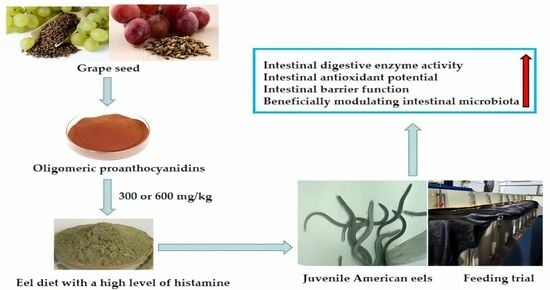
Oligomeric Proanthocyanidins Alleviate the Detrimental Effects of Dietary Histamine on Intestinal Health of Juvenile American Eels (Anguilla rostrata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish, Diet, and Management
2.2. Sample Collection
2.3. Intestinal Digestive Enzyme Analysis
2.4. Intestinal Antioxidant Potential
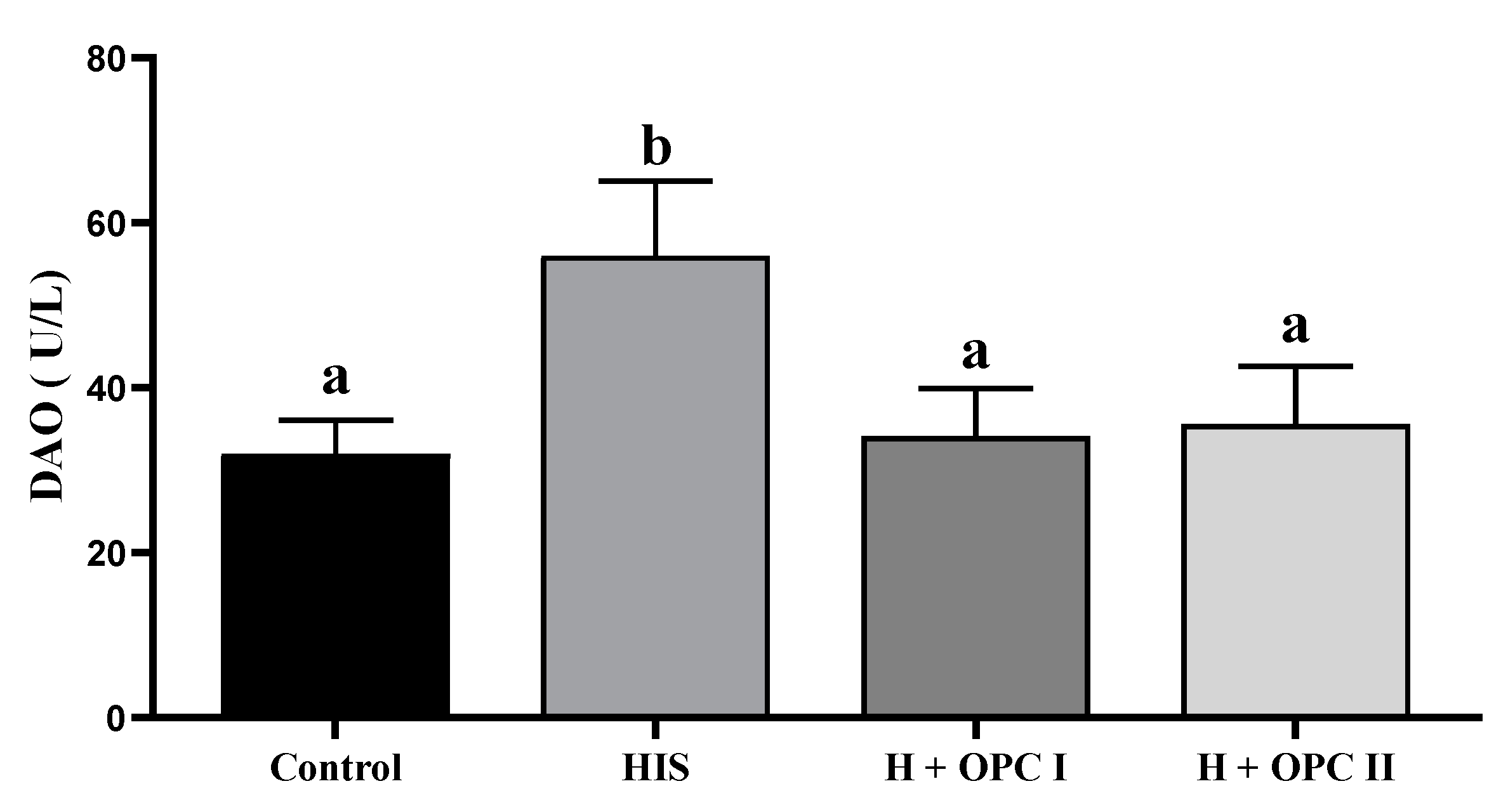
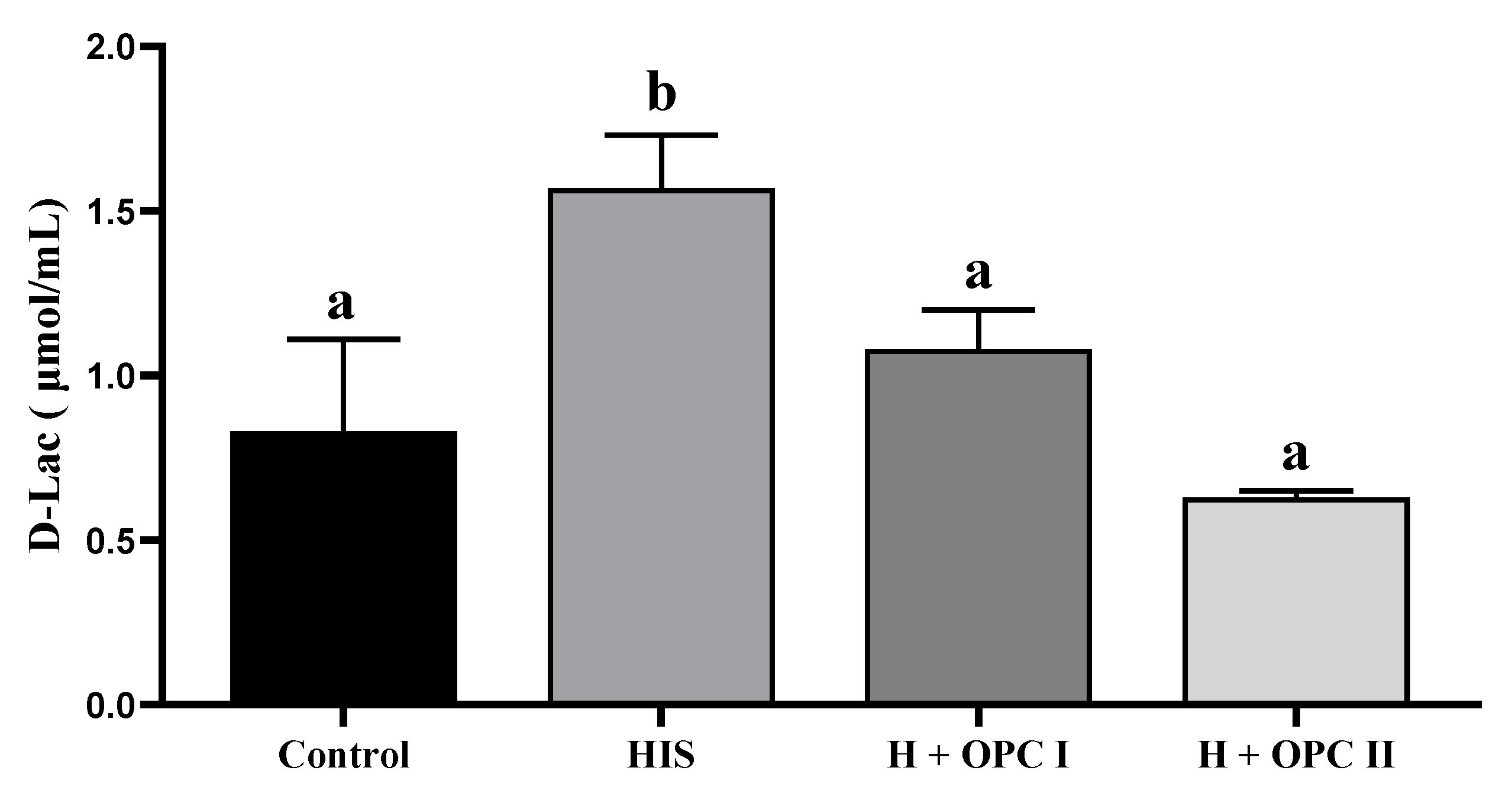
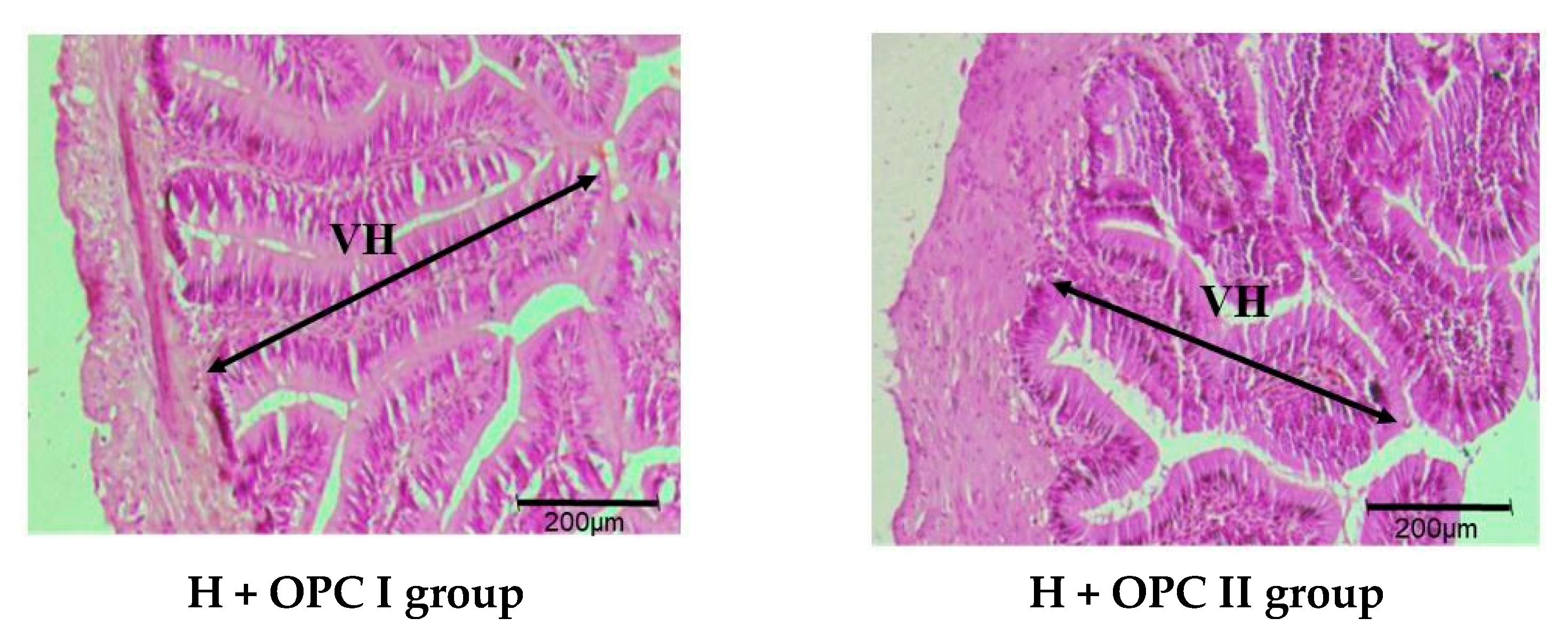
2.5. Parameters Related to Intestinal Barrier Function
2.6. Intestinal Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Digestive Enzyme Activities in the Intestine
3.2. Antioxidant Potential in the Intestine
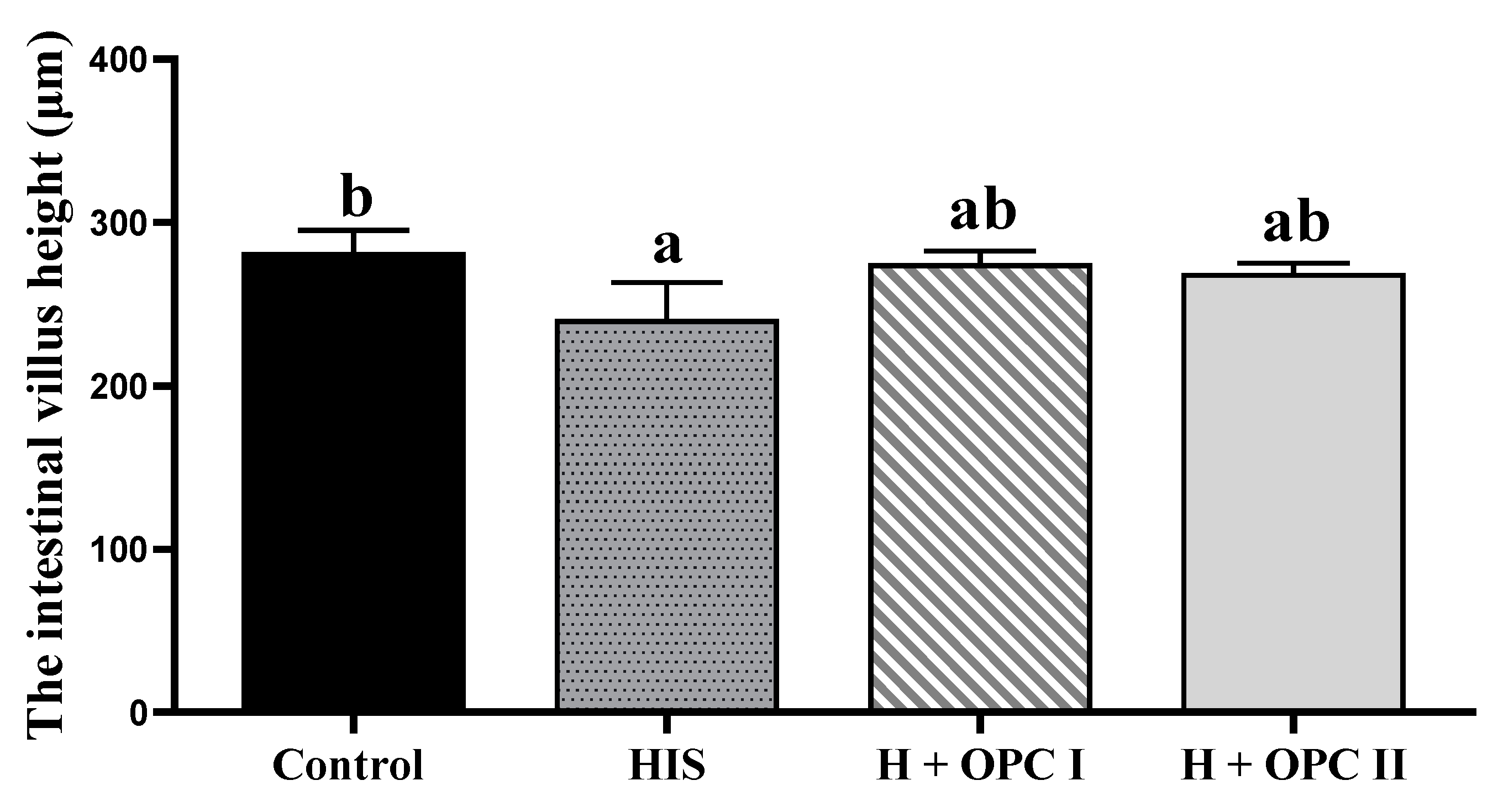
3.3. Parameters Related to Intestinal Barrier Function
3.4. Intestinal Microbiota Analysis
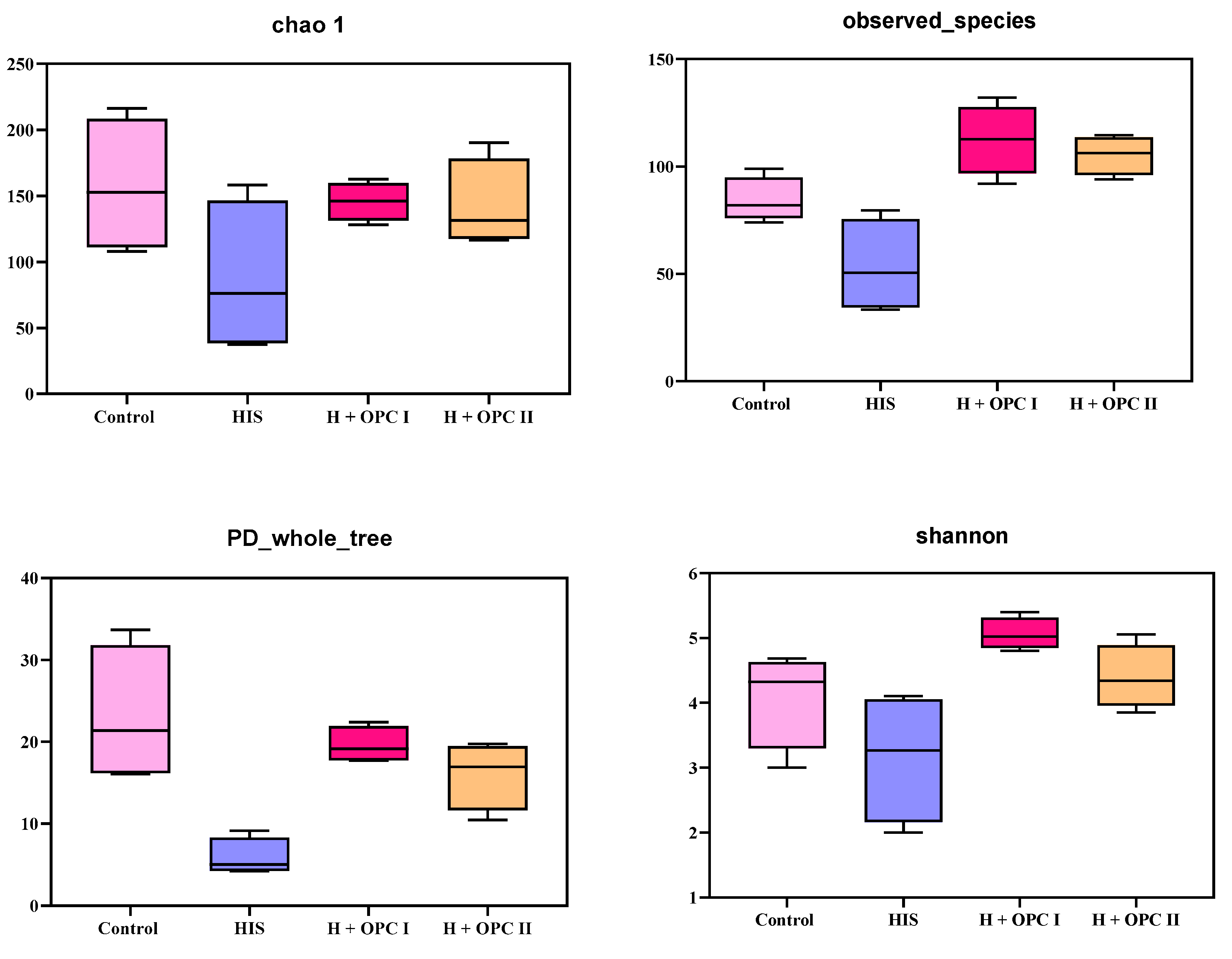
3.4.1. Alpha Diversity of Intestinal Microbiota
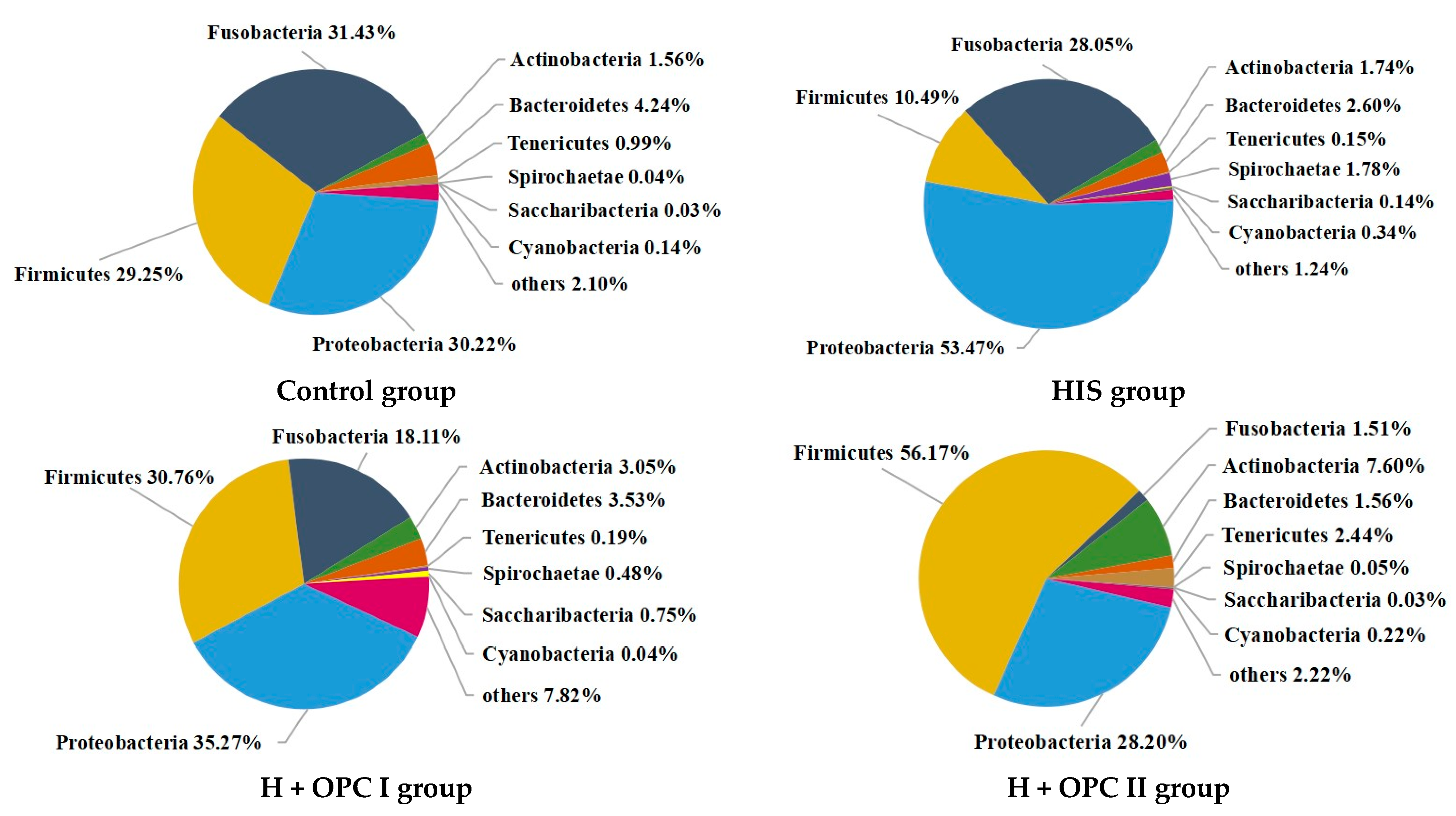
3.4.2. Intestinal Microbiota at the Phylum Level
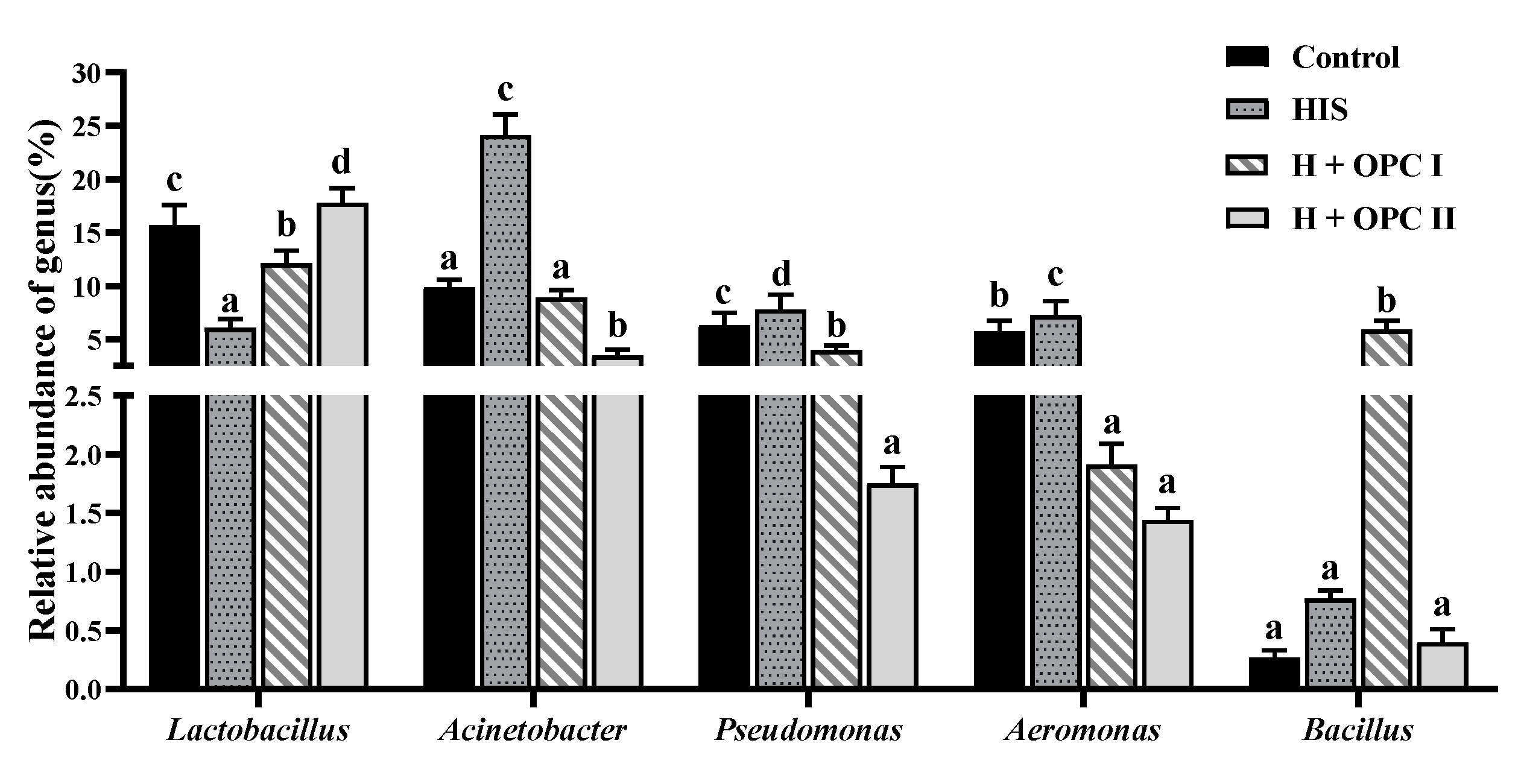
3.4.3. Intestinal Microbiota at the Genus Level
4. Discussion
4.1. Effects of OPC on the Intestinal Digestive Enzyme Activities of Juvenile American Eels Exposed to the High Level of Dietary Histamine
4.2. Effects of OPC on the Intestinal Antioxidant Potential of Juvenile American Eels Exposed to a High Level of Dietary Histamine
4.3. Effects of OPC on the Intestinal Barrier Function of Juvenile American Eels Exposed to a High Level of Dietary Histamine
4.4. Effects of OPC on the Intestinal Microbiota of Juvenile American Eel Exposed to a High Level of Dietary Histamine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Y.; Yuan, Y.M.; Dai, Y.Y.; Gong, Y.C.; Yuan, Y.Q. Development status and trends in the eel farming industry in Asia. N. Am. J. Aquacult. 2022, 84, 3–17. [Google Scholar] [CrossRef]
- Qu, Q.; Cai, G.H.; Chen, X.H.; Zhai, S.W. Comparison of nutritional differences of imported white fish meal and brown fish meal for eel feed. Feed Res. 2021, 44, 95–97. [Google Scholar] [CrossRef]
- Chu, B.Q.; Lin, L.; He, Y. Rapid determination of histamine concentration in fish (Miichthys miiuy) by surface-enhanced Raman spectroscopy and density functional theory. Int. J. Agric. Biol. Eng. 2017, 10, 252–285. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhou, H.; Liu, Y.; Zhu, L.L.; Fan, J.T.; Huang, H.J.; Jiang, W.; Deng, J.M.; Tan, B.P. Dietary histamine impairs the digestive physiology function and muscle quality of hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Antioxidants 2023, 12, 502. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.Y.; Cai, P.Y.; Zhai, S.W.; Chen, X.H. Effect of dietary histamine on growth performance, digestive enzyme activities and antioxidant indices in intestine of juvenile American eels (Anguilla rostrata). Feed Res. 2020, 43, 42–45. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Latif, H.M.R.A. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: An overview. Rev. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Zhai, S.W.; Wang, Y.; He, Y.X.; Chen, X.H. Oligomeric proanthocyanidins counteracts the negative effects of high level of dietary histamine on American eel (Anguilla rostrata). Front. Mar. Sci. 2020, 7, 549145. [Google Scholar] [CrossRef]
- Huang, L.X.; Lu, J.J.; Chen, X.H.; Zhai, S.W. Effect of grape seed proanthocyanidins on serum biochemical indices and free radical levels in intestine of GIFT tilapia exposed to dietary cadmium stress. Feed Res. 2019, 42, 30–33. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, C.Q.; Lu, J.Y.; Sun, Y.H.; Cui, Y.Y. Research progress of proanthocyanidins and anthocyanidins. Phytother. Res. 2023, 37, 2552–2577. [Google Scholar] [CrossRef]
- Yang, L.Y.; Xian, D.H.; Xiong, X.; Lai, R.; Song, J.; Zhong, J.Q. Proanthocyanidins against oxidative stress: From molecular mechanisms to clinical applications. BioMed Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef]
- Rodríguez, P.C.; Villanova, B.G.; Hernández, E.G.; Verardo, V. Grape seeds proanthocyanidins: An overview of in vivo bioactivity in animal models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef]
- Xu, G.L.; Xing, W.; Li, T.L.; Yu, H.H.; Wei, S.B.; Jiang, N.; Ma, Z.H.; Luo, L. Dietary grape seed proanthocyanidins improved growth, immunity, antioxidant, digestive enzymes activities, and intestinal microbiota of juvenile hybrid sturgeon (Acipenser baeri Brandt ♀ × A. schrenckii Brandt ♂). Aquacult. Nutr. 2021, 27, 1983–1995. [Google Scholar] [CrossRef]
- Fu, X.F.; Yan, M.H.; You, C.P. Physiological functions and interactions with gut microbiota of proanthocyanidins. Sci. Technol. Food Ind. 2020, 10, 350–357. [Google Scholar] [CrossRef]
- Bagchi, D.; Swaroop, A.; Preuss, H.G.; Bagchi, M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: An overview. Mutat. Res. 2014, 768, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.W.; Lu, J.J.; Chen, X.H. Effects of dietary grape seed proanthocyanidins on growth performance, some serum biochemical parameters and body composition of tilapia (Oreochromis niloticus) fingerlings. Ital. J. Anim. Sci. 2014, 13, 3357. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, P.; Chen, X.H.; Zhai, S.W. Effects of dietary proanthocyanidins supplementation on growth performance, digestive enzymes activities and microbiota in the intestine of juvenile American eels (Anguilla rostrata) cultured in cement tanks. Isr. J. Aquacult-Bamid. 2020, 72, 1226208. [Google Scholar] [CrossRef]
- Jia, Z.Y.; Tan, Y.T.; Liu, Y.M.; Cai, G.H.; Chen, X.H.; Zhai, S.W. Grape seed proanthocyanidins alleviate the negative effects of dietary cadmium on pearl gentian grouper (Epinephelus fuscoguttatus female × Epinephelus lanceolatus male). Isr. J. Aquacult-Bamid. 2021, 73, 1427267. [Google Scholar] [CrossRef]
- Lange, B.; Currie, K.; Howarth, G.S.; Stone, D.A.J. Grape seed extract and dried macroalgae, Ulva lactuca Linnaeus, improve survival of greenlip abalone, Haliotis laevigata Donovan, at high water temperature. Aquaculture 2014, 433, 348–360. [Google Scholar] [CrossRef]
- Chen, R.N.; Huang, L.X.; Zhai, S.W. Effects of Macleaya cordata extract on growth performance, serum biochemical parameters, and intestinal health of juvenile American eel (Anguilla rostrata). Fishes 2022, 7, 229. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, D.Y.; Zhai, S.W. Revealing the difference of intestinal microbiota composition of cultured European eels (Anguilla anguilla) with different growth rates. Isr. J. Aquacult-Bamid. 2020, 72, 959575. [Google Scholar] [CrossRef]
- Darafsh, F.; Soltani, M.; Abdolhay, H.A.; Mehrejan, M.S. Improvement of growth performance, digestive enzymes and body composition of Persian sturgeon (Acipenser persicus) following feeding on probiotics: Bacillus licheniformis, Bacillus subtilis and Saccharomyces cerevisiae. Aquac. Res. 2020, 51, 957–964. [Google Scholar] [CrossRef]
- Xu, Q.Y. Preliminary Study of Toxic Effects of Dietary Histamine on American Eel (Anguilla rostrata) Juveniles. Master’s Thesis, Jimei University, Xiamen, China, 2021. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, X.; Cheng, Y.; Yang, S. Effect of dietary histamine supplementation on growth, digestive enzyme activities and morphology of intestine and hepatopancreas in the Chinese mitten crab Eriocheir sinensis. SpringerPlus 2016, 5, 552. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, X.L.; Huang, H.J.; Fan, J.T.; Zhou, H.; Deng, J.M.; Tan, B.P. High dietary histamine induces digestive tract oxidative damage in juvenile striped catfish (Pangasianodon hypophthalmus). Antioxidants 2022, 11, 2276. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Z.; Hu, H.; Xiao, K.Y.; Luo, S.M.; Xie, P.R.; Ouyang, J.Y. Effects of plant extract from grape seed and sweet wormwood on intestinal digestive enzyme activities and blood biochemical parameters of eel (Monopterus albus). South China Fish. Sci. 2013, 9, 70–75. [Google Scholar] [CrossRef]
- Lu, J.J.; Zhai, S.W. Effects of grape proanthocyanidins supplementation on growth performance, intestinal digestive enzyme activities, serum lipid levels and hepatopancreas antioxidant capacity of genetic improvement of farmed tilapia (GIFT, Oreochromis niloticus). Chin. J. Anim. Nutr. 2014, 26, 1095–1102. [Google Scholar] [CrossRef]
- Wang, Y. Effects of Peanut Skin Proanthocyanidins Supplemented in Diets of Juvenile American Eel (Anguilla rostrata) on Growth, Antioxidant Capacity and Lipid Metabolism. Master’s Thesis, Jimei University, Xiamen, China, 2022. [Google Scholar]
- Gonzalez, Q.C.; Rodriguez, G.E.; Beltran, D.R.; Pinent, M.; Ardevol, A.; Blay, M.T.; Terra, X. Health-promoting properties of proanthocyanidins for intestinal dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Houseiny, W.; Khalil, A.A. The effects of toxigenic Clostridium perfringens types A and D on survival, as well as innate immune, inflammatory and oxidative stress responses in Nile tilapia. Aquaculture 2020, 529, 735694. [Google Scholar] [CrossRef]
- Holanda, B.F.; Araujo, D.F.D.; Silva, J.N.R.; Pereira, M.G.; Pires, A.D.F.; Assreuy, A.M. Polysaccaride-rich extract of Caesalpina ferrea stem barks attenuates mice acute inflammation induced by zymosan: Oxidative stress modulation. J. Ethnopharmacol. 2021, 267, 113501. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yang, H.L.; Hu, L.H.; Yang, W.; Ai, C.X.; Sun, Y.Z. Autochthonous probiotics alleviate the adverse effects of dietary histamine in juvenile grouper (Epinephelus coioides). Front. Microbiol. 2021, 12, 792718. [Google Scholar] [CrossRef]
- Rada, B.; Boudreau, H.E.; Park, J.J.; Leto, T.L. Histamine stimulates hydrogen peroxide production by bronchial epithelial cells via histamine H1 receptor and dual oxidase. Am. J. Respir. Cell Mol. Biol. 2014, 50, 125–134. [Google Scholar] [CrossRef]
- Mousavi, S.; Sheikhzadeh, N.; Nasrabadi, H.T.; Salteh, S.A.; Khani Oushani, A.; Firouzamandi, M.; Mardani, K. Administration of grape (Vitis vinifera) seed extract to rainbow trout (Oncorhynchus mykiss) modulates growth performance, some biochemical parameters, and antioxidant-relevant gene expression. Fish Physiol. Biochem. 2020, 46, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Lv, X.H.; Zhao, H.X.; Chen, B.; Chen, X.Y.; Huang, W. Antioxidant and intestinal recovery function of condensed tannins in Lateolabrax maculatus responded to in vivo and in vitro oxidative stress. Aquaculture 2022, 547, 737399. [Google Scholar] [CrossRef]
- Li, M. Effects of Condensed Tannins on Growth Performance, Intestinal Structure and Immune Function as Well as the Mechanisms in On-Growing Grass Carp (Ctenopharyngodon idella). Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2019. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Pourmozaffar, S.; Mozanzadeh, M.T.; Adeshina, I.; Seemab, Z.A.; Sarahi, V. Dietary effect of grape seed proanthocyanidin extract on growth performance, serum biochemical parameters, skin mucosal immune response, and antioxidant capacity in goldfish (Carassius auratus). Ann. Anim. Sci. 2023, 23, 215–223. [Google Scholar] [CrossRef]
- Yaser, M.; Barzan, B.K.; Zarei, M.A. Effects of diets containing grape seed proanthocyanidin extract on the growth and oxidative capacity of common carp (Cyprinus carpio). Aquaculture 2021, 540, 736689. [Google Scholar] [CrossRef]
- Deng, C.Z. The Influences of Stocking Density and Grape Proanthocyanidins on Growth Physiology and Biochemistry of Red Tilapia. Master’s Thesis, Jimei University, Xiamen, China, 2015. [Google Scholar]
- Thompson, J.S.; Petersen, P.; Nguyen, B.L.; Quigley, E.M. Serum and intestinal diamine oxidase activity during intestinal adaptation. J. Investig. Surg. 1992, 5, 297–304. [Google Scholar] [CrossRef]
- Xu, R.; Lei, Y.; Shi, J.; Zhou, Y.; Chen, Y.; He, Z. Effects of lactadherin on plasma D-lactic acid and small intestinal MUC2 and claudin-1 expression levels in rats with rotavirus-induced diarrhea. Exp. Ther. Med. 2016, 11, 943–950. [Google Scholar] [CrossRef]
- Wu, Q.J.; Wang, Y.Q.; Qi, Y.X. The protective effect of procyanidin against LPS-induced acute gut injury by the regulations of oxidative state. SpringerPlus 2016, 5, 1645. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhang, S.C.; Cai, R.B.; Ren, S.C.; Zhou, X.J. Effects of chestnut tannins on the performance, intestinal morphology and permeability, and antioxidant status in weaned piglets. China Feed 2018, 597, 44–48. [Google Scholar] [CrossRef]
- Chao, D.; Yan, Z.; Xu, Y.; Zhexiu, C.; Qinghong, L.; Ruirong, H. Effects of grape seed procyanidins on antioxidant function, barrier function, microbial community, and metabolites of cecum in geese. Poult. Sci. 2023, 102, 102878. [Google Scholar]
- Kristiansen, M.; Merrifield, D.L.; Vecino, J.; Myklebust, R.; Ringoe, E. Evaluation of prebiotic and probiotic effects on the intestinal gut microbiota and histology of Atlantic salmon (Salmo salar L.). J. Aquacult. Res. Dev. 2011, s1, 9. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.D.; Liu, Z.H.; Yin, Y.L.; Xu, G.H.; Han, M.L.; Xie, L.W. Effect of dietary histamine on intestinal morphology, inflammatory status, and gut microbiota in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2021, 117, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.B.; Li, W.; Han, M.L.; Xv, G.H.; Xie, L.W.; Yin, Y.L.; Liang, J.Q. Protective effect of Lactobacillus reuteri on intestine and liver in Pseudobagrus fulvidraco induced by histamine. Acta Hydrobiol. Sin. 2019, 43, 94–101. [Google Scholar] [CrossRef]
- Opstvedt, J.; Mundheim, H.; Nygård, E.; Aase, H.; Pike, I.H. Reduced growth and feed consumption of Atlantic salmon (Salmo salar L.) fed fish meal made from stale fish is not due to increased content of biogenic amines. Aquaculture 2000, 188, 323–337. [Google Scholar] [CrossRef]
- Smolinska, S.; Winiarska, E.; Globinska, A.; Jutel, M. Histamine: A mediator of intestinal disorders-a review. Metabolites 2022, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Chen, B.; Zhao, H.X.; Wang, L.J.; Zhu, X.F.; Huang, W. Effects of condensed tannins on growth performance, body composition, apparent digestibility of nutrients, and intestinal morphology of Lateolabtax mculatus. China Anim. Husb. Vet. Med. 2022, 49, 2953–2960. [Google Scholar] [CrossRef]
- Laurent, C.; Besançon, P.; Auger, C.; Rouanet, J.; Caporiccio, B. Grape seed extract affects proliferation and differentiation of human intestinal Caco-2 cells. J. Agric. Food Chem. 2004, 52, 3301–3308. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, e17023. [Google Scholar] [CrossRef]
- Han, F.L.; Xu, C.; Qi, C.L.; Lin, Z.D.; Li, E.C.; Wang, C.L.; Wang, X.D.; Qin, J.G.; Chen, L.Q. Sodium butyrate can improve intestinal integrity and immunity in juvenile Chinese mitten crab (Eriocheir sinensis) fed glycinin. Fish Shellfish Immunol. 2020, 102, 400–411. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, X.; Liu, L.; Wang, X.; Li, H.; Zhu, J.; Cao, Y.; Zhu, H. Gut microbiota modulation by plant polyphenols in koi carp (Cyprinus carpio L.). Front. Microbiol. 2022, 13, 977292. [Google Scholar] [CrossRef]
- Lu, W.Q.; Yu, H.X.; Liang, Y.; Zhai, S.W. Evaluation of methanotroph (Methylococcus capsulatus, Bath) bacteria protein as an alternative to fish meal in the diet of juvenile American eel (Anguilla rostrata). Animals 2023, 13, 681. [Google Scholar] [CrossRef]
- Da, C.R.G.; Ramos, P.L.; Passarini, M.; Vieira, S.M.; Okamoto, D.N.; de Oliveira, L.; Zezzo, L.V.; Marem, A.; Santos, R.R.; Da, C.J.; et al. Cellulolytic and proteolytic ability of bacteria isolated from gastrointestinal tract and composting of a hippopotamus. AMB Express. 2016, 6, 17–26. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Lazado, C.C.; Caipang, C.M.A. Mucosal immunity and probiotics in fish. Fish Shellfish Immunol. 2014, 39, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Olmos Soto, J. Bacillus probiotic enzymes: External auxiliary apparatus to avoid digestive deficiencies, water pollution, diseases, and economic problems in marine cultivated animals. Adv. Food. Nutr. Res. 2017, 80, 15–35. [Google Scholar] [CrossRef]
- Kovács, D.; Palkovicsné Pézsa, N.; Jerzsele, Á.; Süth, M.; Farkas, O. Protective effects of grape seed oligomeric proanthocyanidins in IPEC-J2-Escherichia coli/Salmonella Typhimurium co-culture. Antibiotics 2022, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Chodak, A.D. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]

| Items | Groups | |||
|---|---|---|---|---|
| Control | HIS | H + OPC I | H + OPC II | |
| Amylase (U/mg prot) | 0.40 ± 0.01 | 0.39 ± 0.02 | 0.39 ± 0.01 | 0.43 ± 0.01 |
| Lipase (U/mg prot) | 15.35 ± 0.92 b | 9.08 ± 1.11 a | 16.97 ± 1.68 b | 14.08 ± 0.96 b |
| Protease (U/mg prot) | 182.30 ± 10.30 b | 127.33 ± 13.23 a | 158.32 ± 19.26 ab | 144.99 ± 18.76 ab |
| Items | Groups | |||
|---|---|---|---|---|
| Control | HIS | H + OPC I | H + OPC II | |
| MDA (nmol/mg prot) | 2.11 ± 0.41 a | 2.94 ± 0.54 b | 2.18 ± 0.22 a | 1.58 ± 0.45 a |
| T-AOC (mmol/g prot) | 1.24 ± 0.18 b | 0.84 ± 0.16 a | 1.23 ± 0.06 b | 1.49 ± 0.10 b |
| CAT (U/mg prot) | 8.89 ± 2.01 b | 5.32 ± 0.81 a | 6.02 ± 0.51 a | 5.92 ± 1.46 a |
| SOD (U/mg prot) | 37.21 ± 2.51 | 33.33 ± 4.61 | 37.71 ± 5.27 | 34.19 ± 3.26 |
| GSH-PX (U/mg prot) | 20.89 ± 2.30 c | 13.14 ± 1.37 a | 21.23 ± 1.84 c | 16.80 ± 1.97 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; He, Y.; Xi, F.; Liang, Y.; Zhai, S. Oligomeric Proanthocyanidins Alleviate the Detrimental Effects of Dietary Histamine on Intestinal Health of Juvenile American Eels (Anguilla rostrata). Fishes 2023, 8, 413. https://doi.org/10.3390/fishes8080413
Wang S, He Y, Xi F, Liang Y, Zhai S. Oligomeric Proanthocyanidins Alleviate the Detrimental Effects of Dietary Histamine on Intestinal Health of Juvenile American Eels (Anguilla rostrata). Fishes. 2023; 8(8):413. https://doi.org/10.3390/fishes8080413
Chicago/Turabian StyleWang, Shuo, Yingxia He, Feng Xi, Ying Liang, and Shaowei Zhai. 2023. "Oligomeric Proanthocyanidins Alleviate the Detrimental Effects of Dietary Histamine on Intestinal Health of Juvenile American Eels (Anguilla rostrata)" Fishes 8, no. 8: 413. https://doi.org/10.3390/fishes8080413
APA StyleWang, S., He, Y., Xi, F., Liang, Y., & Zhai, S. (2023). Oligomeric Proanthocyanidins Alleviate the Detrimental Effects of Dietary Histamine on Intestinal Health of Juvenile American Eels (Anguilla rostrata). Fishes, 8(8), 413. https://doi.org/10.3390/fishes8080413