Abstract
In this study, we investigated the functional role of Peroxiredoxin 5 (SmPrx5) in the cuttlefish Sepiella maindroni. The full-length SmPrx5 cDNA is 934 base pairs (bp) in length, comprising a 31 bp 5′ untranslated region (UTR), a 330 bp 3′ UTR, and an open reading frame (ORF) of 573 bp that encodes a polypeptide consisting of 190 amino acids. Sequence analysis revealed the presence of a conserved peroxidase catalytic motif VPGAFTPGCSQTHLPG and the signature domain DGTGLTCSL, indicating that SmPrx5 belongs to the 2-Cys Prx subfamily. Quantitative real-time PCR (RT-qPCR) analysis demonstrated that SmPrx5 is broadly expressed across various tissues in S. maindroni, with particularly high expression levels observed in the testes, hemocytes, liver, and ovaries. Upon challenge with Vibrio alginolyticus, SmPrx5 expression was significantly upregulated in both the liver and hemocytes, peaking at 24 h post-infection and gradually returning to baseline levels within 48 h. Furthermore, the recombinant SmPrx5 protein exhibited notable antioxidant activity in vitro, suggesting its involvement in the oxidative stress response. These findings enhance our understanding of the molecular mechanisms underlying immune defense in marine cephalopods and highlight the potential role of Prx5 in host immunity.
Keywords:
antioxidant activity; mRNA expression; Peroxiredoxin 5 (Prx5); recombinant protein; Sepiella maindroni; Vibrio alginolyticus Key Contribution:
This work provides the first molecular characterization of Prx5 in Sepiella maindroni, revealing its structural features, expression patterns under pathogenic and thermal stress conditions, and functional antioxidant activity. These results enhance our understanding of the antioxidant defense mechanisms in marine cephalopods and offer potential applications for improving cuttlefish aquaculture practices.
1. Introduction
Reactive oxygen species (ROS) have been proven to regulate various cellular processes, including cell differentiation, signal transduction, and ion transport [1]. While low, physiologic amounts of ROS are crucial for regular physiological functions [2], excessive ROS and oxidative stress can induce cell damage, genetic changes, and apoptosis [3]. Antioxidants have been proven to play important physiologic roles by scavenging ROS, helping maintain regular cellular functions [4]. Peroxiredoxins (Prx) are a common family of antioxidant enzymes that have been reported to resist oxidative damage [5]. This family of enzymes has been demonstrated to possess therapeutic benefits against ROS-mediated disorders [2]. The Prx family of enzymes consists of six isoforms classified into two sub-groups: the 2-Cys Prx group, which consists of Prx1 to Prx5, and the 1-Cys Prx group, which contains Prx6 [4].
Prx 5—also called PrxV, ACR1, AOEB166, or PMP20—is the most recently discovered member of the Prx family, and it has been shown to have unique properties [6]. Prx5 is expressed in multiple tissues that face oxidative stress [7]. This protein is also extensively distributed in subcellular fractions, including the cytosol, chondriosomes, nucleus, and peroxisomes [8]. The Prx5 gene has been widely investigated in mammals [9], teleosts [10], and invertebrates [11,12,13], highlighting its importance in physiological processes. Based on its important role as an antioxidant, Prx5 has been investigated as a potential therapeutic target against cancer [14,15], as well as a therapeutic antioxidant for managing metabolic disorders [16].
Cephalopods are characterized by high growth rates and rapid food conversion, which causes a distinguishing short cultivate cycle and a high biomass-to-production ratio, making their cultivation highly cost-effective. However, their culture is currently limited to a small number of species and is only feasible at a small scale [17]. Additionally, most cultures are of individuals captured from the wild [18]. Our lab previously explored a system of breeding Sepiella maindroni (cuttlefish). Cuttlefish is a commercially valuable fish that constitutes a large proportion of Chinese aquaculture. We previously created artificial culture cycles that can rear six cuttlefish generations in captivity. However, cuttlefish farming has been hampered by environment stimuli, including thermal stress, salinity, and pathogens [17]. As with other mollusk species, cuttlefish possess a rudimentary immune system [19] and do not have immunological memory [17], and therefore, they depend on innate immune responses.
The systematic study of non-specific immune-related enzymes can help us to understand the relationship between cephalopod immune function and its systematic evolution. Understanding S. maindroni Prx5 may improve our knowledge of the molecular mechanisms behind antioxidant and immune responses to pathogens and environmental stress, which, in turn, could enhance cultivation practices. Subsequently, this will lead to the development of more effective cuttlefish cultivation methods. In this study, we sought to clone the Prx5 gene, evaluate cuttlefish Prx5 expression patterns, evaluate the effects of pathogen invasion and thermal stress on SmPrx5 expression, and purify the Prx5 recombinant protein and characterize the antioxidant activity in vivo and in vitro.
2. Materials and Methods
2.1. Experimental Animals
Adult cuttlefish, with an average weight of 150.2 g, were bred at an artificial breeding aquafarm in Ningbo City, China (N29°35′, E121°59′). All experiments were carried out at a water temperature of 24 °C and a salinity level of 28 psu. Cuttlefish were fed ad libitum twice daily (08:00 and 16:00) with freshly thawed Exopalaemon carinicauda (mean length 3.5 cm) that was stored at 4 °C in filtered seawater for ≤24 h. Then, 30 min after feeding, feces and food remains were removed. The animals were anesthetized using a 5% ethanol solution. Tissues, including the muscles, ink sacs, liver, ovaries, testes, hemocytes, intestines, and gills, were then gathered and stored in RNAlater (TaKaRa, Tokyo, Japan). Animal handling and collection were carried out following approved guidelines and regulations [20].
2.2. Bacterial Challenge of S. maindroni In Vivo
Vibrio alginolyticus was cultured in seawater medium at a pH of 7.2 at 28 °C for 24 h and then centrifuged at 10,000 rpm for 15 min at 4 °C. The concentration of V. alginolyticus was adjusted to 1 × 107 CFU/mL using sterile PBS (pH 7.2), as determined by OD600 spectrophotometry (Shimadzu UV-1800, Shimadzu Corporation, Kyoto, Japan) with a pre-established calibration curve (R2 = 0.98); it was verified through plate counting on TCBS agar (24 h at 28 °C). It was then stored at 4 °C for <2 h before use [21]. To challenge the cuttlefish with bacterial infection, 100 individuals were randomly divided into two sets (50 individuals/group): the trial set and the control set. A total of 0.1 mL of resuspended bacteria was injected into each cuttlefish in the challenge group, while the control group received 1 × PBS. Five samples were randomly selected from each set at 0, 6, 12, 24, 48, 72, and 96 h after injection. Livers and gills were gathered and preserved as described in Section 2.1.
2.3. Thermal Temperature Stress of S. maindroni
Cuttlefish of an average of 3.1 cm were randomized into six groups for the thermal stress test. After acclimatization for 48 h, three sets were subjected to thermal stimulation by raising the water temperature to 32 °C at 1 °C per hour [22]. The control groups were maintained at a normal culture temperature (24 °C). Following thermal stimulation, five samples were randomly selected at 0, 6, 12, 24, 48, 72, and 96 h, and the livers and gills were collected and stored for later analysis as described in Section 2.1.
2.4. Full-Length cDNA Amplification of SmPrx5
Total RNA was extracted using Trizol (Invitrogen, Waltham, MA, USA). RNA quality was assessed on a 1.2% agarose gel, and the concentration was quantified using a NanoDrop-2000 (Thermo Fisher, Waltham, MA, USA) at 260 nm. A cuttlefish cDNA library was constructed using the Creator Smart cDNA Library Construction Kit (TaKaRa, Japan). The partial cDNA sequence of Prx5 (EST GT618047) was analyzed from this library. Full-length Prx5 was performed using a 3′-Full RACE kit (TaKaRa, Japan) based on the above partial sequence. The purified fragments were ligated into the vector pMD-18T (TaKaRa, Tokyo, Japan) and transformed into competent cells. Recombinants were screened using universal primers (Table 1) before being sequenced.

Table 1.
PCR primers used in the experiment.
2.5. Sequence Analysis of SmPrx5
Sequence alignment was performed using the BLAST program (NCBI BLAST version 2.15.0, https://www.ncbi.nlm.nih.gov/blast/, accessed on 15 April 2025). The deduced amino acid sequences of SmPrx5 were derived using ExPASy (http://www.expasy.org, accessed on 15 April 2025). Protein motif features were elucidated using the SMART Tool (http://smart.emblheidelberg.de/, accessed on 15 April 2025). The signal peptide was predicted by SignalP 5.0 (http://www.cbs.dtu.dk/services/SignalP, accessed on 15 April 2025) [23]. Multiple-sequence alignment of SmPrx5 was conducted using Clustal Omega (version 2.1). A phylogenetic tree was generated using the neighbor-joining (NJ) method via Molecular Evolutionary Genetic Analysis (MEGA) software version 6.0, validated by 1000 bootstrap replications. Prx sequences used in comparison were retrieved from the GenBank database.
2.6. Spatial and Temporal Expression Analysis of SmPrx5
RT-qPCR analysis was performed using an ABI 7300 PCR system (Applied Biosystems, Waltham, MA, USA). The reaction system and response procedures adhered to established protocols. The reactions were carried out in triplicate using the GAPDH gene as the reference gene. SmPrx5 expression was compared with the control using the values derived from employing the 2−ΔΔCt method [24].
2.7. Recombinant Expression and Purification of SmPrx5
Gene-specific primers P7 and P8 were used to amplify the polypeptide of SmPrx5 (Table 1), and the products were transferred into the pMD18-T simple vector (TaKaRa, Tokyo, Japan). NdeI and XhoI (New England Biolabs, Ipswich, MA, USA) were used to digest the production and subcloned into the expression vector pET-21a(+) (Novagen, Shanghai, China), which contains the NdeI and XhoI sites. E. coli Origami (DE3) (Novagen, Shanghai, China) was used to transform the recombinant plasmid. Purified recombinant proteins (designated as rSmPrx5) were separated by 15% SDS-PAGE with a protein marker.
2.8. Antioxidant Properties of SmPrx5 In Vitro
The antioxidant activity of SmPrx5 was determined using a guaiacol peroxidase assay (Nanjing Jiancheng Institute of Biological Engineering, Nanjing, China) with modifications. Reaction kinetics were monitored at 420 nm for 3 min (25 °C) using a UV-vis spectrophotometer (Shimadzu UV-1800). One unit (U) of enzyme activity is defined as the amount of enzyme that catalyzes the decomposition of 1 μmol H2O2 per minute, as calculated using the extinction coefficient ε420 = 6.39 mM−1cm−1 for oxidized guaiacol. Specific activity was expressed as U/mg protein (Bradford assay). Standardization was performed according to the manufacturer’s protocols with parallel controls.
SPSS statistical software package (version 19.0; SPSS, Inc., Chicago, IL, USA) was used for data analysis. The data were expressed as mean ± SD (N = 3). Group differences were compared using a one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test. p < 0.05 was considered statistically significant. Graphs and figures were prepared using Excel, GraphPad Prism 8.0, and Origin 8.0.
3. Results
3.1. Sequence and Phylogenetic Analysis of SmPrx5
SmPrx5 (GenBank accession no: KP657486.1) is 934 bp long and consists of a 31-base-pair 5′ UTR, a 573-base-pair ORF, and a 330-base-pair 3′ UTR. This cDNA encodes 190 amino acids, has a predicted molecular mass of 20.7 kDa, and has a deduced isoelectric point of 8.76. The full length of the nucleotide sequence and the deduced amino acid sequence are shown in Figure 1.

Figure 1.
The nucleotide and deduced amino acid sequence of SmPrx5. Numbers to the left of each row refer to nucleotide or amino acid positions; the initiation codon (ATG) and the termination codon (TAA) are boxed (the TAA needs to be boxed and the red font removed from ATG); the Prx5 family signature is shaded; two active cysteines’ amino acid signature sequences are boxed, the polyadenylation signal sequence (AATAA) appears in red font (the text in italics needs to be removed), and the poly (A) signal sequence is italicized (the text in italics needs to be added).
BLAST analysis revealed that cuttlefish Prx5 shares 66% of its identity with Octopus bimaculoides Prx5, 63% with Crassostrea gigas Prx5, 63% with Branchiostoma tsingtaunese Prx5, and 60% with Boleophthalmus pectinirostris Prx5 (Figure 2). About 30 N-terminal amino acid residues present at the front of SmPrx5 were deduced to specify a mitochondrion-targeting peptide (iPSORT, Version 6.3). They were hypothesized to form intramolecular disulfide bonds.
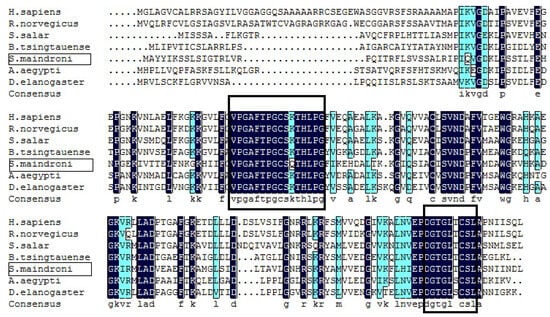
Figure 2.
Multiple alignments of the deduced amino acid sequence of SmPrx5 with other Prx5 genes deposited in GenBank. The dark-blue-shaded sequences indicate positions where all sequences share the same amino acid residues. The light blue boxed sequences highlight conserved motifs or functional domains, while the black boxed sequences represent regions of particular interest, such as potential active sites or unique signature sequences. Gaps are indicated by dashes to improve the alignment.
Phylogenetic analysis showed that peroxiredoxins fall into three clusters (Figure 3). The Prx 1-4 proteins clustered together belonged to the typical 2-Cys group. Prx6 proteins were clustered into the 1-Cys cluster, while SmPrx5 was clustered into the atypical 2-Cys.
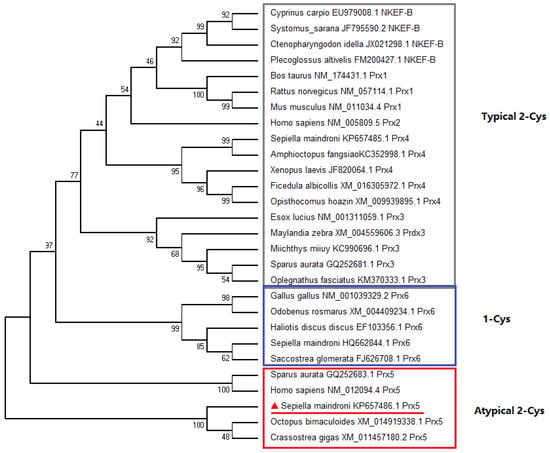
Figure 3.
Consensus neighbor-joining tree based on the sequences of peroxiredoxin from different animals. The accession number of each sequence is presented next to the organism. The subcluster is represented by a box with the name next to it.
3.2. Tissue Distribution of SmPrx5
RT-qPCR analysis of SmPrx5 expression in the liver, muscles, gills, intestines, ink sacs, ovaries, testes, and hemocytes indicates that it is ubiquitously expressed. Its expression was found to be highest in the liver—followed by the gills and hemocytes—and lowest in the muscles (Figure 4).
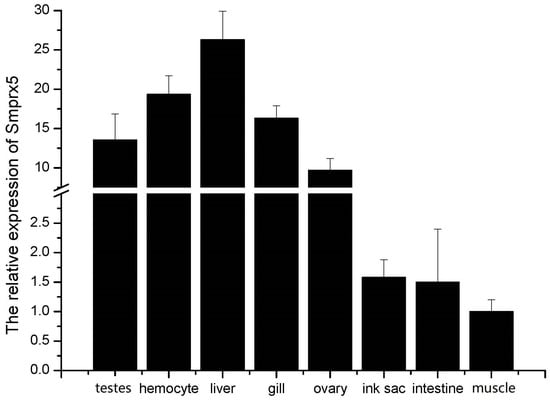
Figure 4.
The expression levels of SmPrx5 in different tissues were determined by SYBR Green RT-qPCR. For each tissue, total RNA was extracted from three independent cuttlefish samples. Each sample was subjected to qPCR analysis in triplicate (technical replicates), and the results were averaged to obtain the mean value. The error bars represent the standard deviation (SD) of the technical replicates.
3.3. SmPrx5 Expression Following Bacterial Challenge and Thermal Stress
Analysis of SmPrx5 expression following environmental and pathogen challenge revealed it was temporally upregulated in the gills and liver (Figure 5). SmPrx5 mRNA levels significantly increased over time and peaked at 12 h in the liver and gills after V. alginolyticus challenge. Although the expression began to fall after peaking, it remained elevated for up to 96 h relative to unchallenged levels (p < 0.05). Upon thermal stress, SmPrx5 expression rose significantly and peaked after 6 h (Figure 6). Expression gradually declined over the next 24 h and then remained relatively high, in relation to pre-challenge levels, until 96 h had passed (p < 0.05) (Figure 6A). The same trend was observed in the gills (p < 0.05) (Figure 6B).
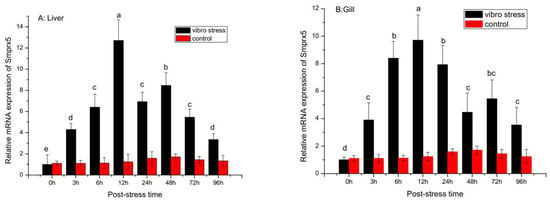
Figure 5.
Temporal expression profile of the SmPrx5 transcript measured by SYBR Green qRT-PCR after V. alginolyticus challenge in the liver (A) and gills (B). S. maindroni larvae challenge by V. alginolyticus, detected at 0, 6, 12, 24, 48, 72, and 96 h. The untreated group was set up as the blank control. Vertical bars represent the mean ± S.D. (n = 3), and bars with different letters are significantly different (p < 0.05).
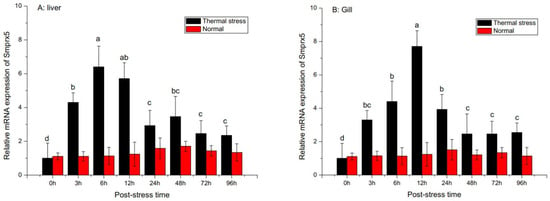
Figure 6.
Temporal expression profile of the SmPrx5 transcript measured by SYBR Green qRT-PCR after thermal stress in the liver (A) and gills (B). The S. maindroni larvae challenge detected at 0, 6, 12, 24, 48, 72, and 96 h. The untreated group was set up as the blank control. Vertical bars represent the mean ± S.D. (n = 5), and bars with different letters are significantly different (p < 0.05).
3.4. Characterization of Recombinant Protein
The pET-21a-SmPrx5 recombinant plasmid was transformed into E. coli Origami (DE3). The whole cell lysate of positive clones induced by IPTG was obtained and separated by SDS-PAGE. This analysis exposed a separate band with a molecular weight of about 20 kDa (Figure 7).
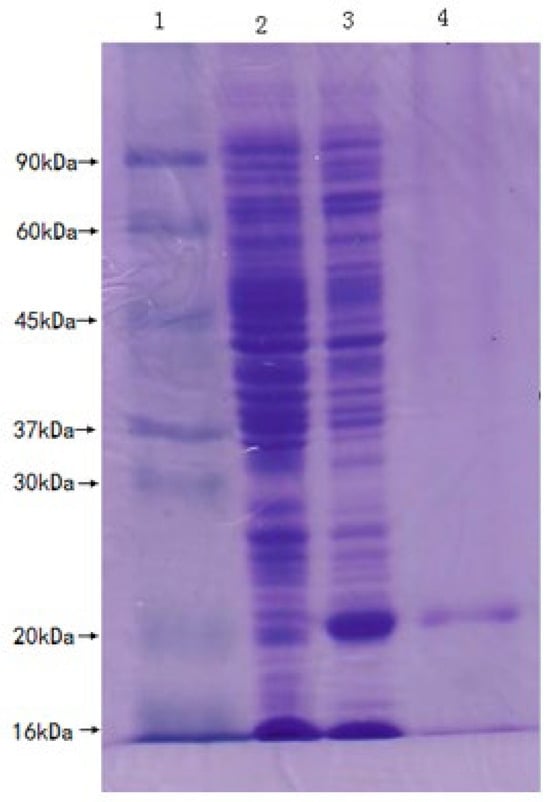
Figure 7.
Expression of the recombinant SmPrx5 protein in E. coli. Lane 1: protein molecular weight standards; lane 2: control without IPTG induction; lane 3: expression of rSmPrx5 protein (20 kDa) after IPTG induction; lane 4: purified rSmPrx5 protein (20 kDa).
3.5. Antioxidant Activity of rSmPrx5
Purified rSmPrx5 was diluted to 500 μg/mL using a BCA protein assay kit (Sangon Biotech, Shanghai, China). The guaiacol assay was used to test the antioxidant activity of rSmPrx5. The experimental groups showed higher reducing activity than the control group (Figure 8).
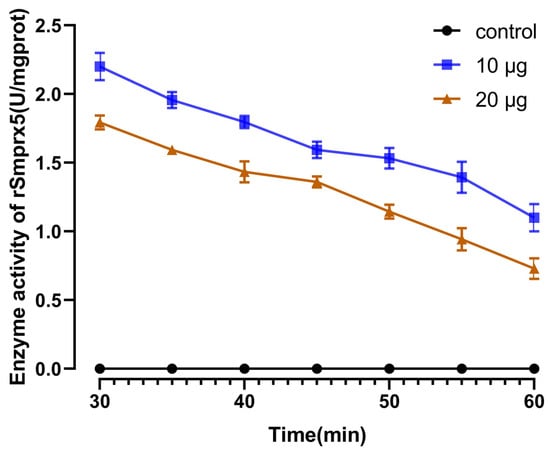
Figure 8.
The antioxidant activity of recombinant SmPrx5 measured by the guaiacol assay. The absorbance at OD420 was determined, and the reaction time was drawn following the reagent kit instructions. The data are expressed as mean ± S.D. (n = 3).
4. Discussion
SmPrx5 contains conserved structural features in relation to Prx5 from other species. SmPrx5 bears a 330-base-pair 3′ UTR. Its ORF encodes 190 amino acids, including 2 conserved Cys residues (Cys78 and Cys179) and a non-conserved Cys residue (Cys103). Cys78 is found in the active site, which could be oxidized to sulfenic acid through peroxide. Once oxidized, the active site combines with Cys78 to shape an intramolecular disulfide bond. Phylogenetic analysis showed that SmPrx5 was clustered with the atypical 2-Cys. This suggested that multiple transcripts can be produced from this gene in a process akin to alternative splicing.
The analysis revealed that SmPrx5 is expressed ubiquitously at varying levels across tissues. A similarly widespread expression of Prx5 was discovered in the rockfish Sebastes schlegelii [25], miiuy croaker Miichthys miiuy [26], rock bream Oplegnathus fasciatus [27], common cutworm Spodoptera litura [13], and Mus musculus [28]. In our study, we found that the highest expression occurs in the liver, at levels identical to those of Prx5 expression in crustaceans, including the ridge-tail white prawn Exopalaemon carinicauda [11], Procambarus clarkia [29], and mud crab Scylla paramamosain [30]. In marine invertebrates, the hepatopancreas plays a vital role in the innate immunity system, and its function is thought to be analogous to that of the mammalian liver and pancreas. This organ plays an important role in metabolism and immunity [27].
We also found a high level of SmPrx5 in gills, where it was expressed at levels similar to those in the bay scallop Argopecten irradians [12] and Zhikong scallop Chlamys farreri [31]. Reflecting its function as a respiratory organ in aquatic organisms, gills are almost entirely exposed to external water. They are therefore in contact with external toxins and pathogens as they extract oxygen from seawater.
The widespread expression of Prx5 across tissues exhibited its importance in a series of physiological processes, including ROS scavenging [32,33], apoptosis [34], cell differentiation [35], proliferation [36], and immunity [12,26,29,37,38,39,40]. However, the expression and modulation of peroxiredoxin function might differ between species and be responsive to environmental and nutritional cues.
SmPrx5 expression following V. alginolyticus challenge was found to increase significantly over time and peaked after 12 h in the liver and gills, indicating that SmPrx5 expression might be responsive to bacterial infection. The higher expression of the SmPrx5 gene in tissues such as the gill and liver, which are expected to be involved in an immune response to environmental challenges, suggests that the SmPrx5 protein plays a role in the context of cuttlefish innate immunity. Following bacterial challenge, SmPrx5 progressively declined for 96 h but remained higher than the pre-challenge level. Together, these results suggest that following bacterial infection, the innate immune system of SmPrx5 is momentarily affected but transiently made up for by a systemic immune response [41].
Oxidative stress is often triggered by environmental stress (thermal, salinity, and pH change). High-temperature stimuli could upregulate Prx transcript levels, thus highlighting the impact of environment cues on gene expression [42]. Several studies have investigated the role of Prx upon thermal stimulation in the 1-Cys group [14]. The purified recombinant SmPrx5 protein effectively scavenges hydrogen peroxide (H2O2) in vitro, demonstrating its antioxidant activity. This suggests that SmPrx5 may play a protective role against oxidative stress and could potentially be explored for therapeutic applications against ROS-induced disorders. Our findings are consistent with previous reports [12,29,30,39,43]. It has been reported that the peroxidase activity of Prx5 depends on its affinity to H2O2. These findings propose that recombinant SmPrx5 protein is an effective antioxidant that might have therapeutic benefits against ROS-induced disorders [44,45,46]. Further research evaluating this possibility is therefore warranted.
5. Conclusions
This study characterized the Peroxiredoxin 5 gene (SmPrx5) from cuttlefish (Sepiella maindroni) and demonstrated its crucial antioxidant role during bacterial infections and thermal stress. SmPrx5 is ubiquitously expressed across tissues, with particularly high levels in the liver, gills, testes, and ovaries. Its expression is significantly upregulated following Vibrio alginolyticus challenge and heat stress, and the recombinant SmPrx5 protein exhibits strong antioxidant activity in vitro. These findings suggest that SmPrx5 plays a vital role in the immune response and adaptation to environmental stress in cuttlefish.
Author Contributions
Conception and design of experiments: W.S., C.S. and C.W. Performing experiments: C.S. and W.S. Data analysis: W.S., C.S. and C.W. Contributing reagents/materials/analysis tools: W.S. and C.S. Writing the paper: C.S., W.S. and C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of Zhejiang Province (LY18C190006) and sponsored by K.C. Wong Magna Fund in Ningbo University.
Institutional Review Board Statement
This work was approved by the Animal Care and Use committee at the School of Marine Sciences, Ningbo University. The animal handling and collection procedures involved in this study were carried out following approved guidelines [20] and regulations (Standardization Administration of China, 2018 GB/T 35892-2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roch, P. Defense mechanisms and disease prevention in farmed marine invertebrates. Aquaculture 1999, 172, 125–145. [Google Scholar] [CrossRef]
- Poynton, R.A.; Hampton, M.B. Peroxiredoxins as biomarkers of oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-J.; Hu, Y.-H.; Zhang, M.; Sun, L. Analysis of the expression and antioxidative property of a peroxiredoxin 6 from Scophthalmus maximus. Fish Shellfish Immunol. 2010, 29, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Schroder, E.; Harris, J.R.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Robinson, M.W.; Hutchinson, A.T.; Dalton, J.P.; Donnelly, S. Peroxiredoxin: A central player in immune modulation. Parasite Immunol. 2010, 32, 305–313. [Google Scholar] [CrossRef]
- Seo, M.S.; Kang, S.W.; Kim, K.; Baines, I.C.; Lee, T.H.; Rhee, S.G. Identification of a New Type of Mammalian Peroxiredoxin That Forms an Intramolecular Disulfide as a Reaction Intermediate. J. Biol. Chem. 2000, 275, 20346–20354. [Google Scholar] [CrossRef]
- Knoops, B.; Loumaye, E.; Van Der Eecken, V. Evolution of the peroxiredoxins. Subcell. Biochem. 2007, 44, 27–40. [Google Scholar]
- Declercq, J.-P.; Evrard, C.; Clippe, A.; Stricht, D.V.; Bernard, A.; Knoops, B. Crystal structure of human peroxiredoxin 5, a novel type of mammalian peroxiredoxin at 1.5 Å resolution. J. Mol. Biol. 2001, 311, 751–759. [Google Scholar] [CrossRef]
- Knoops, B.; Goemaere, J.; Van der Eecken, V.; Declercq, J.-P. Peroxiredoxin 5: Structure, Mechanism, and Function of the Mammalian Atypical 2-Cys Peroxiredoxin. Antioxidants Redox Signal. 2011, 15, 817–829. [Google Scholar] [CrossRef]
- Perez-Sanchez, J.; Bermejo-Nogales, A.; Calduch-Giner, J.A.; Kaushik, S.; Sitja-Bobadilla, A. Molecular characterization and expression analysis of six peroxiredoxin paralogous genes in gilthead sea bream (Sparus aurata): Insights from fish exposed to dietary, pathogen and confinement stressors. Fish Shellfish Immunol. 2011, 31, 294–302. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, P.; Li, J.; Li, J.; Gao, B.; Chen, P. cDNA cloning, characterization and expression analysis of peroxiredoxin 5 gene in the ridgetail white prawn Exopalaemon carinicauda. Mol. Biol. Rep. 2013, 40, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Zhang, S.; Zhang, G. Cloning, genomic structure, and expression analysis of peroxiredoxin V from bay scallop Argopecten irradians. Fish Shellfish Immunol. 2011, 30, 309–316. [Google Scholar] [CrossRef]
- Wan, H.; Kang, T.; Zhan, S.; You, H.; Zhu, F.; Lee, K.S.; Zhao, H.; Jin, B.R.; Li, J. Peroxiredoxin 5 from common cutworm (Spodoptera litura) acts as a potent antioxidant enzyme. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 175, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.-M.; Yoo, J.-W.; Lee, S.; Lee, H.J.; Lee, H.-S.; Lee, D.-S. Peroxiredoxin 5 promotes the epithelial-mesenchymal transition in colon cancer. Biochem. Biophys. Res. Commun. 2017, 487, 580–586. [Google Scholar] [CrossRef]
- Feng, X.; Liu, J.; Fan, S.; Liu, F.; Li, Y.; Jin, Y.; Bai, L.; Yang, Z. The identification of goat peroxiredoxin-5 and the evaluation and enhancement of its stability by nanoparticle formation. Sci. Rep. 2016, 6, 24467. [Google Scholar] [CrossRef]
- Sienko, J.; Gaj, P.; Czajkowski, K.; Nowis, D. Peroxiredoxin-5 is a negative survival predictor in ovarian cancer. Ginekol. Polska 2019, 90, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Martínez, S.; Gestal, C. Pathogens and immune response of cephalopods. J. Exp. Mar. Biol. Ecol. 2013, 447, 14–22. [Google Scholar] [CrossRef]
- Vidal, E.A.; Villanueva, R.; Andrade, J.P.; Gleadall, I.G.; Iglesias, J.; Koueta, N.; Rosas, C.; Segawa, S.; Grasse, B.; Franco-Santos, R.M.; et al. Cephalopod culture: Current status of main biological models and research priorities. Adv. Mar. Biol. 2014, 67, 90–98. [Google Scholar]
- Müller, V.; de Boer, R.J.; Bonhoeffer, S.; Szathmáry, E. An evolutionary perspective on the systems of adaptive immunity. Biol. Rev. 2017, 93, 505–528. [Google Scholar] [CrossRef]
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; de Girolamo, P.; D’Angelo, L.; Mark, F. Guidelines for the care and welfare of cephalopods in research: A consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab Anim. 2015, 49 (Suppl. 2), 1–90. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, Q.; Yao, Z.; Zhang, Z.; Zhang, H.; Wang, Y. Immunoglobulin M gene expression analysis of orange-spotted grouper, Epinephelus coioides, following heat shock and Vibrio alginolyticus challenge. Fish Shellfish Immunol. 2010, 29, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-M.; Lu, Z.-R.; He, H.-J.; Ye, P.-L.; Ying, Z.; Wang, C.-L. Effects of several ecological factors on the hatching of Sepiella maindroni wild and cultured eggs. Chin. J. Appl. Ecol. 2010, 21, 1321–1326. [Google Scholar]
- Schultz, J.; Milpetz, F.; Bork, P.; Ponting, C.P. SMART, a simple modular architecture research tool: Identification of signaling domains. Proc. Natl. Acad. Sci. USA 1998, 95, 5857–5864. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Godahewa, G.I.; Perera, N.C.N.; Nam, B.-H.; Lee, J. Antioxidative properties and structural features of atypical 2-Cys peroxiredoxin from Sebastes schlegelii. Dev. Comp. Immunol. 2018, 82, 152–164. [Google Scholar] [CrossRef]
- Ren, L.; Sun, Y.; Wang, R.; Xu, T. Gene structure, immune response and evolution: Comparative analysis of three 2-Cys peroxiredoxin members of miiuy croaker, Miichthys miiuy. Fish Shellfish Immunol. 2014, 36, 409–416. [Google Scholar] [CrossRef]
- Revathy, K.S.; Umasuthan, N.; Whang, I.; Jung, H.-B.; Lim, B.-S.; Nam, B.-H.; Lee, J. A potential antioxidant enzyme belonging to the atypical 2-Cys peroxiredoxin subfamily characterized from rock bream, Oplegnathus fasciatus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 187, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Kim, S.J.; Kang, S.W.; Lee, K.-K.; Rhee, S.G.; Yu, D.-Y. Molecular Cloning and Characterization of the Mouse Peroxiredoxin V Gene. Biochem. Biophys. Res. Commun. 2000, 270, 356–362. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Abbas, M.N.; Kausar, S.; Chen, Q.; Jiang, C.-X.; Dai, L.-S. Molecular structure and functional characterization of the peroxiredoxin 5 in Procambarus clarkii following LPS and Poly I:C challenge. Fish Shellfish Immunol. 2017, 71, 28–34. [Google Scholar] [CrossRef]
- Tu, D.-D.; Jiang, M.; Gu, W.-B.; Zhou, Y.-L.; Zhu, Q.-H.; Zhou, Z.-K.; Chen, Y.-Y.; Shu, M.-A. Identification and characterization of atypical 2-cysteine peroxiredoxins from mud crab Scylla paramamosain: The first evidence of two peroxiredoxin 5 genes in non-primate species and their involvement in immune defense against pathogen infection. Fish Shellfish Immunol. 2017, 69, 119–127. [Google Scholar] [CrossRef]
- Cong, M.; Ni, D.; Song, L.; Wang, L.; Zhao, J.; Qiu, L.; Li, L. Molecular cloning, characterization and mRNA expression of peroxiredoxin in Zhikong scallop Chlamys farreri. Mol. Biol. Rep. 2008, 36, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, S.; Lee, B.-L. Recent Advances in the Innate Immunity of Invertebrate Animals. BMB Rep. 2005, 38, 128–150. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Murrell, G.A.; Trickett, A.; Landtmeters, M.; Knoops, B.; Wang, M.X. Overexpression of antioxidant enzyme peroxiredoxin 5 protects human tendon cells against apoptosis and loss of cellular function during oxidative stress. Biochim. Biophys. Acta 2004, 1693, 37–45. [Google Scholar] [CrossRef]
- Lu, J.L.; Vallat, J.-M.; Pollard, J.D.; Knoops, B.; Ouvrier, R. Expression of the antioxidant enzyme peroxiredoxin 5 in the human peripheral nervous system. J. Peripher. Nerv. Syst. 2006, 11, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Baković, J.; Yu, B.Y.K.; Silva, D.; Chew, S.P.; Kim, S.; Ahn, S.-H.; Palmer, L.; Aloum, L.; Stanzani, G.; Malanchuk, O.; et al. A key metabolic integrator, coenzyme A, modulates the activity of peroxiredoxin 5 via covalent modification. Mol. Cell. Biochem. 2019, 461, 91–102. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Li, P.; Bao, Y.; Guan, X.; Lian, Z.; Yin, Y.; Ding, C.; Yu, S. Brucella infection regulates peroxiredoxin-5 protein expression to facilitate intracellular survival by reducing the production of nitric oxide and reactive oxygen species. Biochem. Biophys. Res. Commun. 2019, 516, 82–88. [Google Scholar] [CrossRef]
- Pushpamali, W.A.; De Zoysa, M.; Kang, H.S.; Oh, C.H.; Whang, I.; Kim, S.J.; Lee, J. Comparative study of two thioredoxin peroxidases from disk abalone (Haliotis discus discus): Cloning, recombinant protein purification, characterization of antioxidant activities and expression analysis. Fish Shellfish Immunol. 2008, 24, 294–307. [Google Scholar] [CrossRef]
- Radyuk, S.N.; Michalak, K.; Klichko, V.I.; Benes, J.; Orr, W.C. Peroxiredoxin 5 modulates immune response in Drosophila. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2010, 1800, 1153–1163. [Google Scholar] [CrossRef]
- Wang, X.; Hu, B.; Wen, C.; Zhang, M.; Jian, S.; Yang, G. Molecular cloning, expression and antioxidative activity of 2-cys-peroxiredoxin from freshwater mussel Cristaria plicata. Fish Shellfish Immunol. 2017, 66, 254–263. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, F.; Zhang, J.; Wang, B.; Gao, H.; Huang, B.; Jiang, H.; Xiang, J. Molecular cloning, expression of a peroxiredoxin gene in Chinese shrimp Fenneropenaeus chinensis and the antioxidant activity of its recombinant protein. Mol. Immunol. 2007, 44, 3501–3509. [Google Scholar] [CrossRef]
- Arockiaraj, J.; Easwvaran, S.; Vanaraja, P.; Singh, A.; Othman, R.Y.; Bhassu, S. Immunological role of thiol-dependent peroxiredoxin gene in Macrobrachium rosenbergii. Fish Shellfish Immunol. 2012, 33, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Ahn, I.-Y.; Kim, H.; Cheon, J.; Kim, M. Analysis of ESTs and expression of two peroxiredoxins in the thermally stressed Antarctic bivalve Laternula elliptica. Fish Shellfish Immunol. 2008, 25, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.N.; Kausar, S.; Cui, H. The biological role of peroxiredoxins in innate immune responses of aquatic invertebrates. Fish Shellfish Immunol. 2019, 89, 91–97. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, H.J.; Lee, S.R.; Lee, H.S.; Huh, J.W.; Bae, Y.C.; Lee, D.S. Peroxiredoxin 5 inhibits glutamate-induced neuronal cell death through the regulation of calcineurin-dependent mitochondrial dynamics in HT22 cells. Mol. Cell Biol. 2019, 39, e00045-19. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Kam, M.K.; Kim, K.M.; Kim, H.S.; Kwon, O.-S.; Lee, H.-S.; Lee, D.-S. Peroxiredoxin 5 prevents iron overload-induced neuronal death by inhibiting mitochondrial fragmentation and endoplasmic reticulum stress in mouse hippocampal HT-22 cells. Int. J. Biochem. Cell Biol. 2018, 102, 10–19. [Google Scholar] [CrossRef]
- Pirson, M.; Clippe, A.; Knoops, B. The curious case of peroxiredoxin-5: What its absence in aves can tell us and how it can be used. BMC Evol. Biol. 2018, 18, 18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).