean meal levels. Anim. Feed Sci. Technol. 2016, 222, 204–216. [Google Scholar] [CrossRef]
- McCurdy, D.B. Who should have access to what mental health services? The journey from Oz to Ephesus. Conserv. Jud. 1999, 51, 47–63. [Google Scholar]
- Baeverfjord, G.; Krogdahl, Å. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: A comparison with the intestines of fasted fish. J. Fish Dis. 1996, 19, 375–387. [Google Scholar] [CrossRef]
- Salamat, N.; Zarie, M. Using of Fish Pathological Alterations to Assess Aquatic Pollution: A Review. World J. Fish Mar. Sci. 2012, 4, 223–231. [Google Scholar]
- Zimmerli, S.; Bernet, D.; Burkhardt-Holm, P.; Schmidt-Posthaus, H.; Vonlanthen, P.; Wahli, T.; Segner, H. Assessment of fish health status in four Swiss rivers showing a decline of brown trout catches. Aquat. Sci. 2007, 69, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Handy, R.D.; Runnalls, T.; Russell, P.M. Histopathologic biomarkers in three spined sticklebacks, Gasterosteus aculeatus, from several rivers in Southern England that meet the freshwater fisheries directive. Ecotoxicology 2002, 11, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Lukin, A.; Sharova, J.; Belicheva, L.; Camus, L. Assessment of fish health status in the Pechora River: Effects of contamination. Ecotoxicol. Environ. Saf. 2011, 74, 355–365. [Google Scholar] [CrossRef]
- van Dyk, J.C.; Marchand, M.J.; Smit, N.J.; Pieterse, G.M. A histology-based fish health assessment of four commercially and ecologically important species from the Okavango Delta panhandle, Botswana. Afr. J. Aquat. Sci. 2009, 34, 273–282. [Google Scholar] [CrossRef]
- McHugh, K.J.; Smit, N.J.; Van Vuren, J.H.J.; Van Dyk, J.C.; Bervoets, L.; Covaci, A.; Wepener, V. A histology-based fish health assessment of the tigerfish, Hydrocynus vittatus from a DDT-affected area. Phys. Chem. Earth 2011, 36, 895–904. [Google Scholar] [CrossRef]
- Mchugh, K.J.; Van Dyk, J.C.; Weyl, O.L.F.; Smit, N.J. First report of nephrocalcinosis in a wild population of Mugil cephalus L. and Myxus capensis (Valenciennes). J. Fish Dis. 2013, 36, 887–889. [Google Scholar] [CrossRef]
- Torres, L.; Nilsen, E.; Grove, R.; Patiño, R. Health status of Largescale Sucker (Catostomus macrocheilus) collected along an organic contaminant gradient in the lower Columbia River, Oregon and Washington, USA. Sci. Total Environ. 2014, 484, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coz-Rakovac, R.; Strunjak-Perovic, I.; Hacmanjek, M.; Topic Popovic, N.; Lipej, Z.; Sostaric, B. Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the North Adriatic Sea. Vet. Res. Commun. 2005, 29, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Rašković, B.; Jarić, I.; Koko, V.; Spasić, M.; Dulić, Z.; Marković, Z.; Poleksić, V. Histopathological indicators: A useful fish health monitoring tool in common carp (Cyprinus carpio Linnaeus, 1758) culture. Cent. Eur. J. Biol. 2013, 8, 975–985. [Google Scholar] [CrossRef]
- Saraiva, A.; Costa, J.; Serrão, J.; Cruz, C.; Eiras, J.C. A histology-based fish health assessment of farmed seabass (Dicentrarchus labrax L.). Aquaculture 2015, 448, 375–381. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Dezfuli, B.S.; Giari, L.; Simoni, E.; Menegatti, R.; Shinn, A.P.; Manera, M. Gill histopathology of cultured European sea bass, Dicentrarchus labrax (L.), infected with Diplectanum aequans (Wagener 1857) Diesing 1958 (Diplectanidae: Monogenea). Parasitol. Res. 2007, 100, 707–713. [Google Scholar] [CrossRef]
- González-Lanza, C.; Alvarez-Pellitero, P.; Sitjá-Bobadilla, A. Diplectanidae (Monogenea) infestations of sea bass, Dicentrarchus labrax (L.), from the Spanish Mediterranean area-Histopathology and population dynamics under culture conditions. Parasitol. Res. 1991, 77, 307–314. [Google Scholar] [CrossRef]
- Oliver, G. Effect pathogène de la fixation de Diplectanum aequans (Wagener, 1857) Diesing, 1858 (Monogenea, Monopisthocotylea, Diplectanidae) sur les branchies de Dicentrarchus labrax (Linnaeus, 1758), (Pisces, Serranidae). Z. Für Parasitenkd. 1977, 11, 7–11. [Google Scholar] [CrossRef]
- Yardimci, B.; Zafer Pekmezci, G. Gill histopathology in cultured sea bass (Dicentrarchus labrax (L.) co-infected by Diplectanum aequans (Wagener, 1857) and Lernanthropus kroyeri (van Beneden, 1851). Ank. Univ. Vet. Fak. Derg. 2012, 59, 61–64. [Google Scholar] [CrossRef]
- Manera, M.; Dezfuli, B.S. Lernanthropus kroyeri infections in farmed sea bass Dicentrarchus labrax: Pathological features. Dis. Aquat. Org. 2003, 57, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Giari, L.; Manera, M.; Simoni, E.; Dezfuli, B.S. Cellular alterations in different organs of European sea bass Dicentrarchus labrax (L.) exposed to cadmium. Chemosphere 2007, 67, 1171–1181. [Google Scholar] [CrossRef]
- Kurtovic, B.; Teskeredžic, E.; Teskeredžic, Z. Histological comparison of spleen and kidney tissue from farmed and wild European sea bass (Dicentrarchus labrax L.). Acta Adriat. 2008, 49, 147–154. [Google Scholar]
- Roberts, R.J. Fish Pathology; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-444-33282-7. [Google Scholar]
- Smith, C.E.; Holway, J.E.; Hammer, G.L. Sulphamerazine toxicity in cut-throat trout broodfish Salmo clarki (Richardson). J. Fish Biol. 1973, 5, 97–101. [Google Scholar] [CrossRef]
- Braunbeck, T. Cytological alterations in fish hepatocytes following in vivo and in vitro sublethal exposure to xenobiotics—Structural biomarkers of environmental contamination. In Fish Ecotoxicology; Braunbeck, T., Hinton, D.E., Streit, B., Eds.; Birkhäuser Basel: Basel, Switzerland, 1998; pp. 61–140. ISBN 978-3-0348-8853-0. [Google Scholar]
- Biagianti-Risbourg, S.; Bastide, J. Hepatic perturbations induced by a herbicide (atrazine) in juvenile grey mullet Liza ramada (Mugilidae, Teleostei): An ultrastructural study. Aquat. Toxicol. 1995, 31, 217–229. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Simoni, E.; Giari, L.; Manera, M. Effects of experimental terbuthylazine exposure on the cells of Dicentrarchus labrax (L.). Chemosphere 2006, 64, 1684–1694. [Google Scholar] [CrossRef]
- Strmac, M.; Braunbeck, T. Cytological and biochemical effects of a mixture of 20 pollutants on isolated rainbow trout (Oncorhynchus mykiss) hepatocytes. Ecotoxicol. Environ. Saf. 2002, 53, 293–304. [Google Scholar] [CrossRef]
- Bilen, A.M.; Bilen, S. Effect of diet on the fatty acids composition of cultured sea bass (Dicentrarchus labrax) liver tissues a nd histo ology compared with sea bass caught in Eagean Sea. Mar. Sci. Technol. Bull. 2013, 2, 13–19. [Google Scholar]
- Čož-Rakovac, R.; Strunjak-Perović, I.; Topić Popović, N.; Hacmanjek, M.; Šimpraga, B.; Teskeredžić, E. Health status of wild and cultured sea bass in the northern Adriatic Sea. Vet. Med. 2002, 47, 222–226. [Google Scholar] [CrossRef]
- Uran, P.A.; Schrama, J.W.; Rombout, J.H.W.M.; Obach, A.; Jensen, L.; Koppe, W.; Verreth, J.A.J. Soybean meal-induced enteritis in Atlantic salmon (Salmo salar L.) at different temperatures. Aquac. Nutr. 2008, 14, 324–330. [Google Scholar] [CrossRef]
- del Pilar Álvarez-Pellitero, M. Mucosal Intestinal Immunity and Response to Parasite Infections in Ectothermic Vertebrates; Nova Science Publishers: New York, NY, USA, 2013. [Google Scholar]
- Rombout, J.H.W.M.; van den Berg, A.A. Uptake and transport of ferritin in the epithelium of carp (Cyprinus Carpio L.) and the possible immunological implications. Cell Biol. Int. Rep. 1985, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Couto, A.; Kortner, T.M.; Penn, M.; Bakke, A.M.; Krogdahl, Å.; Oliva-Teles, A. Effects of dietary phytosterols and soy saponins on growth, feed utilization efficiency and intestinal integrity of gilthead sea bream (Sparus aurata) juveniles. Aquaculture 2014, 432, 295–303. [Google Scholar] [CrossRef]
- Estensoro, I.; Benedito-Palos, L.; Palenzuela, O.; Kaushik, S.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. The nutritional background of the host alters the disease course in a fish-myxosporean system. Vet. Parasitol. 2011, 175, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourente, G.; Good, J.E.; Thompson, K.D.; Bell, J.G. Effects of partial substitution of dietary fish oil with blends of vegetable oils, on blood leucocyte fatty acid compositions, immune function and histology in European sea bass (Dicentrarchus labrax L.). Br. J. Nutr. 2007, 98, 770–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva-Teles, A. Nutrition and health of aquaculture fish. J. Fish Dis. 2012, 35, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, M.L.; Caffara, M.; Florio, D.; Gustinelli, A.; Marcer, F. Sphaerospora dicentrarchi and S. testicularis (Myxozoa: Sphaerosporidae) in farmed European seabass (Dicentrarchus labrax) from Italy. Folia Parasitol. 2004, 51, 208–210. [Google Scholar] [CrossRef] [Green Version]
- Mladineo, I. Myxosporidean infections in Adriatic cage-reared fish. Bull.-Eur. Assoc. Fish Pathol. 2003, 23, 113–122. [Google Scholar]
- Sitjà Bobadilla, A.; Diamant, A.; Palenzuela, O.; Alvarez Pellitero, P. Short communication Effect of host factors and experimental conditions on the horizontal transmission of Enteromyxum leei (Myxozoa) to bass, Dicentrarchus labrax (L.). J. Fish Dis. 2007, 30, 243–250. [Google Scholar] [CrossRef]
- Khojasteh, S.M.B.; Sheikhzadeh, F.; Mohammadnejad, D.; Azami, A. Histological, Histochemical and Ultrastructural Study of the Intestine of Rainbow Trout (Oncorhynchus mykiss). World Appl. Sci. J. 2009, 6, 1525–1531. [Google Scholar]
- Refaey, M.M.; Li, D.; Tian, X.; Zhang, Z.; Zhang, X.; Li, L.; Tang, R. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of channel catfish Ictalurus punctatus. Aquaculture 2018, 492, 73–81. [Google Scholar] [CrossRef]
- Conforto, E.; Vílchez-Gómez, L.; Parrinello, D.; Parisi, M.G.; Esteban, M.Á.; Cammarata, M.; Guardiola, F.A. Role of mucosal immune response and histopathological study in European eel (Anguilla anguilla L.) intraperitoneal challenged by Vibrio anguillarum or Tenacibaculum soleae. Fish Shellfish. Immunol. 2021, 114, 330–339. [Google Scholar] [CrossRef]
- Mitchell, S.O.; Rodger, H.D. A review of infectious gill disease in marine salmonid fish. J. Fish Dis. 2011, 34, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, F.A.; Mabrok, M.; Machado, M.; Azeredo, R.; Afonso, A.; Esteban, M.A.; Costas, B. Mucosal and systemic immune responses in Senegalese sole (Solea senegalensis Kaup) bath challenged with Tenacibaculum maritimum: A time-course study. Fish Shellfish. Immunol. 2019, 87, 744–754. [Google Scholar] [CrossRef]
- Suanyuk, N.; Rogge, M.; Thune, R.; Watthanaphiromsakul, M.; Champhat, N.; Wiangkum, W. Mortality and pathology of hybrid catfish, Clarias macrocephalus (Günther) × Clarias gariepinus (Burchell), associated with Edwardsiella ictaluri infection in southern Thailand. J. Fish Dis. 2014, 37, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Maftuch, M.; Sanoesi, E.; Farichin, I.; Saputra, B.A.; Ramdhani, L.; Hidayati, S.; Fitriyah, N.; Prihanto, A.A. Histopathology of gill, muscle, intestine, kidney, and liver on Myxobolus sp.-infected Koi carp (Cyprinus carpio). J. Parasit. Dis. 2018, 42, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Bernet, D.; Schmidt-Posthaus, H.; Wahli, T.; Burkhardt-Holm, P. Evaluation of two monitoring approaches to assess effects of waste water disposal on histological alterations in fish. Hydrobiologia 2004, 524, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Øverli, Ø.; Pottinger, T.G.; Carrick, T.R.; Øverli, E.; Winberg, S. Brain monoaminergic activity in rainbow trout selected for high and low stress responsiveness. Brain Behav. Evol. 2001, 57, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Ellis, T.; Yildiz, H.Y.; López-Olmeda, J.; Spedicato, M.T.; Tort, L.; Øverli, Ø.; Martins, C.I.M. Cortisol and finfish welfare. Fish Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef]
- Harris, J.; Bird, D.J. Modulation of the fish immune system by hormones. Vet. Immunol. Immunopathol. 2000, 77, 163–176. [Google Scholar] [CrossRef]
- Schreck, C.B.; Contreras-Sanchez, W.; Fitzpatrick, M.S. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 2001, 197, 3–24. [Google Scholar] [CrossRef]
- Fernandes-de-Castilho, M.; Pottinger, T.G.; Volpato, G.L. Chronic social stress in rainbow trout: Does it promote physiological habituation? Gen. Comp. Endocrinol. 2008, 155, 141–147. [Google Scholar] [CrossRef] [Green Version]
- DeVries, A.C.; Craft, T.K.S.; Glasper, E.R.; Neigh, G.N.; Alexander, J.K. 2006 Curt P. Richter award winner. Social influences on stress responses and health. Psychoneuroendocrinology 2007, 32, 587–603. [Google Scholar] [CrossRef]
- Carbonara, P.; Scolamacchia, M.; Spedicato, M.T.; Zupa, W.; Mckinley, R.S.; Lembo, G. Muscle activity as a key indicator of welfare in farmed European sea bass (Dicentrarchus labrax L. 1758). Aquac. Res. 2015, 46, 2133–2146. [Google Scholar] [CrossRef]
- Rodnick, K.J.; Planas, J.V. The Stress and Stress Mitigation Effects of Exercise: Cardiovascular, Metabolic, and Skeletal Muscle Adjustments; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, ISBN 9780128027288. [Google Scholar]
- Yada, T.; Tort, L. 10–Stress and Disease Resistance: Immune System and Immunoendocrine Interactions. Fish Physiol. 2016, 35, 365–403. [Google Scholar]
- Noakes, D.L.G.; Jones, K.M.M. 9-Cognition, Learning, and Behavior. In Biology of Stress in Fish; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 333–364. [Google Scholar]
- Campagnolo, L.; Hougaard, K.S. Chapter 17-Reproduction and Development. In Adverse Effects of Engineered Nanomaterials, 2nd ed.; Fadeel, B., Pietroiusti, A., Shvedova, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 397–421. ISBN 978-0-12-809199-9. [Google Scholar]
- Paull, G.C.; Filby, A.L.; Giddins, H.G.; Coe, T.S.; Hamilton, P.B.; Tyler, C.R. Dominance hierarchies in zebrafish (Danio rerio) and their relationship with reproductive success. Zebrafish 2010, 7, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Korzan, W.J.; Höglund, E.; Watt, M.J.; Forster, G.L.; Øverli, Ø.; Lukkes, J.L.; Summers, C.H. Memory of opponents is more potent than visual sign stimuli after social hierarchy has been established. Behav. Brain Res. 2007, 183, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, C.; Xie, C.; Tang, R.; Qin, X.; Wang, D.; Li, D. Effect of Stocking Density on Growth, Physiological Responses, and Body Composition of Juvenile Blunt Snout Bream, Megalobrama amblycephala. J. World Aquac. Soc. 2016, 47, 358–368. [Google Scholar] [CrossRef]
- Li, D.; Liu, Z.; Xie, C. Effect of stocking density on growth and serum concentrations of thyroid hormones and cortisol in Amur sturgeon, Acipenser schrenckii. Fish Physiol. Biochem. 2012, 38, 511–520. [Google Scholar] [CrossRef]
- Ni, M.; Wen, H.S.; Li, J.; Chi, M.; Bu, Y.; Ren, Y.; Zhang, M.; Song, Z.; Ding, H. Effects of stocking density on mortality, growth and physiology of juvenile Amur sturgeon (Acipenser schrenckii). Aquac. Res. 2016, 47, 1596–1604. [Google Scholar] [CrossRef]
- Liu, B.; Jia, R.; Han, C.; Huang, B.; Lei, J.L. Effects of stocking density on antioxidant status, metabolism and immune response in juvenile turbot (Scophthalmus maximus). Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2016, 190, 1–8. [Google Scholar] [CrossRef]
- de las Heras, V.; Martos-Sitcha, J.A.; Yúfera, M.; Mancera, J.M.; Martínez-Rodríguez, G. Influence of stocking density on growth, metabolism and stress of thick- lipped grey mullet (Chelon labrosus) juveniles. Aquaculture 2015, 448, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Pickering, A.D.; Stewart, A. Acclimation of the interrenal tissue of the brown trout, Salmo trutta L., to chronic crowding stress. J. Fish Biol. 1984, 24, 731–740. [Google Scholar] [CrossRef]
- Tort, L.; Sunyer, J.O.; Gómez, E.; Molinero, A. Crowding stress induces changes in serum haemolytic and agglutinating activity in the gilthead sea bream Sparus aurata. Vet. Immunol. Immunopathol. 1996, 51, 179–188. [Google Scholar] [CrossRef]
- Mazur, C.F.; Iwama, G.K. Handling and crowding stress reduces number of plaque-forming cells in atlantic salmon. J. Aquat. Anim. Health 1993, 5, 98–101. [Google Scholar] [CrossRef]
- Yin, Z.; Lam, T.J.; Sin, Y.M. The effects of crowding stress on the non-specific immuneresponse in fancy carp (Cyprinus carpio L.). Fish Shellfish. Immunol. 1995, 5, 519–529. [Google Scholar] [CrossRef]
- Huntingford, F.; Adams, C. Behavioural syndromes in farmed fish: Implications for production and welfare. Behaviour 2005, 142, 1207–1221. [Google Scholar] [CrossRef]
- Conte, F.S. Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 2004, 86, 205–223. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress- physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Øverli, Ø.; Sørensen, C.; Pulman, K.G.T.; Pottinger, T.G.; Korzan, W.; Summers, C.H.; Nilsson, G.E. Evolutionary background for stress-coping styles: Relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci. Biobehav. Rev. 2007, 31, 396–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, S.; Millot, S.; Leguay, D.; Chatain, B.; Bégout, M.L. Consistency in European seabass coping styles: A life-history approach. Appl. Anim. Behav. Sci. 2015, 167, 74–88. [Google Scholar] [CrossRef] [Green Version]
- Castanheira, M.F.; Conceição, L.E.C.; Millot, S.; Rey, S.; Bégout, M.L.; Damsgård, B.; Kristiansen, T.; Höglund, E.; Øverli, Ø.; Martins, C.I.M. Coping styles in farmed fish: Consequences for aquaculture. Rev. Aquac. 2017, 9, 23–41. [Google Scholar] [CrossRef]
- Tudorache, C.; Schaaf, M.J.M.; Slabbekoorn, H. Covariation between behaviour and physiology indicators of coping style in zebrafish (Danio rerio). J. Endocrinol. 2013, 219, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millot, S.; Bégout, M.L.; Chatain, B. Risk-taking behaviour variation over time in sea bass Dicentrarchus labrax: Effects of day-night alternation, fish phenotypic characteristics and selection for growth. J. Fish Biol. 2009, 75, 1733–1749. [Google Scholar] [CrossRef] [Green Version]
- Øverli, Ø.; Korzan, W.J.; Larson, E.T.; Winberg, S.; Lepage, O.; Pottinger, T.G.; Renner, K.J.; Summers, C.H. Behavioral and neuroendocrine correlates of displaced aggression in trout. Horm. Behav. 2004, 45, 324–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfonso, S.; Zupa, W.; Manfrin, A.; Fiocchi, E.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Stress coping styles: Is the basal level of stress physiological indicators linked to behaviour of sea bream? Appl. Anim. Behav. Sci. 2020, 231, 105085. [Google Scholar] [CrossRef]
- Carbonara, P.; Alfonso, S.; Zupa, W.; Manfrin, A.; Fiocchi, E.; Pretto, T.; Spedicato, M.T.; Lembo, G. Behavioral and physiological responses to stocking density in sea bream (Sparus aurata): Do coping styles matter? Physiol. Behav. 2019, 212, 112698. [Google Scholar] [CrossRef] [PubMed]
- United Nations. The Sustainable Development Goals Report 2019. United Nations Publication Issued by the Department of Economic and Social Affairs. 2019, p. 64. Available online: https://unstats.un.org/sdgs/report/2019/The-Sustainable-Development-Goals-Report-2019.pdf (accessed on 6 August 2023).
- Petrick, K.; Jérémie, F.; Heloïse, L.; Fabio, F. Blue Economy in the Mediterranean; Union for the Mediterranean: Barcelona, Spain, 2017; pp. 1–71. Available online: https://ufmsecretariat.org/wp-content/uploads/2017/12/UfMS_Blue-Economy_Report.pdf (accessed on 6 August 2023).
- FAO. World Fisheries and Aquaculture; FAO: Rome, Italy, 2018; ISBN 9789251072257. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022; ISBN 978-92-5-136364-5. [Google Scholar]
- FEAP. European Aquaculture Production Report. 2022. Available online: https://feap.info/wp-content/uploads/2022/12/feap-annual-report-feap-2022.pdf (accessed on 6 August 2023).
- Braithwaite, V.A.; Huntingford, F.A. Fish and welfare: Do fish have the capacity for pain perception and suffering? Anim. Welf. 2004, 13, 87–92. [Google Scholar] [CrossRef]
- Cerqueira, M.; Millot, S.; Felix, A.; Silva, T.; Oliveira, G.A.; Oliveira, C.C.V.; Rey, S.; MacKenzie, S.; Oliveira, R. Cognitive appraisal in fish: Stressor predictability modulates the physiological and neurobehavioural stress response in sea bass. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192922. [Google Scholar] [CrossRef] [Green Version]
- Gross, M.R. One species with two biologies: Atlantic salmon (Salmo salar) in the wild and in aquaculture. Can. J. Fish. Aquat. Sci. 1998, 55, 131–144. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Mancera, J.M.; Prunet, P.; Magnoni, L.J. Editorial: Welfare and Stressors in Fish: Challenges Facing Aquaculture. Front. Physiol. 2020, 11, 10–12. [Google Scholar] [CrossRef] [Green Version]
- FEAP. European Aquaculture Production Report. FEAP Production Report-2015. 2021. Available online: https://feap.info/wp-content/uploads/2023/04/2023-04-05-production-report-2023.pdf (accessed on 6 August 2023).
- Bjelland, H.V.; Føre, M.; Lader, P.; Kristiansen, D.; Holmen, I.M.; Fredheim, A.; Grøtli, E.I.; Fathi, D.E.; Oppedal, F.; Utne, I.B.; et al. Exposed Aquaculture In Norway Technologies For Robust Operations In Rough Conditions. In Proceedings of the OCEANS’15 MTS/IEEE Washington, Washington, DC, USA, 19–22 October 2015; ISBN 9780933957435. [Google Scholar]
- Hussey, N.E.; Kessel, S.T.; Aarestrup, K.; Cooke, S.J.; Cowley, P.D.; Fisk, A.T.; Harcourt, R.G.; Holland, K.N.; Iverson, S.J.; Kocik, J.F.; et al. Aquatic animal telemetry: A panoramic window into the underwater world. Science 2015, 348, 1255642. [Google Scholar] [CrossRef] [Green Version]
- Cooke, S.J.; Hinch, S.G.; Wikelski, M.; Andrews, R.D.; Kuchel, L.J.; Wolcott, T.G.; Butler, P.J. Biotelemetry: A mechanistic approach to ecology. Trends Ecol. Evol. 2004, 19, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, A.; Gunnarsson, A.; Árnason, T.; Oddgeirsson, M.; Sigmarsson, A.B.; Gunnarsson, Á. Validation of ECG-derived heart rate recordings in Atlantic cod (Gadus morhua L.) with an implantable data logging system. Anim. Biotelemetry 2019, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Millidine, K.J.; Metcalfe, N.B.; Armstrong, J.D. The use of ventilation frequency as an accurate indicator of metabolic rate in juvenile Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2008, 65, 2081–2087. [Google Scholar] [CrossRef]
- Metcalfe, J.D.; Wright, S.; Tudorache, C.; Wilson, R.P. Recent advances in telemetry for estimating the energy metabolism of wild fishes. J. Fish Biol. 2016, 88, 284–297. [Google Scholar] [CrossRef] [Green Version]
- Brijs, J.; Sandblom, E.; Axelsson, M.; Sundell, K.; Sundh, H.; Huyben, D.; Broström, R.; Kiessling, A.; Berg, C.; Gräns, A. The final countdown: Continuous physiological welfare evaluation of farmed fish during common aquaculture practices before and during harvest. Aquaculture 2018, 495, 903–911. [Google Scholar] [CrossRef]
- Carbonara, P.; Alfonso, S.; Gai, F.; Gasco, L.; Palmegiano, G.; Spedicato, M.T.; Zupa, W.; Lembo, G. Moderate stocking density does not influence the behavioural and physiological responses of rainbow trout (Oncorhynchus mykiss) in organic aquaculture. Aquac. Res. 2020, 51, 3007–3016. [Google Scholar] [CrossRef] [Green Version]
- Lembo, G.; Carbonara, P.; Scolamacchia, M.; Spedicato, M.T.; Bjørnsen, J.E.; Holand, B.; McKinley, R.S. Introduction of a new physiological acoustic electromyogram transmitter. Fish. Manag. Ecol. 2008, 15, 333–338. [Google Scholar] [CrossRef]
- Lembo, G.; Carbonara, P.; Scolamacchia, M.; Spedicato, M.T.; McKinley, R.S. Use of muscle activity indices as a relative measure of well-being in cultured sea bass Dicentrarchus labrax (Linnaeus, 1758). Hydrobiologia 2007, 582, 271–280. [Google Scholar] [CrossRef]
- Jepsen, N.; Schreck, C.; Clements, S.; Thorstad, E.B. A brief discussion on the 2% tag/bodymass rule of thumb. In Aquatic Telemetry: Advances and Applications; Proceedings of the Fifth Conference on Fish Telemetry Held in Europe, Ustica, Italy, 9–13 June 2003; FAO/COISPA: Rome, Italy, 2005; p. 295. [Google Scholar]
- Smircich, M.G.; Kelly, J.T. Extending the 2% rule: The effects of heavy internal tags on stress physiology, swimming performance, and growth in brook trout. Anim. Biotelemetry 2014, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, N.; Koed, A.; Thorstad, E.B.; Baras, E. Surgical implantation of telemetry transmitters in fish: How much have we learned? Hydrobiologia 2002, 483, 239–248. [Google Scholar] [CrossRef]
- Cooke, S.J.; Graeb, B.D.S.; Suski, C.D.; Ostrand, K.G. Effects of suture material on incision healing, growth and survival of juvenile largemouth bass implanted with miniature radio transmitters: Case study of a novice and experienced fish surgeon. J. Fish Biol. 2003, 62, 1366–1380. [Google Scholar] [CrossRef]
- Bridger, C.J.; Booth, R.K. The effects of biotelemetry transmitter presence and attachment procedures on fish physiology and behavior. Rev. Fish. Sci. 2003, 11, 13–34. [Google Scholar] [CrossRef]
- Cooke, S.J.; Woodley, C.M.; Eppard, M.B.; Brown, R.S.; Nielsen, J.L. Advancing the surgical implantation of electronic tags in fish: A gap analysis and research agenda based on a review of trends in intracoelomic tagging effects studies. Rev. Fish Biol. Fish. 2011, 21, 127–151. [Google Scholar] [CrossRef]
- Thiem, J.D.; Taylor, M.K.; McConnachie, S.H.; Binder, T.R.; Cooke, S.J. Trends in the reporting of tagging procedures for fish telemetry studies that have used surgical implantation of transmitters: A call for more complete reporting. Rev. Fish Biol. Fish. 2011, 21, 117–126. [Google Scholar] [CrossRef]
- Gambelli, D.; Naspetti, S.; Zander, K.; Zanoli, R. Organic Aquaculture: Economic, Market and Consumer Aspects. In Organic Aquaculture: Impacts and Future Developments; Lembo, G., Mente, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 41–63. ISBN 978-3-030-05603-2. [Google Scholar]
- Gould, D.; Compagnoni, A.; Lembo, G. Organic Aquaculture; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Borquez, A.; Serrano, E.; Dantagnan, P.; Carrasco, J.; Hernandez, A. Feeding high inclusion of whole grain white lupin (Lupinus albus) to rainbow trout (Oncorhynchus mykiss): Effects on growth, nutrient digestibility, liver and intestine histology and muscle fatty acid composition. Aquac. Res. 2011, 42, 1067–1078. [Google Scholar] [CrossRef] [Green Version]
- Geay, F.; Ferraresso, S.; Zambonino-Infante, J.L.; Bargelloni, L.; Quentel, C.; Vandeputte, M.; Kaushik, S.; Cahu, C.L.; Mazurais, D. Effects of the total replacement of fish-based diet with plant-based diet on the hepatic transcriptome of two European sea bass (Dicentrarchus labrax) half-sibfamilies showing different growth rates with the plant-based diet. BMC Genom. 2011, 12, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Smith, V.J. Fisheries Society of the British Isles (FSBI). Fish Shellfish. Immunol. 2002, 10, 213–214. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Kadri, S. Welfare and Fish. Fish Welf. 2008, 19–31. [Google Scholar] [CrossRef]
- Lembo, G.; Carbonara, P.; Fabris, A.; Manfrin, A.; Zupa, W. Welfare Issues and Veterinary Treatments. In Organic Aquaculture: Impacts and Future Developments; Lembo, G., Mente, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 119–140. ISBN 978-3-030-05603-2. [Google Scholar]
- Prunet, P.; Øverli, O.; Douxfils, J.; Bernardini, G.; Kestemont, P.; Baron, D. Fish welfare and genomics. Fish Physiol. Biochem. 2012, 38, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Raposo de Magalhães, C.S.F.; Cerqueira, M.A.C.; Schrama, D.; Moreira, M.J.V.; Boonanuntanasarn, S.; Rodrigues, P.M.L. A Proteomics and other Omics approach in the context of farmed fish welfare and biomarker discovery. Rev. Aquac. 2020, 12, 122–144. [Google Scholar] [CrossRef]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef]
- Alfonso, S.; Sadoul, B.; Cousin, X.; Bégout, M.L. Spatial distribution and activity patterns as welfare indicators in response to water quality changes in European sea bass, Dicentrarchus labrax. Appl. Anim. Behav. Sci. 2020, 226, 104974. [Google Scholar] [CrossRef]
- Sadoul, B.; Friggens, N.C.; Valotaire, C.; Labbé, L.; Colson, V.; Prunet, P.; Leguen, I. Physiological and behavioral flexibility to an acute CO2 challenge, within and between genotypes in rainbow trout. Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2017, 209, 25–33. [Google Scholar] [CrossRef]
- Stien, L.H.; Bratland, S.; Austevoll, I.; Oppedal, F.; Kristiansen, T.S. A video analysis procedure for assessing vertical fish distribution in aquaculture tanks. Aquac. Eng. 2007, 37, 115–124. [Google Scholar] [CrossRef]
- Sadoul, B.; Evouna Mengues, P.; Friggens, N.C.; Prunet, P.; Colson, V. A new method for measuring group behaviours of fish shoals from recorded videos taken in near aquaculture conditions. Aquaculture 2014, 430, 179–187. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Axelsson, M.; Chabot, D.; Claireaux, G.; Cooke, S.J.; Corner, R.A.; de Boeck, G.; Domenici, P.; Guerreiro, P.M.; Hamer, B.; et al. Conservation physiology of marine fishes: State of the art and prospects for policy. Conserv. Physiol. 2016, 4, cow046. [Google Scholar] [CrossRef] [Green Version]
- Gesto, M.; Zupa, W.; Alfonso, S.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Using acoustic telemetry to assess behavioral responses to acute hypoxia and ammonia exposure in farmed rainbow trout of different competitive ability. Appl. Anim. Behav. Sci. 2020, 230, 105084. [Google Scholar] [CrossRef]
- Oyarzún, R.; Paredes, R.; Saravia, J.; Morera, F.J.; Muñoz, J.L.P.; Ruiz-Jarabo, I.; Mancera, J.M.; Vargas-Chacoff, L. Stocking density affects the growth performance, intermediary metabolism, osmoregulation, and response to stress in Patagonian blennie Eleginops maclovinus. Aquaculture 2020, 515, 734565. [Google Scholar] [CrossRef]
- Halachmi, I.; Guarino, M.; Bewley, J.; Pastell, M. Smart Animal Agriculture: Application of Real-Time Sensors to Improve Animal Well-Being and Production. Annu. Rev. Anim. Biosci. 2019, 7, 403–425. [Google Scholar] [CrossRef]
- Jepsen, N.; Davis, L.E.; Schreck, C.B.; Siddens, B. The Physiological Response of Chinook Salmon Smolts to Two Methods of Radio-Tagging. Trans. Am. Fish. Soc. 2001, 130, 495–500. [Google Scholar] [CrossRef]
- Clark, T.D.; Sandblom, E.; Hinch, S.G.; Patterson, D.A.; Frappell, P.B.; Farrell, A.P. Simultaneous biologging of heart rate and acceleration, and their relationships with energy expenditure in free-swimming sockeye salmon (Oncorhynchus nerka). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2010, 180, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Martos-Sitcha, J.A.; Simó-Mirabet, P.; de las Heras, V.; Calduch-Giner, J.À.; Pérez-Sánchez, J. Tissue-Specific Orchestration of Gilthead Sea Bream Resilience to Hypoxia and High Stocking Density. Front. Physiol. 2019, 10, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zupa, W.; Carbonara, P.; Spedicato, M.T.; Lembo, G. Modelling swimming activities and energetic costs in European sea bass (Dicentrarchus labrax L., 1758) during critical swimming tests. Mar. Freshw. Behav. Physiol. 2015, 48, 341–357. [Google Scholar] [CrossRef]
- Alfonso, S.; Zupa, W.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Using Telemetry Sensors Mapping the Energetic Costs in European Sea Bass (Dicentrarchus labrax), as a Tool for Welfare Remote Monitoring in Aquaculture. Front. Anim. Sci. 2022, 3, 885850. [Google Scholar] [CrossRef]
- Wilson, S.M.; Hinch, S.G.; Eliason, E.J.; Farrell, A.P.; Cooke, S.J. Calibrating acoustic acceleration transmitters for estimating energy use by wild adult Pacific salmon. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2013, 164, 491–498. [Google Scholar] [CrossRef]
- Zupa, W.; Alfonso, S.; Gai, F.; Gasco, L.; Spedicato, M.T.; Lembo, G.; Carbonara, P. Calibrating accelerometer tags with oxygen consumption rate of rainbow trout (Oncorhynchus mykiss) and their use in aquaculture facility: A case study. Animals 2021, 11, 1496. [Google Scholar] [CrossRef]
- Norin, T.; Clark, T.D. Measurement and relevance of maximum metabolic rate in fishes. J. Fish Biol. 2016, 88, 122–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brett, J.R. The Respiratory Metabolism and Swimming Performance of Young Sockeye Salmon. J. Fish. Res. Board Can. 1964, 21, 1183–1226. [Google Scholar] [CrossRef]
- Claireaux, G.; Couturier, C.; Groison, A.L. Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J. Exp. Biol. 2006, 209, 3420–3428. [Google Scholar] [CrossRef] [Green Version]
- Claireaux, G.; Lefrançois, C. Linking environmental variability and fish performance: Integration through the concept of scope for activity. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 2031–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rome, L.C.; Choi, I.H.; Lutz, G.; Sosnicki, A. The influence of temperature on muscle function in the fast swimming scup. I. Shortening velocity and muscle recruitment during swimming. J. Exp. Biol. 1992, 163, 259–279. [Google Scholar] [CrossRef]
- Burgetz, I.J.; Rojas-Vargas, A.; Hinch, S.G.; Randall, D.J. Initial recruitment of anaerobic metabolism during sub-maximal swimming in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 1998, 201, 2711–2721. [Google Scholar] [CrossRef]
- Ellerby, D.J.; Altringham, J.D.; Williams, T.; Block, B.A. Slow muscle function of Pacific bonito (Sarda chiliensis) during steady swimming. J. Exp. Biol. 2000, 203, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Brijs, J.; Sandblom, E.; Axelsson, M.; Sundell, K.; Sundh, H.; Kiessling, A.; Berg, C.; Gräns, A. Remote physiological monitoring provides unique insights on the cardiovascular performance and stress responses of freely swimming rainbow trout in aquaculture. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lembo, G.; Mente, E. Organic Aquaculture. In Impacts and Future Developments; Springer: Pennsylvania, PA, USA, 2019; ISBN 9783030056025. [Google Scholar]
- Kaushik, S.J.; Seiliez, I. Protein and amino acid nutrition and metabolism in fish: Current knowledge and future needs. Aquac. Res. 2010, 41, 322–332. [Google Scholar] [CrossRef]
- Palmegiano, G.B.; Gai, F.; Gasco, L.; Lembo, G.; Spedicato, M.T.; Trotta, P.; Zoccarato, I. Partial replacement of fish meal by T-Iso in gilthead sea bream (Sparus aurata) juveniles diets. Ital. J. Anim. Sci. 2009, 8, 869–871. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Mai, K.S.; Tan, B.P.; Liu, Y.J. Partial replacement of fishmeal by soybean meal in diets for juvenile cobia (Rachycentron canadum). Aquac. Nutr. 2005, 11, 175–182. [Google Scholar] [CrossRef]
- Teletchea, F. Domestication of marine fish species: Update and perspectives. J. Mar. Sci. Eng. 2015, 3, 1227–1243. [Google Scholar] [CrossRef] [Green Version]
- Vandeputte, M.; Gagnaire, P.A.; Allal, F. The European sea bass: A key marine fish model in the wild and in aquaculture. Anim. Genet. 2019, 50, 195–206. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Obach, A.; Arantzamendi, L.; Montero, D.; Robaina, L.; Rosenlund, G. Dietary lipid sources for seabream and seabass: Growth performance, tissue composition and flesh quality. Aquac. Nutr. 2003, 9, 397–407. [Google Scholar] [CrossRef]
- Bonvini, E.; Bonaldo, A.; Mandrioli, L.; Sirri, R.; Dondi, F.; Bianco, C.; Fontanillas, R.; Mongile, F.; Gatta, P.P.; Parma, L. Effects of feeding low fishmeal diets with increasing soybean meal levels on growth, gut histology and plasma biochemistry of sea bass. Animal 2018, 12, 923–930. [Google Scholar] [CrossRef]
- Whyte, J.J.; Jung, R.E.; Schmitt, C.J.; Tillitt, D.E. Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit. Rev. Toxicol. 2000, 30, 347–570. [Google Scholar] [CrossRef]
- Teles, M.; Gravato, C.; Pacheco, M.; Santos, M.A. Juvenile sea bass biotransformation, genotoxic and endocrine responses to β-naphthoflavone, 4-nonylphenol and 17β-estradiol individual and combined exposures. Chemosphere 2004, 57, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Mie, A.; Andersen, H.R.; Gunnarsson, S.; Kahl, J.; Kesse-Guyot, E.; Rembiałkowska, E.; Quaglio, G.; Grandjean, P. Human health implications of organic food and organic agriculture: A comprehensive review. Environ. Health A Glob. Access Sci. Source 2017, 16, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huntingford, F.A. Implications of domestication and rearing conditions for the behaviour of cultivated fishes. J. Fish Biol. 2004, 65, 122–142. [Google Scholar] [CrossRef]
- McEwen, B.S.; Stellar, E. Stress and individual. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Auperin, B.; Geslin, M. Plasma cortisol response to stress in juvenile rainbow trout is influenced by their life history during early development and by egg cortisol content. Gen. Comp. Endocrinol. 2008, 158, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Fast, M.D.; Hosoya, S.; Johnson, S.C.; Afonso, L.O.B. Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish Shellfish. Immunol. 2008, 24, 194–204. [Google Scholar] [CrossRef]
- Chrousos, G.P. The Hypothalamic–Pituitary–Adrenal Axis and Immune-Mediated Inflammation. N. Engl. J. Med. 1995, 332, 1351–1362. Available online: https://www.nejm.org/doi/10.1056/NEJM199505183322008?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 6 August 2023). [CrossRef]
- Sloman, K. Physiological effects of dominance hierarchies: Laboratory artefacts or natural phenomena? J. Fish Biol. 2002, 61, 1–23. [Google Scholar] [CrossRef]
- Currie, S.; Leblanc, S.; Watters, M.A.; Gilmour, K.M. Agonistic encounters and cellular angst: Social interactions induce heat shock proteins in juvenile salmonid fish. Proc. R. Soc. B Biol. Sci. 2010, 277, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Magnhagen, C.; Borcherding, J. Risk-taking behaviour in foraging perch: Does predation pressure influence age-specific boldness? Anim. Behav. 2008, 75, 509–517. [Google Scholar] [CrossRef]
- Rosenthal, G.G.; Martinez, T.Y.F.; García de León, F.J.; Ryan, M.J. Shared preferences by predators and females for male ornaments in swordtails. Am. Nat. 2001, 158, 146–154. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Hirschenhauser, K.; Carneiro, L.A.; Canario, A.V.M. Social modulation of androgen levels in male teleost fish. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2002, 132, 203–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutte, C.; Taborsky, M.; Brinkhof, M.W.G. What sets the odds of winning and losing? Trends Ecol. Evol. 2006, 21, 16–21. [Google Scholar] [CrossRef]
- Galhardo, L.; Oliveira, R.F. Psychological stress and welfare in fish. Annu. Rev. Biomed. Sci. 2009, 11, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, D.C.; Spencer, R.L.; Weiss, S.M.; Blanchard, R.J.; McEwen, B.; Sakai, R.R. Visible burrow system as a model of chronic social stress: Behavioral and neuroendocrine correlates. Psychoneuroendocrinology 1995, 20, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Höglund, E.; Kolm, N.; Winberg, S. Stress-induced changes in brain serotonergic activity, plasma cortisol and aggressive behavior in Arctic charr (Salvelinus alpinus) is counteracted by L-DOPA. Physiol. Behav. 2001, 74, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.I.M.; Trenovski, M.; Schrama, J.W.; Verreth, J.A.J. Comparison of feed intake behaviour and stress response in isolated and non-isolated African catfish. J. Fish Biol. 2006, 69, 629–636. [Google Scholar] [CrossRef]
- Edeline, E.; Haugen, T.O.; Weltzien, F.A.; Claessen, D.; Winfield, I.J.; Stenseth, N.C.; Asbjørn Vøllestad, L. Body downsizing caused by non-consumptive social stress severely depresses population growth rate. Proc. R. Soc. B Biol. Sci. 2010, 277, 843–851. [Google Scholar] [CrossRef]
- Filby, A.L.; Paull, G.C.; Bartlett, E.J.; Van Look, K.J.W.; Tyler, C.R. Physiological and health consequences of social status in zebrafish (Danio rerio). Physiol. Behav. 2010, 101, 576–587. [Google Scholar] [CrossRef]
- Rivers, J.J.; Josephs, R.A. Dominance and health: The role of social rank in physiology and illness. In The Social Psychology of Power; Guilford Press: New York, NY, USA, 2010; pp. 87–112. ISBN 978-1-60623-619-2. [Google Scholar]
- Golub, M.S.; Sassenrath, E.N.; Goo, G.P. Plasma cortisol levels and dominance in peer groups of rhesus monkey weanlings. Horm. Behav. 1979, 12, 50–59. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Sloman, K.A.; Metcalfe, N.B.; Taylor, A.C.; Gilmour, K.M. Plasma cortisol concentrations before and after social stress in rainbow trout and brown trout. Physiol. Biochem. Zool. 2001, 74, 383–389. [Google Scholar] [CrossRef]
- Goldan, O.; Popper, D.; Karplus, I. Food competition in small groups of juvenile gilthead sea bream (Sparus aurata). Isr. J. Aquac.-Bamidgeh 2003, 55, 94–106. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Nazzaro-Alvarez, J.; Jardí-Pons, A.; Reig, L.; Carella, F.; Carrassón, M.; Roque, A. Linking stocking densities and feeding strategies with social and individual stress responses on gilthead seabream (Sparus aurata). Physiol. Behav. 2020, 213, 112723. [Google Scholar] [CrossRef]
- Castanheira, M.F.; Herrera, M.; Costas, B.; Conceição, L.E.C.; Martins, C.I.M. Linking cortisol responsiveness and aggressive behaviour in gilthead seabream Sparus aurata: Indication of divergent coping styles. Appl. Anim. Behav. Sci. 2013, 143, 75–81. [Google Scholar] [CrossRef]
- Bell, I.R.; Hardin, E.E.; Baldwin, C.M.; Schwartz, G.E. Increased Limbic System Symptomatology and Sensitizability of Young Adults with Chemical and Noise Sensitivities. Environ. Res. 1995, 70, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.N.; Blight, C.M. Algorithmic behaviour and spatial memory are used by two intertidal fish species to solve the radial maze. Anim. Behav. 1999, 58, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Batzina, A.; Karakatsouli, N. The presence of substrate as a means of environmental enrichment in intensively reared gilthead seabream Sparus aurata: Growth and behavioral effects. Aquaculture 2012, 370, 54–60. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Caballero-Froilán, J.C.; Jiménez-García, M.; Capó, X.; Tejada, S.; Saraiva, J.L.; Sureda, A.; Moranta, D. Enriched environments enhance cognition, exploratory behaviour and brain physiological functions of Sparus aurata. Sci. Rep. 2020, 10, 11252. [Google Scholar] [CrossRef]
- Føre, M.; Frank, K.; Norton, T.; Svendsen, E.; Alfredsen, J.A.; Dempster, T.; Eguiraun, H.; Watson, W.; Stahl, A.; Sunde, L.M.; et al. Precision fish farming: A new framework to improve production in aquaculture. Biosyst. Eng. 2018, 173, 176–193. [Google Scholar] [CrossRef]
- Stickney, R.R. Polyculturepolyculturein Aquaculture. In Sustainable Food Production; Christou, P., Savin, R., Costa-Pierce, B.A., Misztal, I., Whitelaw, C.B.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1366–1368. ISBN 978-1-4614-5797-8. [Google Scholar]
- Thomas, M.; Pasquet, A.; Aubin, J.; Nahon, S.; Lecocq, T. When more is more: Taking advantage of species diversity to move towards sustainable aquaculture. Biol. Rev. 2021, 96, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Nhan, D.K.; Phong, L.T.; Verdegem, M.J.C.; Duong, L.T.; Bosma, R.H.; Little, D.C. Integrated freshwater aquaculture, crop and livestock production in the Mekong delta, Vietnam: Determinants and the role of the pond. Agric. Syst. 2007, 94, 445–458. [Google Scholar] [CrossRef]
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-González, J.A.; Yarish, C.; Neefus, C. Integrating seaweeds into marine aquaculture systems: A key toward sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Flickinger, D.L.; Dantas, D.P.; Proença, D.C.; David, F.S.; Valenti, W.C. Phosphorus in the culture of the Amazon river prawn (Macrobrachium amazonicum) and tambaqui (Colossoma macropomum) farmed in monoculture and in integrated multitrophic systems. J. World Aquac. Soc. 2020, 51, 1002–1023. [Google Scholar] [CrossRef]
- Medeiros, M.V.; Aubin, J.; Camargo, A.F.M. Life cycle assessment of fish and prawn production: Comparison of monoculture and polyculture freshwater systems in Brazil. J. Clean. Prod. 2017, 156, 528–537. [Google Scholar] [CrossRef] [Green Version]
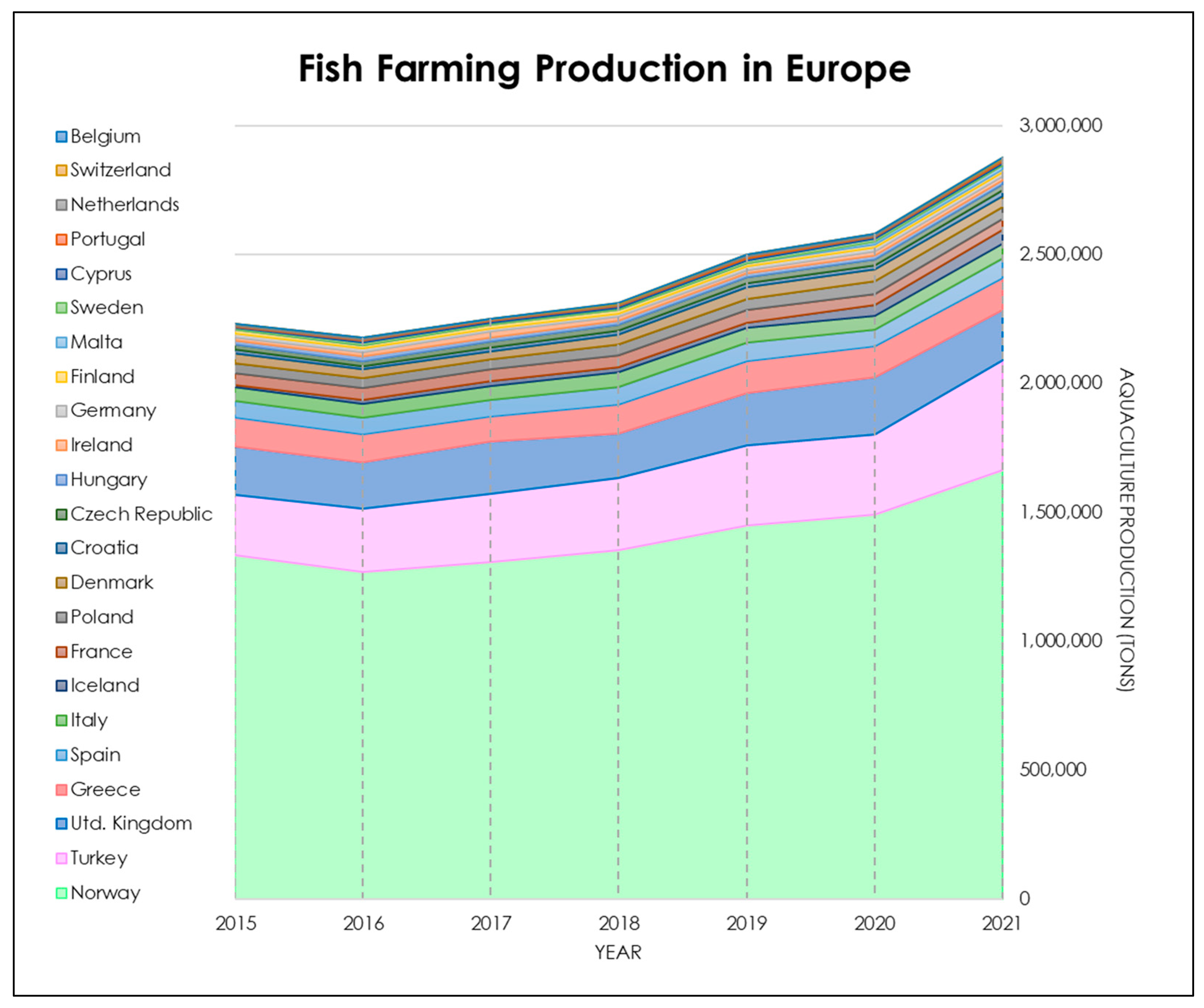
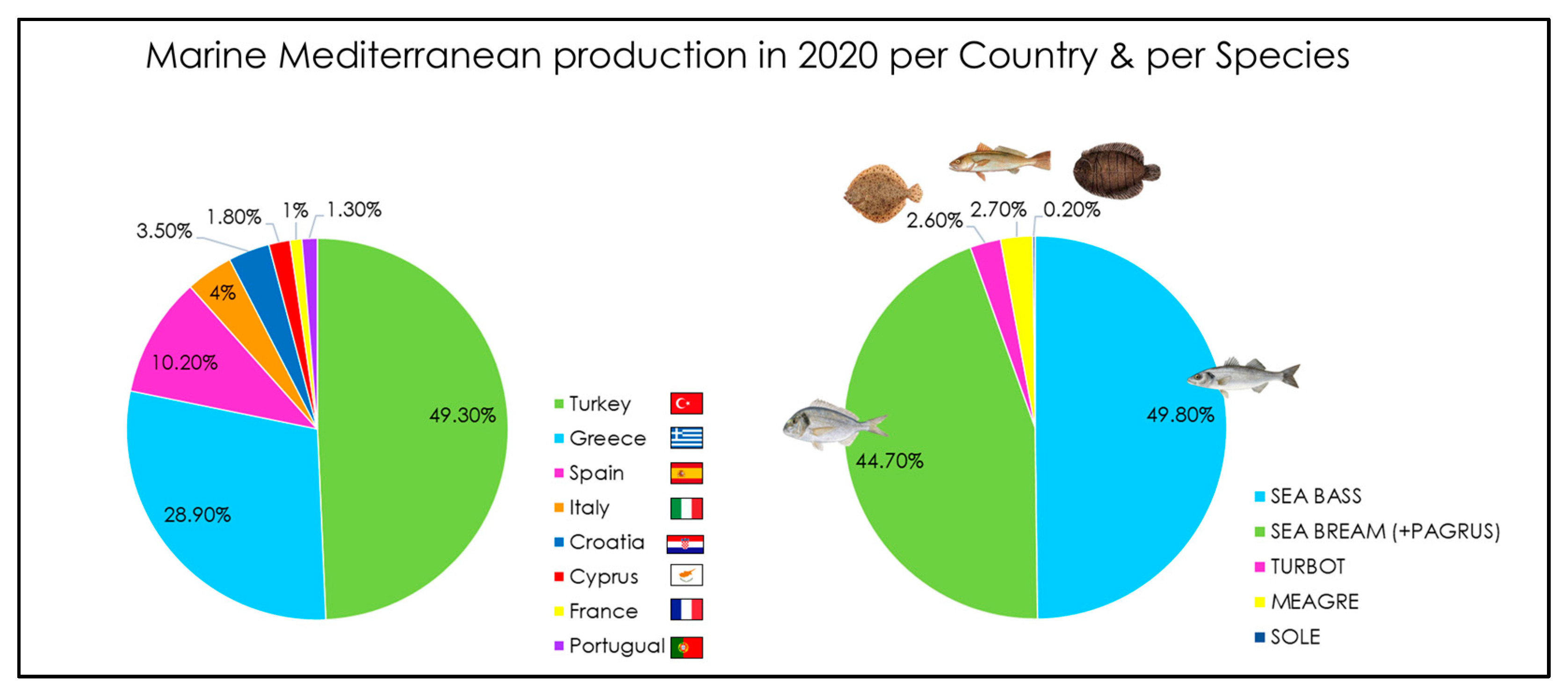


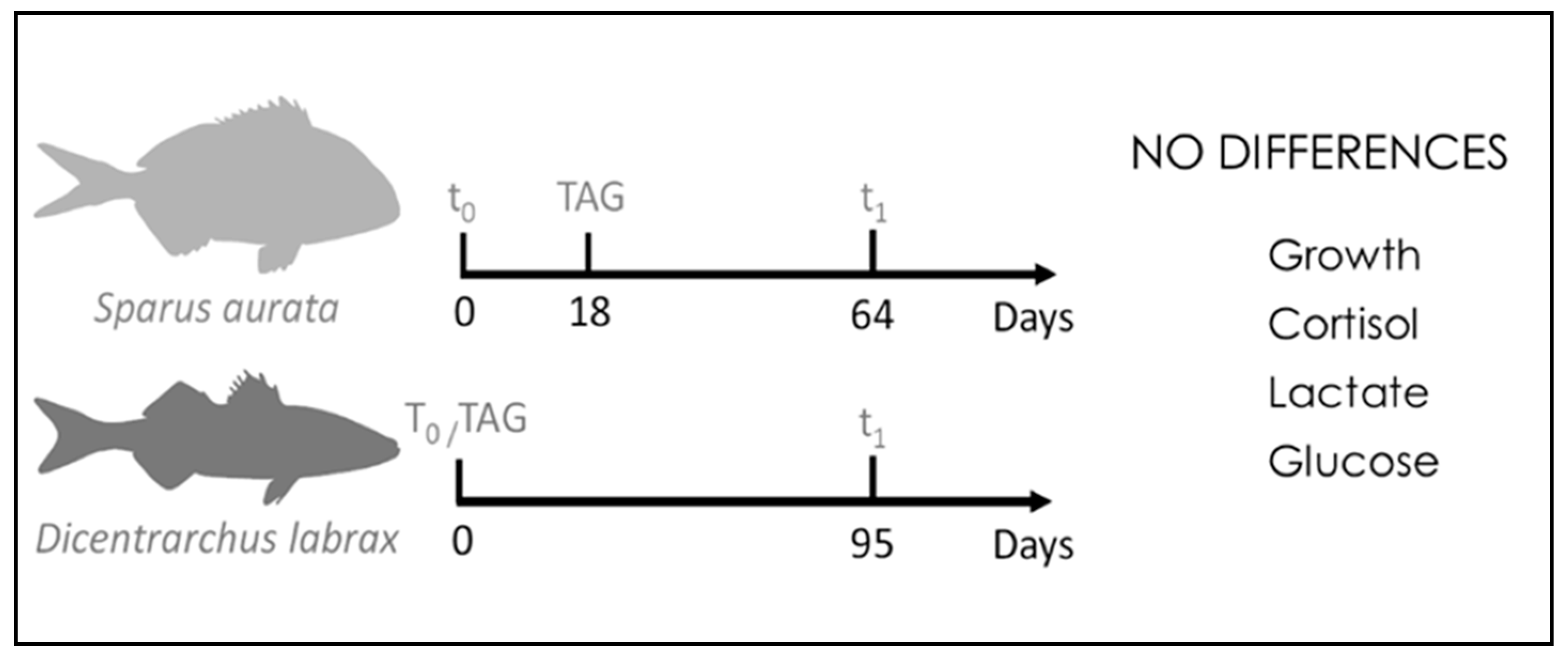
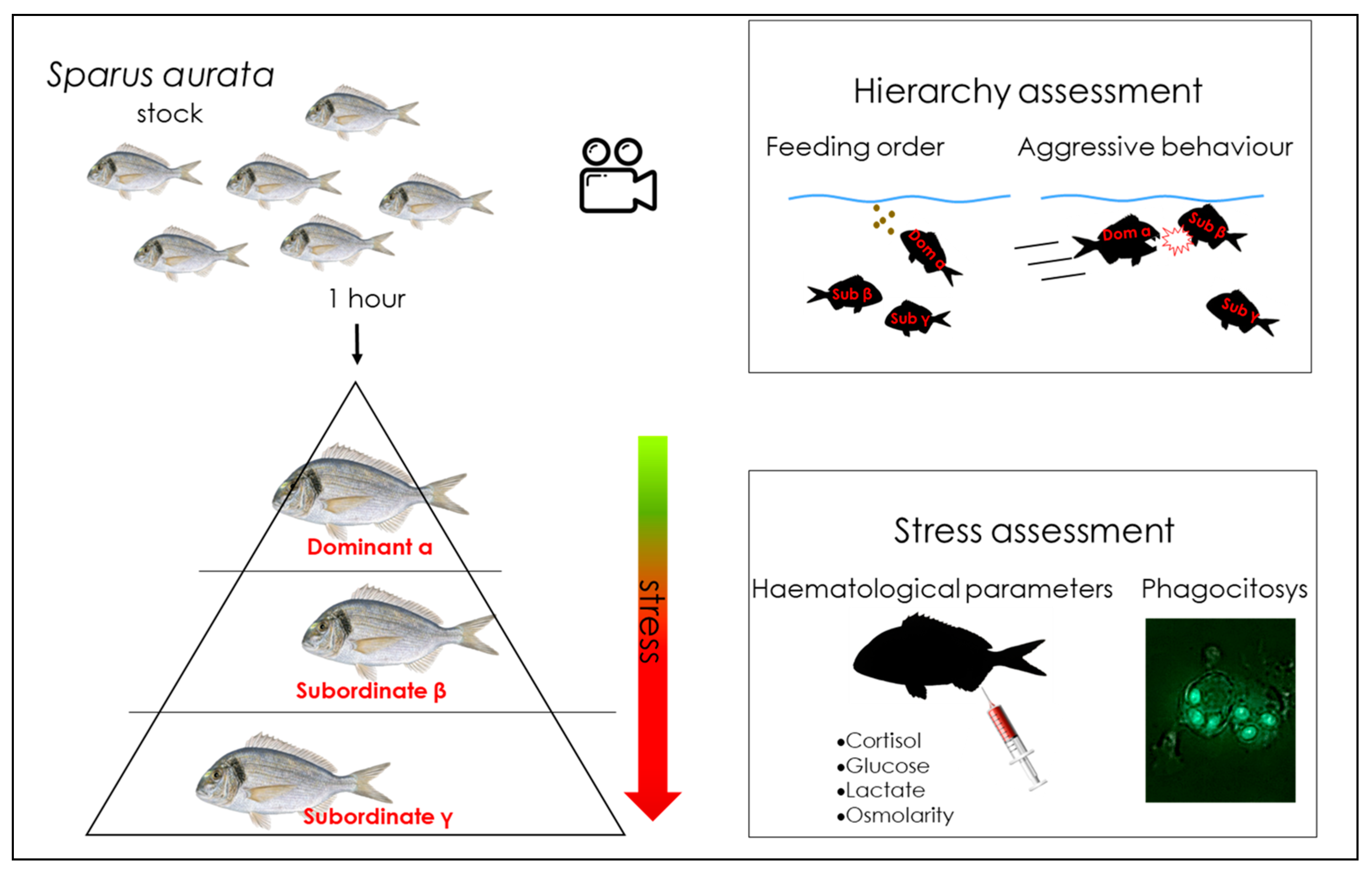
| Paper | Species | Tools | Methods | Results |
|---|---|---|---|---|
| [31] | S. aurata D. labrax | Acoustic accelerometer tags | Telemetry; growth; physiological approach | No significant differences among the tagged and untagged fish groups. |
| [32] | D. labrax | Acoustic accelerometer tag; Blažka swimming chamber; diets | Telemetry; morphometry; physiological, immunological approach | Organic diet does not affect the welfare of the European sea bass. Previously calibrated acoustic transmitters are a promising |
| [33] | D. labrax | Different diets; radio transmitters; Blažka chamber | Telemetry; morphometric, physiological, immunological approach | EMG, recovery ratio, growth Cortisol, glucose, and lysozyme proved to be sensitive to assessing welfare |
| [34] | S. aurata | Aquariums-arena; recording camera | Behavioural observation; physiological, immunological approach | Links between behaviour, stress physiological profile and immunity, in relation to social hierarchy |
| [35] | S. aurata | Aquariums-arena; recording camera | Behavioural observation; physiological, immunological approach | Exploration time is fundamental for hierarchy; demonstrated social rank and physiological immunological profile relation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dara, M.; Carbonara, P.; La Corte, C.; Parrinello, D.; Cammarata, M.; Parisi, M.G. Fish Welfare in Aquaculture: Physiological and Immunological Activities for Diets, Social and Spatial Stress on Mediterranean Aqua Cultured Species. Fishes 2023, 8, 414. https://doi.org/10.3390/fishes8080414
Dara M, Carbonara P, La Corte C, Parrinello D, Cammarata M, Parisi MG. Fish Welfare in Aquaculture: Physiological and Immunological Activities for Diets, Social and Spatial Stress on Mediterranean Aqua Cultured Species. Fishes. 2023; 8(8):414. https://doi.org/10.3390/fishes8080414
Chicago/Turabian StyleDara, Mariano, Pierluigi Carbonara, Claudia La Corte, Daniela Parrinello, Matteo Cammarata, and Maria Giovanna Parisi. 2023. "Fish Welfare in Aquaculture: Physiological and Immunological Activities for Diets, Social and Spatial Stress on Mediterranean Aqua Cultured Species" Fishes 8, no. 8: 414. https://doi.org/10.3390/fishes8080414
APA StyleDara, M., Carbonara, P., La Corte, C., Parrinello, D., Cammarata, M., & Parisi, M. G. (2023). Fish Welfare in Aquaculture: Physiological and Immunological Activities for Diets, Social and Spatial Stress on Mediterranean Aqua Cultured Species. Fishes, 8(8), 414. https://doi.org/10.3390/fishes8080414